Abstract
Weaning from invasive mechanical ventilation is an important part of the management of respiratory failure patients. Patients can be classified into those who wean on the first attempt (simple weaning), those who require up to three attempts (difficult weaning) and those who require more than three attempts (prolonged weaning). The process of weaning includes adequately treating the underlying cause of respiratory failure, assessing the readiness to wean, evaluating the response to a reduction in ventilatory support, and eventually liberation from mechanical ventilation and extubation or decannulation. Post-extubation respiratory failure is a contributor to poorer outcomes. Identifying and addressing modifiable risk factors for post-extubation respiratory failure is important; noninvasive ventilation and high-flow nasal cannulae may be useful bridging aids after extubation. Factors to consider in the pathophysiology of prolonged mechanical ventilation include increased respiratory muscle load, reduced respiratory muscle capacity and reduced respiratory drive. Management of these patients involves a multidisciplinary team, to first identify the cause of failed weaning attempts, and subsequently optimise the patient's physiology to improve the likelihood of being successfully weaned from invasive mechanical ventilation.
Shareable abstract
Prolonged weaning from mechanical ventilation requires a comprehensive multidisciplinary management strategy, focusing on each potential cause of respiratory failure https://bit.ly/40uEf1a
Educational aims
Describe the process of weaning a patient from invasive mechanical ventilation.
Explain the pathophysiology of weaning failure.
Provide management strategies that should be applied when managing a patient undergoing prolonged mechanical ventilation.
Introduction
Weaning from mechanical ventilation is a key task for any critical care specialist with 3% of all hospital admissions requiring mechanical ventilation [1]. Understanding the weaning process is essential not only for critical care specialists, but all hospital physicians, as they are likely to be caring for patients after they have been weaned.
Weaning should be considered in four steps: 1) adequately treating the underlying cause of respiratory failure, 2) assessment of the readiness to wean, 3) evaluation of the response to a reduction in ventilatory support, and 4) liberation from mechanical ventilation and extubation or decannulation. Early weaning is important, and the longer a patient receives invasive ventilation the more likely they are to develop complications, such as ventilator-associated pneumonia (VAP) [2, 3] and long-term weaning failure [4], both of which are associated with a greater mortality [5, 6]. In addition, the cost of care clearly rises with each day of mechanical ventilation [7, 8]. This review will first summarise the process of weaning from mechanical ventilation. It will then explore in more detail the pathophysiology of patients requiring prolonged weaning and management strategies to improve outcomes in patients who are difficult to wean.
Weaning classification
The 2005 International Consensus Conference in Intensive Care Medicine [9] provided a classification of weaning based on the time taken to successfully wean. Weaning success is defined as being liberated from any form of invasive mechanical support. Patients may be breathing spontaneously, or they may be supported by noninvasive positive pressure therapy. Simple weaning patients (group 1) are those who proceed from initiation of weaning to successful extubation on the first attempt without difficulty. Difficult weaning patients (group 2) are those who fail initial weaning and require up to three spontaneous breathing trials (SBTs) or up to 7 days from the first SBT to achieve successful weaning. Prolonged weaning patients (group 3) are those who fail at least three weaning attempts or require >7 days of weaning after the first SBT [9]. Of all patients receiving invasive mechanical ventilation, 24% did not have a weaning attempt, 57% weaned in <24 h (group 1), 10% weaned between 1 and 7 days (group 2), while 9% required >7 days to wean from mechanical ventilation (group 3) [10]. The “Weaning according to a New Definition” (WIND) study suggested further subdividing group 3 into 3a (prolonged weaning leading to a successful wean 7 days or more after the first attempt) and 3b (prolonged weaning without success) [10, 11]. The recent WEAN SAFE study, which analysed weaning outcomes in 5869 patients across 50 countries, reported that 35% of patients remained on mechanical ventilation at 90 days. They reported that 65% of patients had a weaning time of <1 day, 10% had a weaning time of 2–6 days, 10% a weaning time of >6 days, and 15% had weaning failure [12]. A limitation of assessing the incidence of weaning failure lies in the varying definitions of failure used in the literature [13]; however, an aggregate of several studies reported a weaning failure rate of 31% [9]. Similarly, other cohort studies of patients referred to regional weaning centres reported a weaning failure rate of 30–40% [14–16]. In this review, weaning failure after endotracheal intubation is defined as either a failed SBT, reintubation and/or resumption of ventilator support following successful extubation, or death within 48 h following extubation [9]. Weaning failure after prolonged weaning is defined as death prior to discharge from hospital or the need for invasive mechanical ventilation after discharge. It is important to note that there is considerable worldwide variation in weaning practices [12, 17, 18].
Process of weaning
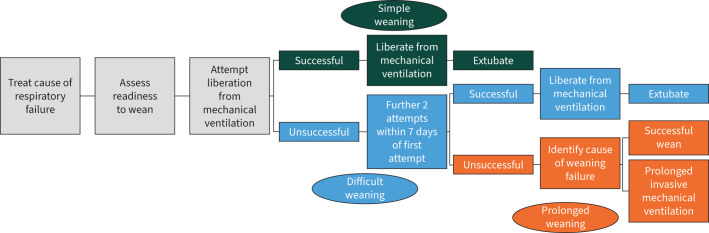
The process of weaning from mechanical ventilation (figure 1) can be separated into four steps:
-
1)
Adequately treating the underlying cause of respiratory failure
-
2)
Assessment of the readiness to wean
-
3)
Evaluation of the response to a reduction in ventilatory support
-
4)
Liberation from mechanical ventilation and extubation or decannulation
FIGURE 1.
The process of weaning from invasive mechanical ventilation.
Exploring the management of the underlying cause of respiratory failure in patients requiring mechanical ventilation is beyond the scope of this review. Suffice to say, however, that without the underlying cause being diagnosed and managed, any attempt to wean from mechanical ventilation is unlikely to be successful.
Assessing the readiness to wean
The frequent assessment of the readiness of a patient to wean is a key part of the weaning process and its importance is emphasised by the negative sequelae of delayed weaning [12]. Data suggest that clinicians frequently underestimate the ability of patients to successfully wean from mechanical ventilation; many patients are extubated on the first day of readiness assessments [19] and approximately half of self-extubations do not require reintubation [20]. This is relevant to all clinicians as a prospective cohort study reported that mortality was more than double when extubation was delayed [21]. It is essential that readiness to wean is assessed at least daily, to ensure early weaning is initiated as soon as the patient is ready to wean. A daily assessment of weaning readiness has been demonstrated to be a predictor of weaning success in itself [22]. Despite this, there is wide international variation in the practice of assessing the readiness to wean on a daily basis [18]. Assessment of the readiness to wean incorporates clinical assessment as well as measurement of objective markers. Clinical assessment includes confirming that the patient has adequate cough function and an absence of excessive tracheobronchial secretions. In addition, the clinician should ensure that the acute phase of the primary cause of respiratory failure for which the patient was intubated has resolved. Key objective markers to be assessed daily are displayed in table 1.
TABLE 1.
Key factors to be assessed daily when considering the readiness of a patient to wean
| Clinical assessment |
| Adequate cough |
| Absence of excessive tracheobronchial secretions |
| Resolution of the acute phase of the disease for which the patient was intubated |
| Objective measurements |
| Stable cardiovascular status (i.e. heart rate ≤140 beats·min−1, systolic blood pressure 90–160 mmHg, no or minimal vasopressors) |
| Stable metabolic status |
| Adequate oxygenation |
| Arterial oxygen saturation >90% on FIO2 ≤0.4 (or PaO2/FIO2 ≥150 mmHg) |
| PEEP ≤8 cmH2O |
| Adequate pulmonary function |
| Respiratory rate ≤35 breaths·min−1 |
| Maximal inspiratory pressure ≤ −20 to −25 cmH2O |
| Tidal volume >5 mL·kg−1 |
| Vital capacity >10 mL·kg−1 |
| Rapid shallow breathing index (respiratory rate/tidal volume) ≤105 breaths·min−1·L−1 |
| No significant respiratory acidosis |
| Adequate mentation |
| No sedation or adequate mentation on sedation (or stable neurological patient) |
| Tympanic temperature between 36°C and 38°C |
| No relevant electrolyte disorder and haemoglobin level 70–80 g·L−1 |
FIO2: inspiratory oxygen fraction; PaO2: arterial oxygen tension; PEEP: positive end-expiratory pressure. Reproduced and modified from [9] with permission.
It is important to emphasise that these criteria are only indicators of potential successful weaning and not absolute prerequisites that must be met before weaning attempts. The clinician will need to use these criteria to guide their own clinical practice. Patients not satisfying all of the criteria can often be weaned successfully [22]. Indeed, the Weaning Task Force identified 462 weaning predictors in 65 observational studies and evaluated the ability of each variable to predict the outcome of an SBT [23]. The pooled likelihood ratios of the predictors were all found to have a low predictive power. At the time, the task force concluded that weaning predictors should be disregarded and the weaning process initiated with an SBT. Although there is now more evidence to support the use of weaning predictors (table 1), predicting successful weaning remains difficult and clinical judgement is an important element of the decision-making process.
Lung and diaphragm ultrasound have more recently been investigated as a predictive tool in weaning, as diaphragm dysfunction may play a significant role in weaning failure [24, 25]. In fact, impaired neuromechanical efficiency of the diaphragm has been demonstrated to predict weaning failure [26]. A meta-analysis and subsequent studies support the use of ultrasound to indicate those patients who are ready for a weaning trial [27–30]. However, these studies report differing ultrasound techniques and indices and so this tool needs comprehensive validation before being used in routine clinical practice.
Response to a reduction in ventilatory support
Once a patient is considered to be ready for a weaning trial, their response to a reduction in ventilatory support should be assessed. The purpose of the SBT is to determine the patient's ability to breathe spontaneously off the ventilator. It is performed either by reducing the applied airway pressure, to provide low levels of pressure support (5–10 cmH2O), or by using a T-piece trial. A T-piece trial involves disconnecting the patient from the ventilator and attaching a T-piece to the endotracheal tube with supplemental oxygen as required. SBTs tend to last at least 30 min, increasing up to 120 min in patients considered to be at high risk of extubation failure [31, 32]. Of note, the patient's perspective about their ability to breathe unassisted has been demonstrated to improve the predictive value of the SBT [33]. Timely weaning trials are important; delay from meeting eligibility for a weaning trial to actual liberation attempt was associated with subsequent weaning failure [12, 18]. Failure of the SBT is indicated by any form of clinical instability during the trial. This can include haemodynamic compromise, cardiac arrhythmias, tachypnoea, hypoxia or hypercapnia on blood gases, increased respiratory effort, altered mental status, and agitation or anxiety. If it is not clear that the patient has passed at 120 min, the SBT should be considered a failure.
The literature indicates that there are no clear differences between pressure support and T-piece trials in their effectiveness in successfully weaning patients from mechanical ventilation. Various studies report conflicting results. A Cochrane review of nine studies with 1208 patients concluded no clear difference between pressure support and T-piece trials in achieving successful weaning (77% versus 73%, p=0.16), intensive care unit (ICU) mortality (9% versus 12%, p=0.32) or VAP (5% versus 8%, p=0.72) [34], albeit the evidence was judged to be of low quality. A subsequent systematic review comparing the two techniques also concluded that there was insufficient evidence to recommend one technique over the other [35]. A more recent randomised trial comparing 30-min pressure support versus 120-min T-piece SBT in patients deemed ready for weaning after at least 24 h of mechanical ventilation reported that the 30-min pressure support trial resulted in more successful extubations compared with the 120-min SBT group (82% versus 74%, p=0.001) [36]. In addition, hospital mortality was lower (10% versus 15%, p=0.02). There were no differences in reintubation rate or ICU and hospital length of stay [36]. However, it is difficult to draw practice-changing clinical implications from this study as the use of noninvasive ventilation (NIV) and high-flow oxygen therapy with nasal cannula (HFNC) to prevent post-extubation failure was not controlled. A large randomised study of 969 patients considered to be at high risk of extubation failure reported no difference in ventilator-free days or weaning outcome between pressure support trials and T-piece SBTs [37]. It is therefore acceptable to attempt to wean patients using either pressure support or T-piece SBTs, and the choice of strategy will largely be directed by local expertise. Once the patient has successfully completed a SBT, patients can then progress to be liberated from invasive ventilation. A recent multicentre randomised study has reported that reconnection to a ventilator for 60 min following a successful SBT reduces the risk of reintubation within 48 h [38]. Importantly, synchronised intermittent mandatory ventilation should be avoided in weaning from mechanical ventilation based on the outcomes of two landmark studies [19, 39].
Extubation
Extubation is the final step of the weaning process and involves removing the endotracheal tube. Patients who have successfully completed a SBT should be extubated if there are no contraindications. Contraindications include ongoing acute phase of the respiratory failure resulting in intubation, poor neurological status, excessive secretions, airway obstruction, cardiovascular instability or ongoing use of paralytic agents [40, 41]. Poor cough strength and excessive endotracheal secretions were associated with patients who failed extubation after a successful SBT [42]. In patients with upper airway obstruction, demonstration of air leak around the endotracheal tube after deflation of the cuff was adequate to proceed with extubation [43–45].
All patients should be reassessed for the above contraindications prior to extubation, even if they have successfully passed an SBT. When planning to extubate a patient, all equipment required to reintubate the patient should be readily available at the bedside. Patients should ideally be extubated in the upright sitting position. Immediately prior to extubation, the endotracheal tube and oral cavity should be suctioned. The patient should then be asked to take a deep breath and then exhale; as the patient exhales, the cuff should be deflated and the tube removed. After removal of the tube, the oral cavity should be suctioned and the patient asked to cough out any secretions. The patient should be provided with supplemental oxygen therapy and closely observed for several hours after extubation. Frequent airway suction is likely to be required during this time period [46].
Prevention of post-extubation respiratory failure
Post-extubation respiratory failure (PERF) is defined as the need for reintubation within 7 days of extubation. It is associated with considerably higher mortality compared with successfully extubated patients [47–49]. In addition, preventing PERF is likely to result in a lower likelihood of prolonged weaning, although reintubation after extubation has not been investigated widely as a risk factor for prolonged weaning. The cause of PERF is often excessive secretion load, fatigue, respiratory muscle weakness or fluid overload, and the patient should receive regular clinical review and be closely monitored. Factors that increase the risk of PERF include increasing age (age >65 years), pre-existing left ventricular dysfunction, anaemia, higher Acute Physiology and Chronic Health Evaluation (APACHE) II score and longer duration of mechanical ventilation [50]. The management of patients who are at high risk of PERF is not clearly defined. NIV and HFNC have been investigated as potential “bridging” support to reduce the risk of PERF in selected patients.
Noninvasive ventilation
NIV may be an effective tool to facilitate extubation. In patients with COPD who failed an SBT, extubation onto NIV compared with continued invasive ventilation with further SBTs resulted in a shorter time on mechanical ventilation (10 versus 17 days, p=0.021), shorter length of ICU stay (15 versus 24 days p=0.005) and a higher rate of weaning success (p=0.002) [51]. The evidence for the use of NIV in patients with conditions other than COPD is less clear. A randomised study in all-cause patients who had failed SBTs on three or more days demonstrated that when compared with continued invasive ventilation with SBTs, NIV resulted in shorter duration of invasive mechanical ventilation (10 versus 20 days, p=0.003), shorter ICU (14 versus 25 days, p=0.002) and hospital (28 versus 41 days, p=0.026) length of stay and higher ICU survival (90% versus 59%, p=0.045) [52]. A randomised trial comparing NIV with oxygen immediately post extubation in simple weaning patients who received mechanical ventilation for >3 days reported that NIV reduced the 48-h re-extubation rate (5% versus 39%, p=0.016) and the in-hospital mortality rate (0% versus 22%, p=0.041), without any difference in length of ICU stay [53]. A randomised study investigating chronic respiratory failure patients who had failed a SBT reported that when compared with invasive mechanical ventilation with SBTs, extubation onto NIV resulted in shorter duration of mechanical ventilation (5 versus 8 days, p=0.004) but without any difference in length of ICU or hospital stay, or in-hospital mortality [54]. Contrary to these studies, two large multicentre randomised studies have failed to demonstrate additional benefit of NIV. Esteban et al. [55] reported a trial that was stopped early, as it was found that extubation onto NIV compared with standard medical therapy in simple weaning patients with PERF resulted in a higher ICU mortality (25% versus 14%, p=0.048). The recent UK BREATHE trial compared protocolised weaning with early extubation to NIV against invasive ventilation in patients who had failed a SBT. The authors reported that NIV did not shorten the time to liberation from mechanical ventilation or improve mortality [56], but there was a reduction in invasive ventilator days and ICU length of stay with similar tracheostomy rate and mortality. A non-randomised prospective study in prolonged weaning patients with respiratory failure due to various causes reported that the addition of NIV as an adjunct to the weaning process had no effect on mechanical ventilation days, ICU length of stay or 1-year mortality, compared with weaning without NIV [57]. Clinicians must remember to carefully review the target population enrolled in these trials (e.g. under 65 years, non-obese and only moderate APACHE II scores) as this will guide the clinician as to which patients may benefit from early extubation using NIV if developing local protocols. Both of these trials contained a broad spectrum of conditions causing respiratory failure without preference to COPD and these trials are more reflective of the general ICU population. NIV has an important role in prolonged weaning patients and can facilitate decannulation onto long-term NIV [58]. Two meta-analyses, 10 years apart, comparing early extubation onto NIV with continued invasive ventilation have demonstrated that NIV decreases hospital mortality, incidence of VAP and ICU length of stay [59, 60]. The benefit of NIV was most pronounced in patients with chronic respiratory disease. Therefore, it appears that in patients with chronic respiratory disease, particularly those who are difficult-to-wean, the value of NIV in facilitating weaning is clear. It is less clear in patients without chronic respiratory disease and in these patients, NIV use should be limited to those units with a multidisciplinary weaning team with clinical expertise and established clinical protocols.
High-flow oxygen therapy with nasal cannula
HFNC is a more recent addition to the armoury in the management of respiratory failure. Two recent large studies have reported on the value of HFNC in preventing PERF. A randomised study comparing HFNC and conventional oxygen therapy in low-risk patients reported that HFNC reduced the risk of reintubation at 72 h (5% versus 12%, p=0.004) and PERF (8% versus 14%, p=0.03) [61]. A randomised study comparing HFNC with NIV in patients at high risk of PERF demonstrated non-inferiority of HFNC compared with NIV in preventing reintubation (23% versus 19%) and PERF (27% versus 40%) [62]. As there are potential patient comfort benefits of HFNC over NIV, this is a useful study to consider in clinical practice. Finally, the addition of HFNC to NIV in high-risk patients, when compared with HFNC alone, has also been demonstrated to reduce the risk of reintubation by day seven (12% versus 18%, p=0.02), PERF by day seven (21% versus 29%, p=0.01) and PERF until ICU discharge (12% versus 20%, p=0.009) [63].
Pathophysiology of difficult and prolonged weaning
The longer a patient spends on mechanical ventilation, the more likely they are to suffer adverse outcomes [8, 64]. Worldwide observational studies indicate that of patients who experience weaning failure, approximately 50% remain dependent on invasive ventilation at 1 year [65, 66] and that they have a 40–75% 1-year mortality [65–69]. Of patients who were successfully liberated after prolonged weaning, 42% required reinstitution of mechanical ventilation, and the longer the time spent on ventilation, the more likely they were to require reinstitution [70]. In addition, prolonged mechanical ventilation attracts increased healthcare-related costs. The in-hospital cost for patients who required >3 weeks of mechanical ventilation is three-fold greater than those who did not [8, 71, 72], and uses more than one third of the intensive care budget [73]. It is therefore imperative that difficult-to-wean patients are offered the highest probability of weaning from invasive ventilation to improve their outcome. As the literature tends to consider groups 2 and 3 of the 2005 International Consensus Conference in Intensive Care Medicine classification [9] together, they are also discussed together in this section. The two groups can be considered a spectrum of pathophysiological states that result in a failure to wean successfully after the first attempt. These difficult-to-wean patients comprise ∼20% of all patients weaning from mechanical ventilation, and therefore a sizable and important subgroup of patients whose management is far more complex, challenging and expensive than their small proportion would suggest [10].
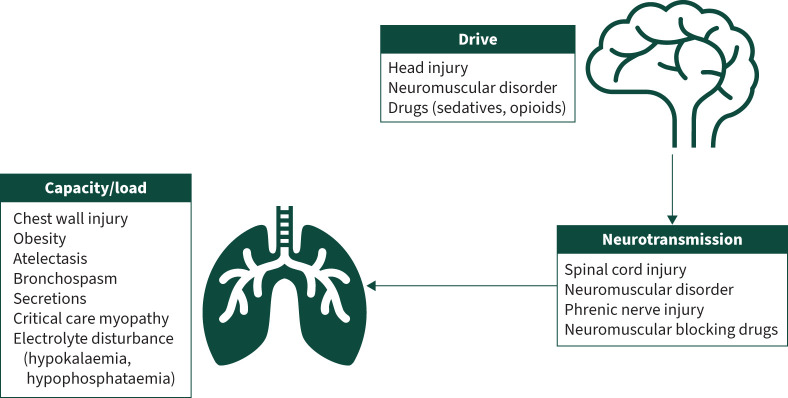
The pathophysiology leading to difficult weaning can be best understood by considering the factors that contribute to adequate alveolar ventilation. In difficult-to-wean patients, the interaction between respiratory muscle load, drive and capacity should be considered, in relation to impairment of the neural respiratory pathway (figure 2). The resolution of the initial insult leading to the need for mechanical ventilation is the overriding contributor to successful weaning, and so consideration of the pathophysiology of difficult weaning assumes resolution of this initial insult.
FIGURE 2.
Potential causes of weaning failure that should be considered and addressed when managing an individual requiring prolonged weaning.
Respiratory muscle load
Respiratory muscle load is the combination of resistive load, elastic load and threshold load [74]. Excessive secretions or bronchospasm can cause airway obstruction leading to increased resistive load. Elastic load is increased by atelectasis, pulmonary fibrosis, alveolar oedema and chest wall dysfunction. As a result of airflow limitation in severe exacerbations of asthma and COPD, gas-trapping will lead to increased intrinsic positive end-expiratory pressure (PEEP), resulting in increased threshold load [75, 76].
Respiratory muscle capacity
Respiratory muscle capacity is a measure of inspiratory muscle strength and endurance [74]. Thus, any condition causing respiratory muscle dysfunction will result in reduced respiratory capacity. In the critical care environment, most frequently this will be observed in patients suffering from critical care neuromyopathy and electrolyte disturbances such as hypokalaemia [77].
Respiratory muscle drive
Respiratory muscle drive is the transfer of neural signals from the respiratory centre in the medulla through the spinal cord and on to the respiratory muscles, and is required to control the respiratory rate and tidal volume in response to changes in H+ ion concentration in the cerebrospinal fluid [74]. Ongoing neurological dysfunction secondary to the initial insult or use of sedation, opioids and antiepileptic medication can lead to a depression of neural respiratory drive. In addition, metabolic alkalosis (e.g. caused by loop diuretics) can also result in reduced respiratory drive [78]. Interruption in the transmission of neural drive signals, such as observed in spinal cord injury (above C3), phrenic nerve injury or the use of neuromuscular blocking agents or aminoglycosides [79, 80], will also result in reduced drive. It is important for the clinician to assess respiratory muscle load, drive and capacity in all difficult-to-wean patients and adequately address and treat reversible conditions.
Management strategies for prolonged weaning
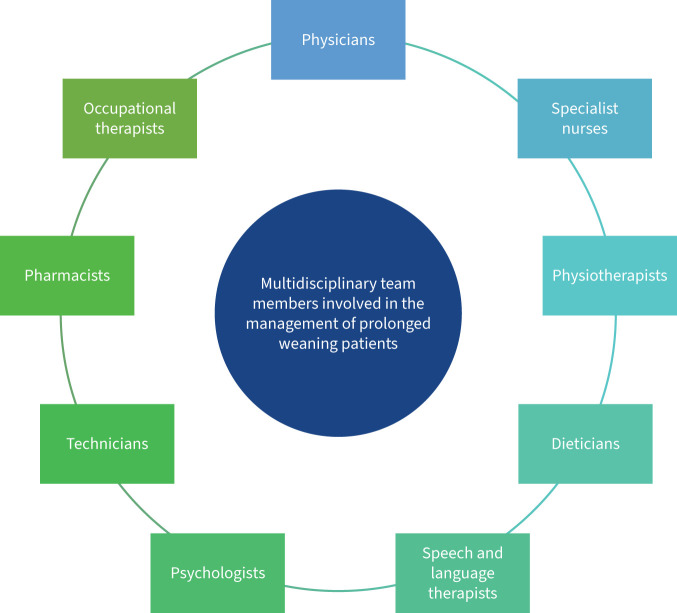
The management of patients requiring prolonged weaning requires input from a multidisciplinary team (figure 3), to maximise the likelihood of being liberated from invasive ventilation. Of patients who require prolonged mechanical ventilation, 55–65% are successfully weaned [14, 81]. A systematic approach should be adopted to address each factor influencing the efficacy of weaning efforts (figure 4), albeit there are limited data on difficult-to-wean patient cohorts. Patients requiring prolonged weaning are ideally cared for in specialised weaning units. Care provided at these units is significantly less costly than care provided by acute ICUs [82, 83].
FIGURE 3.
Members of the multidisciplinary team who are involved in the management of prolonged weaning patients.
FIGURE 4.
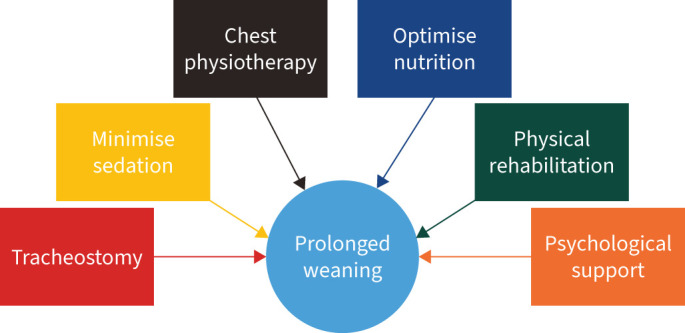
Management strategies to improve the likelihood of weaning from prolonged mechanical ventilation.
Predicting weaning success
Predicting weaning success in prolonged weaning patients is even more difficult than in simple weaning patients. Indeed, a successful SBT may not be a useful marker of weaning success [53] and ICU admission severity scores are not predictive of weaning success [54, 55] in difficult-to-wean patients. A single centre observational cohort study of all-cause critically unwell patients reported on their weaning practice. All patients underwent a trial of pressure support ventilation once preset screening criteria were met (arterial oxygen tension to inspiratory oxygen fraction ratio >26.3 kPa, PEEP ≤5 cmH2O, Richmond Agitation–Sedation Scale ≥3 (more awake than “moderate sedation”) and cardiovascular stability). They reported that the failure to sustain pressure support for 24 h (due to hypoventilation, rapid shallow breathing unresponsive to titration of pressure support, or deterioration in pulmonary gas exchange) was associated with a two-fold increase in the requirement for mechanical ventilation for greater than 7 days (risk ratio 2.12, p=0.002) and a three-fold increase in ICU mortality (risk ratio 2.94, p=0.002). Multivariate regression demonstrated that only preweaning arterial pH (risk ratio 0.996, p=0.002) was associated with failure of a pressure support trial [84]. These data suggest that patients with lower arterial pH and those who are unable to tolerate early pressure support trials should be highlighted early in the course of their critical illness as possible difficult-to-wean patients, and a more cautious approach to weaning be taken.
Tracheostomy management
If a patient has not been successfully weaned, they should be considered for tracheostomy. Conversion from endotracheal intubation to tracheostomy results in improved physiology (increased tidal volume and maximum inspiratory and expiratory pressure, and decreased airway resistance and respiratory frequency to tidal volume ratio) [85]. The timing of tracheostomy remains controversial. Although the complications of prolonged endotracheal intubation are clear, tracheostomy insertion itself poses risk. Various observational studies have demonstrated that early tracheostomy can hasten liberation from invasive ventilation and improve weaning success, but not necessarily survival [86–89]. A large randomised study that compared early and late tracheostomy investigated a primary outcome of VAP incidence, and reported no difference. Secondary outcomes demonstrated that early tracheostomy when compared with late tracheostomy resulted in more ventilator-free days (11 days versus 6 days, p=0.02), and weaning success (77% versus 68%, p0.002) [90]. A Cochrane review demonstrated that although early tracheostomy was associated with lower mortality, the evidence was of insufficient quality to make a definitive conclusion [91]. A potential reason for this lack of clarity about timing of tracheostomy is the definition of “early” tracheostomy. The literature has defined early tracheostomy as anything from within 10 days [87–89, 91] to up to 21 days [87, 89] of endotracheal intubation. Despite this, the majority of units in the UK and Europe perform tracheostomies in difficult-to-wean patients within the first 14 days post-intubation [92, 93].
Once a patient has been tracheostomised, the goal is eventual decannulation. There is limited evidence to guide the development of protocols to advise on the method of decannulation and care tends to be personalised [94]. Tracheostomy tubes should be downsized, and patients should receive pressure support ventilation. As clinical stability is achieved, cuff deflation trials should be performed, with increasing duration and frequency. If successful, a one-way speaking valve can be introduced [95]. If cuff deflation trials are successful on a regular basis, the tube can be changed to a cuffless tube. In parallel, daytime self-ventilation periods should be increased aiming to achieve self-ventilation throughout the daytime. Once this is achieved, the tracheostomy tube can be capped, with overnight pressure support ventilation. Once this is achieved, the tracheostomy tube can be capped overnight, and NIV delivered at higher pressure support than required invasively. Once the patient can tolerate both daytime spontaneous ventilation and overnight NIV without any cardiorespiratory instability, decannulation can be performed, with continuation of NIV as required [96–98].
Minimise risk of VAP
VAP is one the most common causes of prolonged weaning, and is associated with prolonged length of stay and increased mortality [5]. An evidence synthesis of several guidelines on VAP prevention (European Task Force on VAP, the US Centers for Disease Control and Prevention, the Canadian Critical Care Society, and the American Thoracic Society and Infectious Diseases Society of America) reported that the following factors may be useful to reduce the risk of VAP: 1) reducing the risk of PERF, 2) early tracheostomy, 3) use of subglottic secretion drainage, 4) nursing the patient in the semi-recumbent position, 5) minimising sedation use, and 6) oral care combining chlorhexidine rinse and mechanical cleaning [99]. Respiratory muscle weakness as a result of prolonged mechanical ventilation can result in inadequate secretion clearance, further increasing the risk of VAP. Respiratory physiotherapy is important for the optimisation of secretion clearance, but very little objective data exist to demonstrate the impact this has on weaning outcome.
Optimise nutrition
Although the importance of adequate nutrition in the critical care population is clear, an optimal nutritional strategy for patients requiring prolonged mechanical ventilation remains elusive. It appears that adequate early feeding is particularly important in those who go on to require prolonged ventilation [100]. A randomised study that compared permissive underfeeding with target feeding in patients admitted to critical care for more than 48 h demonstrated no difference in 28-day all-cause mortality (18% versus 23%, p=0.34), mechanical ventilation duration (11 days versus 13 days, p=0.1) or hospital length of stay (70 days versus 67 days, p=0.8). However, in-hospital mortality was less in the permissive underfeeding group (30% versus 42%, p=0.04) [101]. Similarly, although optimising trace elements such as magnesium enhances respiratory muscle function [102], there does not appear to be any clinical impact of this on the weaning population [103].
Physical rehabilitation
As a consequence of the catabolic phase-induced skeletal muscle loss in critical care [104], critical care-acquired weakness is highly prevalent in the prolonged weaning cohort [105] and a risk factor for the requirement for prolonged weaning [106]. Physical rehabilitation, particularly of the respiratory muscles, is therefore a crucial part of the weaning process [107, 108]. A randomised study compared a multimodal physical rehabilitation programme with usual care in prolonged mechanical ventilation patients. They demonstrated that the physical rehabilitation programme resulted in shorter time on ventilation compared with usual care (median: 17 versus 56 days, p=0.07) and a higher rate of successful weaning (87% versus 41%, p<0.01) [109]. A retrospective cohort study reported on prolonged weaning patients who underwent an incremental intensive physiotherapy programme. Weaning success was higher in patients who progressed further in the incremental programme, compared with those who did not (72% versus 56%, p<0.001). Logistic regression also demonstrated that further progression through the incremental programme was an independent predictor of successful weaning (odds ratio 2.2, p<0.001) [110]. A recent study demonstrated that the implementation of a protocolised physical therapy programme in prolonged weaning patients resulted in shorter time on mechanical ventilation and improved survival, although no effect on weaning success rate [111]. Similarly, a randomised study reported that inspiratory muscle training in prolonged weaning patients resulted in improved weaning success and survival [112]. Despite limited strong evidence for the inclusion of physical therapy in the management of prolonged weaning [113], there are no negative data and therefore, given the potential for benefit, this is a worthwhile intervention.
Psychological support
Critical care patients suffer from high levels of psychological morbidity [114]. A prospective cohort study of patients requiring prolonged ventilation reported that patients who failed weaning attempts had a particularly high prevalence of psychological symptoms, such as anxiety and fear [115] and delirium [116]. The presence of psychological morbidity can impact on the patient's ability to engage with the weaning process [117, 118]. Despite this, there are limited studies demonstrating the benefit of psychological interventions on outcome in critical care. A single interventional study was identified which demonstrated the value of intervention from a clinical psychologist on long-term psychological sequelae following critical care admission [119]. A recent randomised study failed to demonstrate any benefit of cognitive behavioural therapy on post-traumatic stress outcomes following critical care admission [120]. There are no studies investigating psychological interventions in the prolonged weaning population. Historical data have demonstrated that biofeedback techniques may have some benefit in difficult and prolonged weaning patients [121, 122].
Outstanding challenges to prolonged weaning
Early predictors for the readiness to wean in difficult-to-wean patients are required and this will become an increasingly important research priority. Although the use of diaphragm ultrasound is a potential clinical tool, comprehensive validation is required for this to be adopted into wider clinical practice. The use of NIV, outside of chronic respiratory disease, and HFNC in preventing post-extubation failure need further investigation to determine their safety, clinical efficacy and cost-effectiveness. In difficult-to-wean patients, the most appropriate timing of tracheostomy remains elusive, whilst the implementation of physical rehabilitation and psychological interventions needs to be further investigated. Poor sleep quality and architecture have been demonstrated in patients receiving prolonged mechanical ventilation [123]. Although this is not particularly surprising given that poor sleep quality has been widely reported in critical care patients [124–126], the impact of this specifically on weaning outcomes remains to be elucidated. Interventions aiming to improve sleep quality may result in shorter time to liberation from invasive mechanical ventilation. Finally, with the advent of artificial intelligence, the utility of machine learning in weaning needs to be further explored. It may have a role in predicting readiness to wean, but this is a relatively new technology with unclear results [127, 128].
Summary
Weaning from invasive mechanical ventilation is a key part of the management of the critical care patient and its early implementation has direct impact on outcomes. It remains difficult to predict accurately when a patient is ready to undergo a weaning attempt, and a combination of objective parameters and clinical judgement should be employed. SBTs can be conducted using either pressure support ventilation or a T-piece, depending on local expertise. To reduce the risk of PERF, extubation onto NIV in COPD has a clear benefit; less so in conditions other than COPD. HFNC has the potential to be useful in preventing PERF, but its safety and efficacy need further evaluation. Patients requiring prolonged mechanical ventilation suffer from profound and complex pathophysiology and require a multidisciplinary approach to prepare them for weaning attempts. As well as correcting the initial insult leading to the respiratory failure and daily assessment of the readiness to wean, these patients will require intensive input from physiotherapy, dietetics, occupational therapy and psychological services. Several specifics of weaning patients from invasive mechanical ventilation remain elusive, leaving many questions for future research.
Key points
Weaning from invasive mechanical ventilation is an important task in the management of a patient with respiratory failure. Weaning can be split into simple, difficult and prolonged, based on the number of attempts taken to liberate the patient from mechanical ventilation.
Early and frequent assessments of the readiness to wean, and early weaning attempts when the patient is ready, are important to improve overall outcomes.
The risk of PERF may be reduced with the use of NIV or HFNC after extubation.
Factors to be considered in the pathophysiology of prolonged mechanical ventilation include increased respiratory muscle load, reduced respiratory muscle capacity and reduced respiratory drive.
The management of a prolonged weaning patient requires a comprehensive multidisciplinary assessment to 1) identify the cause of failed weaning attempts and 2) optimise the patient's physiology to improve their likelihood of successfully weaning from invasive mechanical ventilation.
Self-evaluation questions
- The definition of prolonged weaning is:
- Liberation from invasive mechanical ventilation after first attempt.
- Liberation from invasive mechanical ventilation after up to three attempts within 7 days of the first attempt.
- The requirement for invasive mechanical ventilation for >7 days after first weaning attempt, or more than three weaning attempts.
- Risk factors for the development of PERF include all of the following except:
- Pre-existing left ventricular dysfunction
- Anaemia
- Lower APACHE II score
- Longer duration of mechanical ventilation
- Increasing age
- For which of the following methods to assess readiness to wean is there a stronger evidence base?
- A reduction in pressure support
- T-piece trial
- Neither
Suggested answers
1. c.
2. c.
3. c.
Footnotes
Conflict of interest: The authors have nothing to disclose.
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med 2010; 38: 1947–1953. doi: 10.1097/CCM.0b013e3181ef4460 [DOI] [PubMed] [Google Scholar]
- 2.Ding C, Zhang Y, Yang Z, et al. Incidence, temporal trend and factors associated with ventilator-associated pneumonia in mainland China: a systematic review and meta-analysis. BMC Infect Dis 2017; 17: 468. doi: 10.1186/s12879-017-2566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobin MJ. Mechanical ventilation. N Engl J Med 1994; 330: 1056–1061. doi: 10.1056/NEJM199404143301507 [DOI] [PubMed] [Google Scholar]
- 4.Windisch W, Dellweg D, Geiseler J, et al. Prolonged weaning from mechanical ventilation. Dtsch Arztebl Int 2020; 117: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safdar N, Dezfulian C, Collard HR, et al. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 2005; 33: 2184–2193. doi: 10.1097/01.CCM.0000181731.53912.D9 [DOI] [PubMed] [Google Scholar]
- 6.Funk G-C, Anders S, Breyer M-K, et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J 2010; 35: 88–94. doi: 10.1183/09031936.00056909 [DOI] [PubMed] [Google Scholar]
- 7.Kaier K, Heister T, Wolff J, et al. Mechanical ventilation and the daily cost of ICU care. BMC Health Serv Res 2020; 20: 267. doi: 10.1186/s12913-020-05133-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill AD, Fowler RA, Burns KE, et al. Long-term outcomes and health care utilization after prolonged mechanical ventilation. Ann Am Thorac Soc 2017; 14: 355–362. doi: 10.1513/AnnalsATS.201610-792OC [DOI] [PubMed] [Google Scholar]
- 9.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29: 1033–1056. doi: 10.1183/09031936.00010206 [DOI] [PubMed] [Google Scholar]
- 10.Beduneau G, Pham T, Schortgen F, et al. Epidemiology of weaning outcome according to a new definition. The WIND study. Am J Respir Crit Care Med 2017; 195: 772–783. doi: 10.1164/rccm.201602-0320OC [DOI] [PubMed] [Google Scholar]
- 11.Jeong BH, Lee KY, Nam J, et al. Validation of a new WIND classification compared to ICC classification for weaning outcome. Ann Intensive Care 2018; 8: 115. doi: 10.1186/s13613-018-0461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham T, Heunks L, Bellani G, et al. Weaning from mechanical ventilation in intensive care units across 50 countries (WEAN SAFE): a multicentre, prospective, observational cohort study. Lancet Respir Med 2023; 11: 465–476. doi: 10.1016/S2213-2600(22)00449-0 [DOI] [PubMed] [Google Scholar]
- 13.Rose L, McGinlay M, Amin R, et al. Variation in definition of prolonged mechanical ventilation. Respir Care 2017; 62: 1324–1332. doi: 10.4187/respcare.05485 [DOI] [PubMed] [Google Scholar]
- 14.Mifsud Bonnici D, Sanctuary T, Warren A, et al. Prospective observational cohort study of patients with weaning failure admitted to a specialist weaning, rehabilitation and home mechanical ventilation centre. BMJ Open 2016; 6: e010025. doi: 10.1136/bmjopen-2015-010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MG, Quinnell TG, Oscroft NS, et al. Hospital outcomes and long-term survival after referral to a specialized weaning unit. Br J Anaesth 2017; 118: 563–569. doi: 10.1093/bja/aex031 [DOI] [PubMed] [Google Scholar]
- 16.Ghiani A, Tsitouras K, Paderewska J, et al. Incidence, causes, and predictors of unsuccessful decannulation following prolonged weaning. Ther Adv Chronic Dis 2022; 13: 20406223221109655. doi: 10.1177/20406223221109655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns KEA, Raptis S, Nisenbaum R, et al. International practice variation in weaning critically ill adults from invasive mechanical ventilation. Ann Am Thorac Soc 2018; 15: 494–502. doi: 10.1513/AnnalsATS.201705-410OC [DOI] [PubMed] [Google Scholar]
- 18.Burns KEA, Rizvi L, Cook DJ, et al. Ventilator weaning and discontinuation practices for critically ill patients. JAMA 2021; 325: 1173–1184. doi: 10.1001/jama.2021.2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med 1994; 150: 896–903. doi: 10.1164/ajrccm.150.4.7921460 [DOI] [PubMed] [Google Scholar]
- 20.Epstein SK, Nevins ML, Chung J. Effect of unplanned extubation on outcome of mechanical ventilation. Am J Respir Crit Care Med 2000; 161: 1912–1916. doi: 10.1164/ajrccm.161.6.9908068 [DOI] [PubMed] [Google Scholar]
- 21.Coplin WM, Pierson DJ, Cooley KD, et al. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med 2000; 161: 1530–1536. doi: 10.1164/ajrccm.161.5.9905102 [DOI] [PubMed] [Google Scholar]
- 22.Ely EW, Baker AM, Evans GW, et al. The prognostic significance of passing a daily screen of weaning parameters. Intensive Care Med 1999; 25: 581–587. doi: 10.1007/s001340050906 [DOI] [PubMed] [Google Scholar]
- 23.MacIntyre NR, Cook DJ, Ely EW Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001; 120: Suppl. 6, 375S–395S. doi: 10.1378/chest.120.6_suppl.375S [DOI] [PubMed] [Google Scholar]
- 24.Dres M, Demoule A. Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit Care 2018; 22: 73. doi: 10.1186/s13054-018-1992-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bureau C, Van Hollebeke M, Dres M. Managing respiratory muscle weakness during weaning from invasive ventilation. Eur Respir Rev 2023; 32: 220205. doi: 10.1183/16000617.0205-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doorduin J, Roesthuis LH, Jansen D, et al. Respiratory muscle effort during expiration in successful and failed weaning from mechanical ventilation. Anesthesiology 2018; 129: 490–501. doi: 10.1097/ALN.0000000000002256 [DOI] [PubMed] [Google Scholar]
- 27.Qian Z, Yang M, Li L, et al. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: a systematic review and meta-analysis. BMJ Open 2018; 8: e021189. doi: 10.1136/bmjopen-2017-021189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palkar A, Mayo P, Singh K, et al. Serial diaphragm ultrasonography to predict successful discontinuation of mechanical ventilation. Lung 2018; 196: 363–368. doi: 10.1007/s00408-018-0106-x [DOI] [PubMed] [Google Scholar]
- 29.Abdelwahed WM, Abd Elghafar MS, Amr YM, et al. Prospective study: diaphragmatic thickness as a predictor index for weaning from mechanical ventilation. J Crit Care 2019; 52: 10–15. doi: 10.1016/j.jcrc.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Tenza-Lozano E, Llamas-Alvarez A, Jaimez-Navarro E, et al. Lung and diaphragm ultrasound as predictors of success in weaning from mechanical ventilation. Crit Ultrasound J 2018; 10: 12. doi: 10.1186/s13089-018-0094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallverdu I, Calaf N, Subirana M, et al. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 1998; 158: 1855–1862. doi: 10.1164/ajrccm.158.6.9712135 [DOI] [PubMed] [Google Scholar]
- 32.Vitacca M, Vianello A, Colombo D, et al. Comparison of two methods for weaning patients with chronic obstructive pulmonary disease requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med 2001; 164: 225–230. doi: 10.1164/ajrccm.164.2.2008160 [DOI] [PubMed] [Google Scholar]
- 33.Perren A, Previsdomini M, Llamas M, et al. Patients’ prediction of extubation success. Intensive Care Med 2010; 36: 2045–2052. doi: 10.1007/s00134-010-1984-4 [DOI] [PubMed] [Google Scholar]
- 34.Ladeira MT, Vital FM, Andriolo RB, et al. Pressure support versus T-tube for weaning from mechanical ventilation in adults. Cochrane Database Syst Rev 2014; 5: CD006056. doi: 10.1002/14651858.CD006056.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrini JA, Moraes RB, Maccari JG, et al. Spontaneous breathing trials with T-piece or pressure support ventilation. Respir Care 2016; 61: 1693–1703. doi: 10.4187/respcare.04816 [DOI] [PubMed] [Google Scholar]
- 36.Subira C, Hernandez G, Vazquez A, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA 2019; 321: 2175–2182. doi: 10.1001/jama.2019.7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thille AW, Gacouin A, Coudroy R, et al. Spontaneous-breathing trials with pressure-support ventilation or a T-piece. N Engl J Med 2022; 387: 1843–1854. doi: 10.1056/NEJMoa2209041 [DOI] [PubMed] [Google Scholar]
- 38.Fernandez MM, Gonzalez-Castro A, Magret M, et al. Reconnection to mechanical ventilation for 1 h after a successful spontaneous breathing trial reduces reintubation in critically ill patients: a multicenter randomized controlled trial. Intensive Care Med 2017; 43: 1660–1667. doi: 10.1007/s00134-017-4911-0 [DOI] [PubMed] [Google Scholar]
- 39.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 1995; 332: 345–350. doi: 10.1056/NEJM199502093320601 [DOI] [PubMed] [Google Scholar]
- 40.MacIntyre NR. Evidence-based assessments in the ventilator discontinuation process. Respir Care 2012; 57: 1611–1618. doi: 10.4187/respcare.02055 [DOI] [PubMed] [Google Scholar]
- 41.Torrini F, Gendreau S, Morel J, et al. Prediction of extubation outcome in critically ill patients: a systematic review and meta-analysis. Crit Care 2021; 25: 391. doi: 10.1186/s13054-021-03802-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khamiees M, Raju P, DeGirolamo A, et al. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest 2001; 120: 1262–1270. doi: 10.1378/chest.120.4.1262 [DOI] [PubMed] [Google Scholar]
- 43.Fisher MM, Raper RF. The ‘cuff-leak' test for extubation. Anaesthesia 1992; 47: 10–12. doi: 10.1111/j.1365-2044.1992.tb01943.x [DOI] [PubMed] [Google Scholar]
- 44.Sandhu RS, Pasquale MD, Miller K, et al. Measurement of endotracheal tube cuff leak to predict postextubation stridor and need for reintubation. J Am Coll Surg 2000; 190: 682–687. doi: 10.1016/S1072-7515(00)00269-6 [DOI] [PubMed] [Google Scholar]
- 45.Jaber S, Chanques G, Matecki S, et al. Post-extubation stridor in intensive care unit patients. Risk factors evaluation and importance of the cuff-leak test. Intensive Care Med 2003; 29: 69–74. doi: 10.1007/s00134-002-1563-4 [DOI] [PubMed] [Google Scholar]
- 46.El-Khatib MF, Bou-Khalil P. Clinical review: liberation from mechanical ventilation. Crit Care 2008; 12: 221. doi: 10.1186/cc6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thille AW, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011; 39: 2612–2618. doi: 10.1097/CCM.0b013e3182282a5a [DOI] [PubMed] [Google Scholar]
- 48.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997; 112: 186–192. doi: 10.1378/chest.112.1.186 [DOI] [PubMed] [Google Scholar]
- 49.Dadam MM, Pereira AB, Cardoso MR, et al. Effect of reintubation within 48 hours on mortality in critically ill patients after planned extubation. Respir Care 2024; 69: 829–838. doi: 10.4187/respcare.11077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulkarni AP, Agarwal V. Extubation failure in intensive care unit: predictors and management. Indian J Crit Care Med 2008; 12: 1–9. doi: 10.4103/0972-5229.40942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128: 721–728. doi: 10.7326/0003-4819-128-9-199805010-00004 [DOI] [PubMed] [Google Scholar]
- 52.Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168: 70–76. doi: 10.1164/rccm.200209-1074OC [DOI] [PubMed] [Google Scholar]
- 53.Ornico SR, Lobo SM, Sanches HS, et al. Noninvasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial. Crit Care 2013; 17: R39. doi: 10.1186/cc12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girault C, Daudenthun I, Chevron V, et al. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160: 86–92. doi: 10.1164/ajrccm.160.1.9802120 [DOI] [PubMed] [Google Scholar]
- 55.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350: 2452–2460. doi: 10.1056/NEJMoa032736 [DOI] [PubMed] [Google Scholar]
- 56.Perkins GD, Mistry D, Gates S, et al. Effect of protocolized weaning with early extubation to noninvasive ventilation vs invasive weaning on time to liberation from mechanical ventilation among patients with respiratory failure: the Breathe randomized clinical trial. JAMA 2018; 320: 1881–1888. doi: 10.1001/jama.2018.13763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sancho J, Servera E, Jara-Palomares L, et al. Noninvasive ventilation during the weaning process in chronically critically ill patients. ERJ Open Res 2016; 2: 00061-2016. doi: 10.1183/23120541.00061-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ceriana P, Nava S, Vitacca M, et al. Noninvasive ventilation during weaning from prolonged mechanical ventilation. Pulmonology 2019; 25: 328–333. doi: 10.1016/j.pulmoe.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 59.Yeung J, Couper K, Ryan EG, et al. Non-invasive ventilation as a strategy for weaning from invasive mechanical ventilation: a systematic review and Bayesian meta-analysis. Intensive Care Med 2018; 44: 2192–2204. doi: 10.1007/s00134-018-5434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burns KE, Adhikari NK, Keenan SP, et al. Use of non-invasive ventilation to wean critically ill adults off invasive ventilation: meta-analysis and systematic review. BMJ 2009; 338: b1574. doi: 10.1136/bmj.b1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernandez G, Vaquero C, Gonzalez P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA 2016; 315: 1354–1361. doi: 10.1001/jama.2016.2711 [DOI] [PubMed] [Google Scholar]
- 62.Hernandez G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016; 316: 1565–1574. doi: 10.1001/jama.2016.14194 [DOI] [PubMed] [Google Scholar]
- 63.Thille AW, Muller G, Gacouin A, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA 2019; 322: 1465–1475. doi: 10.1001/jama.2019.14901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Na SJ, Ko RE, Nam J, et al. Factors associated with prolonged weaning from mechanical ventilation in medical patients. Ther Adv Respir Dis 2022; 16: 17534666221117005. doi: 10.1177/17534666221117005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keng LT, Chung KP, Lin SY, et al. Significant clinical factors associated with long-term mortality in critical cancer patients requiring prolonged mechanical ventilation. Sci Rep 2017; 7: 2148. doi: 10.1038/s41598-017-02418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jubran A, Grant BJB, Duffner LA, et al. Long-term outcome after prolonged mechanical ventilation. A long-term acute-care hospital study. Am J Respir Crit Care Med 2019; 199: 1508–1516. doi: 10.1164/rccm.201806-1131OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saiphoklang N, Auttajaroon J. Incidence and outcome of weaning from mechanical ventilation in medical wards at Thammasat University Hospital. PLoS One 2018; 13: e0205106. doi: 10.1371/journal.pone.0205106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leroy G, Devos P, Lambiotte F, et al. One-year mortality in patients requiring prolonged mechanical ventilation: multicenter evaluation of the ProVent score. Crit Care 2014; 18: R155. doi: 10.1186/cc13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herer B. Outcomes of tracheostomized subjects undergoing prolonged mechanical ventilation in an intermediate-care facility. Respir Care 2018; 63: 282–288. doi: 10.4187/respcare.05602 [DOI] [PubMed] [Google Scholar]
- 70.Sansone GR, Frengley JD, Horland A, et al. Effects of reinstitution of prolonged mechanical ventilation on the outcomes of 370 patients in a long-term acute care hospital. J Intensive Care Med 2018; 33: 527–535. doi: 10.1177/0885066616683669 [DOI] [PubMed] [Google Scholar]
- 71.Loss SH, de Oliveira RP, Maccari JG, et al. The reality of patients requiring prolonged mechanical ventilation: a multicenter study. Rev Bras Ter Intensiva 2015; 27: 26–35. doi: 10.5935/0103-507X.20150006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Criner G. Long-term ventilator-dependent patients: new facilities and new models of care. The American perspective. Pulmonology 2012; 18: 214–216. doi: 10.1016/j.rppneu.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 73.Scheinhorn DJ, Chao DC, Stearn-Hassenpflug M. Approach to patients with long-term weaning failure. Respir Care Clin N Am 2000; 6: 437–461. doi: 10.1016/S1078-5337(05)70080-0 [DOI] [PubMed] [Google Scholar]
- 74.Shah NM, Murphy PB. Hypercapnic failure in non-COPD. In: Heunks L, Demoule A, Windisch W, eds. Pulmonary Emergencies (ERS Monograph). Sheffield, European Respiratory Society, 2016; pp. 86–100. doi: 10.1183/2312508X.erm7416 [DOI] [Google Scholar]
- 75.Ranieri VM, Dambrosio M, Brienza N. Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failure. Eur Respir J 1996; 9: 1283–1292. doi: 10.1183/09031936.96.09061283 [DOI] [PubMed] [Google Scholar]
- 76.Stather DR, Stewart TE. Clinical review: mechanical ventilation in severe asthma. Crit Care 2005; 9: 581–587. doi: 10.1186/cc3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press 2010; 8: 72–81. doi: 10.5049/EBP.2010.8.2.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oppersma E, Doorduin J, van der Hoeven JG, et al. The effect of metabolic alkalosis on the ventilatory response in healthy subjects. Respir Physiol Neurobiol 2018; 249: 47–53. doi: 10.1016/j.resp.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 79.Paradelis AG, Triantaphyllidis C, Giala MM. Neuromuscular blocking activity of aminoglycoside antibiotics. Methods Find Exp Clin Pharmacol 1980; 2: 45–51. [PubMed] [Google Scholar]
- 80.Liu M, Kato M, Hashimoto Y. Neuromuscular blocking effects of the aminoglycoside antibiotics arbekacin, astromicin, isepamicin and netilmicin on the diaphragm and limb muscles in the rabbit. Pharmacology 2001; 63: 142–146. doi: 10.1159/000056125 [DOI] [PubMed] [Google Scholar]
- 81.Fradkin M, Elyashiv M, Camel A, et al. A historical cohort study on predictors for successful weaning from prolonged mechanical ventilation and up to 3-year survival follow-up in a rehabilitation center. Respir Med 2024; 227: 107636. doi: 10.1016/j.rmed.2024.107636 [DOI] [PubMed] [Google Scholar]
- 82.Lone NI, Walsh TS. Prolonged mechanical ventilation in critically ill patients: epidemiology, outcomes and modelling the potential cost consequences of establishing a regional weaning unit. Crit Care 2011; 15: R102. doi: 10.1186/cc10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carpenè N, Vagheggini G, Panait E, et al. A proposal of a new model for long-term weaning: respiratory intensive care unit and weaning center. Respir Med 2010; 104: 1505–1511. doi: 10.1016/j.rmed.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 84.Glover G, Connolly B, Di Gangi S, et al. An observational cohort study to determine efficacy, adherence and outcome of the early initiation of pressure support ventilation during mechanical ventilation. BMJ Open Respir Res 2014; 1: e000028. doi: 10.1136/bmjresp-2014-000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim CK, Ruan SY, Lin FC, et al. Effect of tracheostomy on weaning parameters in difficult-to-wean mechanically ventilated patients: a prospective observational study. PLoS One 2015; 10: e0138294. doi: 10.1371/journal.pone.0138294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boynton JH, Hawkins K, Eastridge BJ, et al. Tracheostomy timing and the duration of weaning in patients with acute respiratory failure. Crit Care 2004; 8: R261–R267. doi: 10.1186/cc2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hsu CL, Chen KY, Chang CH, et al. Timing of tracheostomy as a determinant of weaning success in critically ill patients: a retrospective study. Crit Care 2005; 9: R46–R52. doi: 10.1186/cc3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alhajhusain A, Ali AW, Najmuddin A, et al. Timing of tracheotomy in mechanically ventilated critically ill morbidly obese patients. Crit Care Res Pract 2014; 2014: 840638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen HC, Song L, Chang HC, et al. Factors related to tracheostomy timing and ventilator weaning: findings from a population in Northern Taiwan. Clin Respir J 2018; 12: 97–104. doi: 10.1111/crj.12492 [DOI] [PubMed] [Google Scholar]
- 90.Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA 2010; 303: 1483–1489. doi: 10.1001/jama.2010.447 [DOI] [PubMed] [Google Scholar]
- 91.Andriolo BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev 2015; 1: CD007271. doi: 10.1002/14651858.CD007271.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krishnan K, Elliot SC, Mallick A. The current practice of tracheostomy in the United Kingdom: a postal survey. Anaesthesia 2005; 60: 360–364. doi: 10.1111/j.1365-2044.2004.04106.x [DOI] [PubMed] [Google Scholar]
- 93.Kluge S, Baumann HJ, Maier C, et al. Tracheostomy in the intensive care unit: a nationwide survey. Anesth Analg 2008; 107: 1639–1643. doi: 10.1213/ane.0b013e318188b818 [DOI] [PubMed] [Google Scholar]
- 94.Singh RK, Saran S, Baronia AK. The practice of tracheostomy decannulation – a systematic review. J Intensive Care 2017; 5: 38. 10.1186/s40560-017-0234-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.St John RE. Advances in artificial airway management. Crit Care Nurs Clin North Am 1999; 11: 7–17. [PubMed] [Google Scholar]
- 96.Christopher KL. Tracheostomy decannulation. Respir Care 2005; 50: 538–541. [PubMed] [Google Scholar]
- 97.O'Connor HH, White AC. Tracheostomy decannulation. Respir Care 2010; 55: 1076–1081. [PubMed] [Google Scholar]
- 98.D'Cruz RF, Hart N, Kaltsakas G. Weaning: a practical approach. In: Heunks L, Schultz MJ, eds. ERS Practical Handbook of Invasive Mechanical Ventilation. Sheffield, European Respiratory Society, 2019; pp. 227–234. [Google Scholar]
- 99.Lorente L, Blot S, Rello J. Evidence on measures for the prevention of ventilator-associated pneumonia. Eur Respir J 2007; 30: 1193–1207. doi: 10.1183/09031936.00048507 [DOI] [PubMed] [Google Scholar]
- 100.Wei X, Day AG, Ouellette-Kuntz H, et al. The association between nutritional adequacy and long-term outcomes in critically ill patients requiring prolonged mechanical ventilation: a multicenter cohort study. Crit Care Med 2015; 43: 1569–1579. doi: 10.1097/CCM.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 101.Arabi YM, Tamim HM, Dhar GS, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011; 93: 569–577. doi: 10.3945/ajcn.110.005074 [DOI] [PubMed] [Google Scholar]
- 102.Dhingra S, Solven F, Wilson A, et al. Hypomagnesemia and respiratory muscle power. Am Rev Respir Dis 1984; 129: 497–498. doi: 10.1164/arrd.1984.129.3.497 [DOI] [PubMed] [Google Scholar]
- 103.Johnson D, Gallagher C, Cavanaugh M, et al. The lack of effect of routine magnesium administration on respiratory function in mechanically ventilated patients. Chest 1993; 104: 536–541. doi: 10.1378/chest.104.2.536 [DOI] [PubMed] [Google Scholar]
- 104.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013; 310: 1591–1600. doi: 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 105.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002; 288: 2859–2867. doi: 10.1001/jama.288.22.2859 [DOI] [PubMed] [Google Scholar]
- 106.De Jonghe B, Bastuji-Garin S, Sharshar T, et al. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med 2004; 30: 1117–1121. doi: 10.1007/s00134-004-2174-z [DOI] [PubMed] [Google Scholar]
- 107.Chiang LL, Wang LY, Wu CP, et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther 2006; 86: 1271–1281. doi: 10.2522/ptj.20050036 [DOI] [PubMed] [Google Scholar]
- 108.De Jonghe B, Bastuji-Garin S, Durand MC, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med 2007; 35: 2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8 [DOI] [PubMed] [Google Scholar]
- 109.Verceles AC, Wells CL, Sorkin JD, et al. A multimodal rehabilitation program for patients with ICU acquired weakness improves ventilator weaning and discharge home. J Crit Care 2018; 47: 204–210. doi: 10.1016/j.jcrc.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schreiber AF, Ceriana P, Ambrosino N, et al. Physiotherapy and weaning from prolonged mechanical ventilation. Respir Care 2019; 64: 17–25. doi: 10.4187/respcare.06280 [DOI] [PubMed] [Google Scholar]
- 111.Bickenbach J, Fritsch S, Cosler S, et al. Effects of structured protocolized physical therapy on the duration of mechanical ventilation in patients with prolonged weaning. J Crit Care 2024; 80: 154491. doi: 10.1016/j.jcrc.2023.154491 [DOI] [PubMed] [Google Scholar]
- 112.da Silva Guimarães B, de Souza LC, Cordeiro HF, et al. Inspiratory muscle training with an electronic resistive loading device improves prolonged weaning outcomes in a randomized controlled trial. Crit Care Med 2021; 49: 589–597. doi: 10.1097/CCM.0000000000004787 [DOI] [PubMed] [Google Scholar]
- 113.Dunn H, Quinn L, Corbridge SJ, et al. Mobilization of prolonged mechanical ventilation patients: an integrative review. Heart Lung 2017; 46: 221–233. doi: 10.1016/j.hrtlng.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clancy O, Edginton T, Casarin A, et al. The psychological and neurocognitive consequences of critical illness. A pragmatic review of current evidence. J Intensive Care Soc 2015; 16: 226–233. doi: 10.1177/1751143715569637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen YJ, Jacobs WJ, Quan SF, et al. Psychophysiological determinants of repeated ventilator weaning failure: an explanatory model. Am J Crit Care 2011; 20: 292–302. doi: 10.4037/ajcc2011886 [DOI] [PubMed] [Google Scholar]
- 116.Jeon K, Jeong BH, Ko MG, et al. Impact of delirium on weaning from mechanical ventilation in medical patients. Respirology 2016; 21: 313–320. doi: 10.1111/resp.12673 [DOI] [PubMed] [Google Scholar]
- 117.Mendel JG, Khan FA. Psychological aspects of weaning from mechanical ventilation. Psychosomatics 1980; 21: 465–471. doi: 10.1016/S0033-3182(80)73656-3 [DOI] [PubMed] [Google Scholar]
- 118.Elsehrawy MG, Saleh AM. Psychosocial predictors of ventilator weaning outcomes among patients in intensive care units. Heliyon 2024; 10: e24385. doi: 10.1016/j.heliyon.2024.e24385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peris A, Bonizzoli M, Iozzelli D, et al. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients. Crit Care 2011; 15: R41. doi: 10.1186/cc10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wade DM, Mouncey PR, Richards-Belle A, et al. Effect of a nurse-led preventive psychological intervention on symptoms of posttraumatic stress disorder among critically ill patients: a randomized clinical trial. JAMA 2019; 321: 665–675. doi: 10.1001/jama.2019.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Corson JA, Grant JL, Moulton DP, et al. Use of biofeedback in weaning paralyzed patients from respirators. Chest 1979; 76: 543–545. doi: 10.1378/chest.76.5.543 [DOI] [PubMed] [Google Scholar]
- 122.Holliday JE, Hyers TM. The reduction of weaning time from mechanical ventilation using tidal volume and relaxation biofeedback. Am Rev Respir Dis 1990; 141: 1214–1220. doi: 10.1164/ajrccm/141.5_Pt_1.1214 [DOI] [PubMed] [Google Scholar]
- 123.Huttmann SE, Wilms K, Hamm C, et al. Assessment of sleep in patients receiving invasive mechanical ventilation in a specialized weaning unit. Lung 2017; 195: 361–369. doi: 10.1007/s00408-017-9988-2 [DOI] [PubMed] [Google Scholar]
- 124.Little A, Ethier C, Ayas N, et al. A patient survey of sleep quality in the intensive care unit. Minerva Anestesiol 2012; 78: 406–414. [PubMed] [Google Scholar]
- 125.Pisani MA, Friese RS, Gehlbach BK, et al. Sleep in the intensive care unit. Am J Respir Crit Care Med 2015; 191: 731–738. doi: 10.1164/rccm.201411-2099CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients – a clinical review. Ann Intensive Care 2015; 5: 3. doi: 10.1186/s13613-015-0043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fritsch SJ, Riedel M, Marx G, et al. Development of a machine learning model for prediction of the duration of unassisted spontaneous breathing in patients during prolonged weaning from mechanical ventilation. J Crit Care 2024; 82: 154795. doi: 10.1016/j.jcrc.2024.154795 [DOI] [PubMed] [Google Scholar]
- 128.Kwong MT, Colopy GW, Weber AM, et al. The efficacy and effectiveness of machine learning for weaning in mechanically ventilated patients at the intensive care unit: a systematic review. Biodes Manuf 2019; 2: 31–40. doi: 10.1007/s42242-018-0030-1 [DOI] [Google Scholar]