Abstract
Introduction
Post-intubation hypotension (PIH) is a common complication of intubations performed in the emergency department (ED). Identification of patients at high-risk for PIH is a major challenge. We aimed to determine whether pre-intubation metabolic acidosis affects the incidence of PIH in the ED.
Methods
This was a single-center, retrospective, observational study of consecutive patients requiring emergent endotracheal intubation (ETI) from November 1, 2016 to March 31, 2022 at Hyogo Emergency Medical Center, an urban ED. The primary outcome was PIH, defined as a decreased systolic blood pressure (sBP) of <90 mmHg, required initiation of any vasopressor, or a decrease in sBP by ≥ 20 % within 30 min following intubation. Patients were divided into two groups: those with pre-intubation metabolic acidosis (metabolic acidosis group), defined as pH < 7.3 and base excess (BE) < −4 mmol/L on arterial blood gas analysis, and those with no metabolic acidosis (without-metabolic acidosis group). The association between PIH and pre-intubation metabolic acidosis was examined using multivariable logistic regression models. A receiver operating characteristic (ROC) curve was produced to assess the predictive value of pre-intubation BE for PIH.
Results
The study included 311 patients. PIH occurred in 65.5 % (74/113) of patients in the metabolic acidosis group and 29.3 % (58/198) of patients in the without-metabolic acidosis group. Multivariable logistic regression demonstrated that metabolic acidosis was associated with PIH (odds ratio 4.06, 95 % confidence interval 2.31–7.11). In the ROC analysis, the optimal cut-off point for BE was −4.1 (sensitivity = 71 %, specificity = 70 %), with the area under the ROC curve 0.74.
Conclusion
Pre-intubation metabolic acidosis was significantly associated with PIH. Physicians.
Keywords: Adverse events, Emergency department, Metabolic acidosis, Post-intubation
Abbreviation
- PIH
post-intubation hypotension
- ED
emergency department
- ETI
endotracheal intubation
- sBP
systolic blood pressure
- OR
adjusted odds ratio
- CA
cardiac arrest
- CI
confidence interval
- BE
base excess
- SpO2
oxyhemoglobin saturation by pulse oximetry.
1. Introduction
During endotracheal intubation (ETI) in the emergency department (ED), several complications, including hypotension, hypoxia, arrhythmia, and cardiac arrest (CA), can occur [1,2]. In particular, post-intubation hypotension (PIH) is a common complication, presenting significant hypotension just after intubations [3]. Patients who develop PIH in the ED are associated with higher in-hospital mortality and longer length of hospital stay [[4], [5], [6]]. To identify patients at high-risk for PIH, previous studies have evaluated clinical factors such as shock index, comorbidities, indications for ETI, age, induction agent choice, and laboratory data [[7], [8], [9], [10], [11], [12], [13]].
Metabolic acidosis has been shown to cause cardiovascular instability in basic animal research [[14], [15], [16]] and is generally recognized by physicians as a factor complicating airway management [17]. However, validation of the direct association between pre-intubation metabolic acidosis and PIH in clinical practice has been insufficient. Although one study identified severe acidemia (pH < 7.2) as a risk factor for PIH [12], the study cohort was limited to those who were unable to maintain acid-base homeostasis due to mixed metabolic-respiratory acidosis. Therefore, the association between respiratory-compensated metabolic acidosis and PIH remains unclear. While another study linked pre-intubation metabolic acidosis to peri-intubation CA in the ED, PIH was not evaluated as the outcome in that study [18].
We hypothesized that the incidence of PIH in the ED would be higher in patients with metabolic acidosis compared to those without metabolic acidosis. This study aimed to assess the association between metabolic acidosis and PIH in the ED.
2. Materials and methods
2.1. Study design and participants
We conducted a single-center, retrospective, observational study of consecutive patients requiring emergency airway management from November 1, 2016, to March 31, 2022, in an urban ED at Hyogo Emergency Medical Center, a tertiary emergency medical center in Japan with an average annual emergency patient volume of 1000 transported by emergency vehicles, focusing on potentially critically ill or injured. The Hyogo Emergency Medical Center Ethics Committee approved the study (2023003) and waived the requirement for written informed consent. Study results are presented according to the STROBE guidelines for observational studies.
All adult patients (>17 years old) who required emergent ETI in the ED were eligible for this study. Patients were excluded if they were in CA upon ED arrival or developed CA before the intubation procedure, received mechanical circulatory support (such as veno-arterial extracorporeal membrane oxygenation or intra-aortic balloon pump), were administered vasopressors or inotropes (including norepinephrine, vasopressin, dobutamine, phenylephrine, or epinephrine) 60 min prior to intubation, or were missing arterial blood gas analysis data or blood pressure measurements.
2.2. Clinical setting
The ED in our center is staffed by emergency medicine residents supervised by board-certified emergency physicians. Pulse oximetry, continuous electrocardiography, and blood pressure measurement (invasive arterial blood pressure or non-invasive blood pressure) were used to monitor all patients. Intubations were performed by emergency attending physicians or residents (transitional-year residents, emergency medicine residents, or residents in other specialties) under the supervision of the emergency attending physicians. Equipment, including endotracheal tubes, direct or video laryngoscopes, suction tubes, and bag-valve masks, were routinely prepared before intubation, and preoxygenation was performed in all cases. Necessity of intubation, choice of intubation strategy (rapid sequence intubation or others), and selection of sedative drugs, neuromuscular blockers, and vasopressors or inotropes were determined at the physicians’ discretion. Successful endotracheal tube placement was confirmed by auscultation, end-tidal CO2 detector, and subsequent chest X-ray or fluoroscopic images.
Data collection.
Patients’ characteristics (age, sex, weight, and height), comorbidities, outpatient medications, indications for intubation, clinical outcomes (in-hospital mortality, length of intensive care unit (ICU) stay, and length of hospital stay), and vital signs were collected from the electronic medical record system. Vital signs, including blood pressure, heart rate, oxygen saturation (SpO2) level, Glasgow Coma Scale score, and respiratory rate, were collected immediately before the first intubation attempt (pre-intubation), immediately after successful intubation (post-intubation), and 30 min after intubation. Furthermore, peri-intubation characteristics such as laboratory data, induction medication, the use of sodium bicarbonate, and time interval from ED arrival to intubation were collected.
2.3. Definitions
The primary outcome was PIH. In our study, we defined PIH as follows: 1) systolic blood pressure (sBP) of ≥90 mmHg before intubation and sBP of <90 mmHg after intubation; 2) sBP of ≥90 mmHg before intubation and sBP of ≥90 mmHg after intubation with the need to initiate any vasopressor or inotropes within 30 min following intubations; or 3) sBP of <90 mmHg before intubation and ≥20 % decrease in sBP between before and after intubation. For sBP after intubation, the lower sBP of the either two points (post-intubation and 30 min after intubation) was used [6,8,13,19]. PIH was collected as a binary variable. The primary exposure of interest was pre-intubation metabolic acidosis, which was defined as pH < 7.3 and base excess (BE) < −4 mmol/L on arterial blood gas analysis [20]. We compared two groups: the metabolic acidosis group, defined as patients who had pre-intubation metabolic acidosis; and the without-metabolic acidosis group, defined as enrolled patients without metabolic acidosis. The secondary outcomes were in-hospital mortality, length of ICU stay, and length of hospital stay.
2.4. Data analysis
Continuous variables and ordinal variables were described using medians with interquartile ranges (IQR). Categorical variables were described with frequency and percentages. Measures of associations are presented with odds ratios (OR) and 95 % confidence intervals (CI). Categorical variables were compared using chi-square test; continuous variables were compared using Mann Whitney U test. A p-vales of <0.05 were considered significant (two sided). Multivariable logistic regression analysis was performed with PIH as the dependent variable adjusted for the following known risk factors; shock index >0.8 before intubation, age, sex, use of non-depolarizing neuromuscular blocking agents, the use of sedative agents, chronic renal disease, and intubation for respiratory failure [6,12,13]. The same analysis method was applied to in-hospital mortality. Length of ICU stay and length of hospital stay were compared using Mann-Whitney U test.
Since the definition of PIH has not yet been firmly established, the difference in definitions can contribute to the variation in research findings regarding PIH [19]. Therefore, we performed the same analysis method for the following two definitions of PIH (PIHα, PIHβ), respectively: PIHα was defined as 1) sBP of ≥90 mmHg before intubation and sBP of <90 mmHg after intubation, or 2) sBP of ≥90 mmHg before intubation and sBP of ≥90 mmHg after intubation with the need to initiate any vasopressor or inotropes; PIHβ was 1) sBP of ≥90 mmHg before intubation and sBP of <90 mmHg after intubation.
To assess the predictive value of BE for PIH, a receiver operating characteristic (ROC) curve of pre-intubation BE as a predictor of PIH was examined. Area under the curve (AUC) value, cut-off value, sensitivity, and specificity were evaluated.
Additionally, subgroup analysis was conducted among the two groups regarding intubation indication (trauma patients or medical patients). Subgroups were adjusted with multivariable logistic analysis using the same variables. Statistical analysis was performed using STATA 17 (Stata Corp. College Station, Texas, USA).
3. Results
3.1. Baseline characteristics and intubation factors
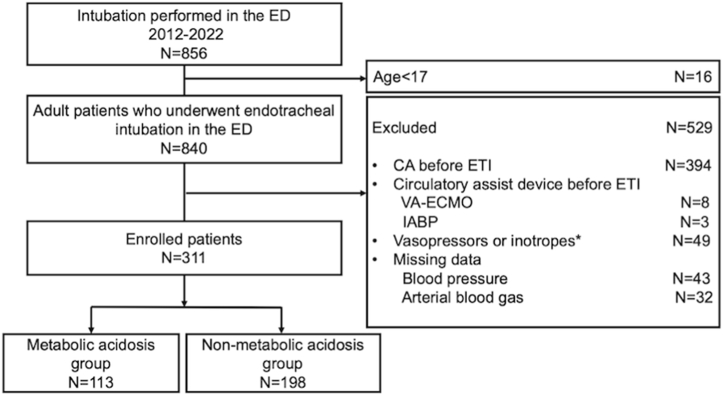
During the study period, 840 adult patients were intubated in the ED. After excluding 529 cases (394 due to CA, 49 for vasopressor support, 11 for circulatory assist devices, and 75 with missing data), 311 cases were included (Fig. 1). Of these patients, 113 patients (36.3 %) had metabolic acidosis (metabolic acidosis group), and 198 patients (63.7 %) did not (without-metabolic acidosis group).
Fig. 1.
Flow diagram of the study population.
ED, emergency department; CA, cardiac arrest; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; ETI, endotracheal intubation. ∗ Vasopressors or inotropes included norepinephrine, vasopressin, dobutamine, phenylephrine, and epinephrine 60 min prior to ETI.
Table 1 shows patients’ characteristics and a comparison between the two groups.
Table 1.
Baseline patient characteristics.
| Metabolic acidosis group (n = 113) | Without-metabolic acidosis group (n = 198) | |
|---|---|---|
| Age, years | 69 (53–79) | 65.5 (46–75) |
| Female sex, n (%) | 33 (29.2) | 68 (34.3) |
| Weight, kg | 60 (50–70) | 60 (53.4–70) |
| Height, cm | 163 (158–170) | 166.3 (155–170) |
| Body mass index, kg/m2 | 22.2 (19.6–25.5) | 22.9 (20.8–25.1) |
| Comorbidities, n (%) | ||
| COPD | 10(8.9) | 7 (3.5) |
| Coronary artery disease | 12(10.6) | 13 (6.8) |
| Heart failure | 7 (6.2) | 6 (3.0) |
| Cerebrovascular disease | 12 (10.6) | 21 (10.6) |
| Malignancy | 6 (5.3) | 10 (5.1) |
| Diabetes mellitus | 21 (18.6) | 22 (11.1) |
| Hypertension | 35 (31.0) | 63 (31.8) |
| End-stage liver disease | 1 (0.9) | 5 (2.5) |
| Chronic kidney disease | 9 (8.0) | 8 (4.0) |
| Outpatient medications, n (%) | ||
| Diuretics | 14 (12.4) | 10 (5.1) |
| β-blockers | 15 (13.3) | 15 (7.6) |
| ACE-Inhibitor | 16 (14.2) | 32 (16.2) |
| Ca-channel blocker | 24 (21.2) | 42 (21.2) |
| Nitrate | 3 (2.7) | 2 (1.0) |
| Antiarrhythmic agent | 3 (2.7) | 2 (1.0) |
| Indication for ETI, n (%) | ||
| Non-cardiogenic respiratory failure | 20 (17.7) | 16 (8.1) |
| Cardiogenic pulmonary edema | 15 (13.3) | 3 (1.5) |
| Airway obstruction | 36 (31.9) | 73 (36.9) |
| Medical shock | 13 (11.5) | 14 (7.1) |
| Trauma | 26 (23.0) | 82 (41.4) |
| Others | 3 (2.7) | 10 (5.1) |
COPD, chronic obstructive pulmonary disease; ACE, angiotensin-converting enzyme; ETI, endotracheal intubation.
Data are presented as medians (interquartile range) for continuous variables and n (%) for categorical variables.
The metabolic acidosis group was older than the without-metabolic acidosis group and a higher proportion of patients in the metabolic acidosis group took diuretics. Indications for ETI differed between the groups.
Table 2 shows the peri-intubation characteristics. The frequency of use of sedative drugs (69.0 % [78/113] vs. 85.9 % [170/198]) and non-depolarizing neuromuscular blocking agents (69.0 % [78/113] vs. 86.4 % [171/198]) were less in the metabolic acidosis group. Fewer amounts of rocuronium (mg/body weight) were used in the metabolic acidosis group (0.79 [IQR: 0–1.11] mg/kg vs. 0.98 [IQR: 0.77–1.10] mg/kg). Regarding blood gas analysis, pH (7.24 [IQR: 7.05–7.36] vs. 7.37 [IQR: 7.27–7.42]) and BE (−8.3 [IQR: 15.3 to −3.7] mmol/L vs. −2.6 [IQR: 5.9 to −0.5] mmol/L) were lower in the metabolic acidosis group, respectively. The lactate level was higher in the metabolic acidosis group (5.7 [IQR: 3.1–9.8] mmol/L vs. 2.9 [IQR: 1.8–4.7] mmol/L).
Table 2.
Peri-intubation characteristics.
| Metabolic acidosis group (n = 113) | Without-metabolic acidosis group (n = 198) | |
|---|---|---|
| Induction medication | ||
| Fentanyl, mcg | 60 (0–100) | 100 (50–200) |
| Use of sedative drug, n (%) | 78 (69.0) | 170 (85.9) |
| Choice of sedatives, n (%) | ||
| Propofol | 16 (14.2) | 76 (38.4) |
| Ketamine | 19 (16.8) | 33 (16.7) |
| Midazolam | 41 (36.3) | 58 (29.3) |
| Others | 4 (3.5) | 5 (2.5) |
| aUse of nNMBs, n (%) | 78 (69.0) | 171 (86.4) |
| Rocuronium, mg | 50 (0–60) | 50 (50–70) |
| Rocuronium mg/body weight, mg/kg | 0.79 (0–1.11) | 0.98 (0.77–1.10) |
| Pre-intubation Vital signs | ||
| Heart rate, beat/min | 113 (87–132) | 103.5 (84–119) |
| sBP, mmHg | 120 (92–150) | 131 (112–160) |
| sBP<90, n (%) | 28 (24.8) | 26 (13.1) |
| Shock index | 0.95 (0.66–1.19) | 0.76 (0.60–0.95) |
| Shock index >0.8, n (%) | 28 (24.8) | 26 (13.1) |
| SpO2, % | 98 (90–100) | 100 (96–100) |
| Respiratory rate, breaths/min | 20 (12–30) | 20 (16–25) |
| Glasgow Coma Scale score | 6 (3–9) | 6 (3–13) |
| Post-intubation vital signs | ||
| Heart rate, beats/min | 114.5 (94.5–132) | 106.5 (91–126.5) |
| sBP, mmHg | 104 (81–129) | 126 (100–151.5) |
| sBP<90, n (%) | 38 (33.6) | 32 (16.2) |
| Blood gas analysis | ||
| pH | 7.24 (7.05–7.36) | 7.37 (7.27–7.42) |
| HCO3−, mmol/L | 19.1 (14.9–22.5) | 22.3 (19.3–24.4) |
| PaCO2, mmHg | 43.4 (32.7–64.8) | 39.5 (27.1–44.9) |
| Correlated anion gap, mEq/L | 19.1 (15.1–23.8) | 15.5 (12.5–19.4) |
| SBE, mmol/L | −8.3 (−15.3 to −3.7) | −2.6 (−5.9 to −0.5) |
| Lactate level, mmol/L | 5.7 (3.1–9.8) | 2.9 (1.8–4.7) |
| Initiation of vasopressor or inotropes, n (%) | 54 (47.8) | 33 (16.7) |
| Use of sodium bicarbonate, n (%) | 12(10.6) | 2(1.0) |
| Time from arrival to intubation, min | 20 (12–30) | 33 (22–55) |
| Rapid sequence intubation, n (%) | 90 (79.7) | 141 (71.2) |
nNMBs, non-depolarizing neuromuscular blocking agents; sBP, systolic blood pressure; SI, shock index; SpO2, oxygen saturation; PaCO2, partial pressure of carbon dioxide; SBE, standard base excess.
Data are presented as medians (interquartile range) for continuous variables and n (%) for categorical variables.
Only rocuronium was used as the non-depolarizing neuromuscular blocking agent.
3.2. Patient outcomes between the metabolic acidosis and without-metabolic acidosis groups
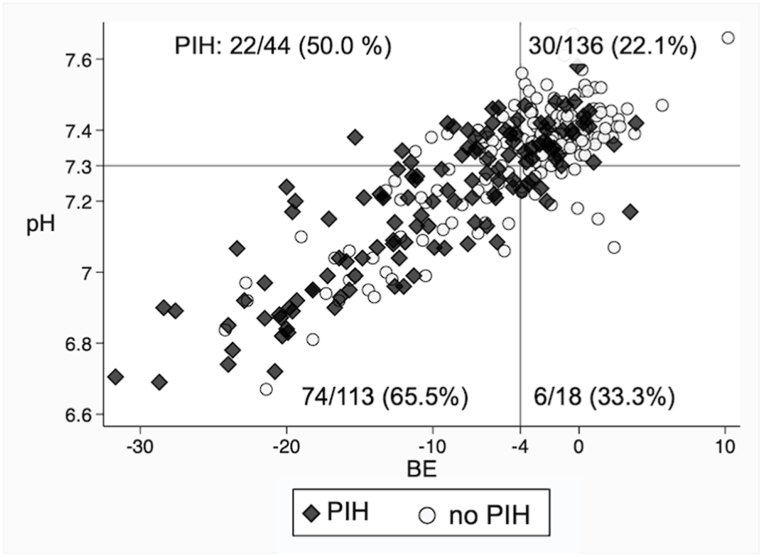
Fig. 2 illustrates a comparison of pH and BE values between patients with PIH and those without PIH. Table 3 shows a comparison of primary and secondary outcomes between the metabolic acidosis group and the without-metabolic acidosis group. Univariable logistic regression analysis (65.5 % [74/113] vs. 29.3 % [58/198], OR 4.58, 95 % CI 2.79–5.21) and multivariable logistic regression analysis (OR 4.06, 95 % CI 2.31–7.11) showed that the metabolic acidosis group was associated with a higher incidence of PIH.
Fig. 2.
Comparison of pH and BE values between patients with PIH and those without PIH.
BE, base excess; PIH, post-intubation hypotension. The figure is divided into four quadrants based on a pH threshold of 7.3 and s BE threshold of −4 mmol/L. Black diamond plots represent patients in the PIH group, while white round plots indicate patients without PIH.
Table 3.
Comparison of primary and secondary outcomes between metabolic acidosis and without-metabolic acidosis groups.
| Outcome | Metabolic acidosis | Without-metabolic | Unadjusted | Adjusteda |
|---|---|---|---|---|
| Variables | N = 113 | acidosis N = 198 | OR (95 % CI) | OR (95 % CI) |
| Primary outcomes | ||||
| PIH, n (%) | 74 (65.5) | 58 (29.3) | 4.58 (2.79–5.21) | 4.06 (2.31–7.11) |
| PIHα, n (%) | 48 (42.5) | 39 (19.7) | 3.01 (1.80–5.02) | 2.98 (1.67–5.33) |
| PIHβ, n (%) | 31 (27.4) | 27 (13.6) | 2.39 (1.34–4.27) | 2.50 (1.30–4.80) |
| Secondary outcomes | ||||
| In-hospital death, n (%) | 36 (31.9) | 45 (22.7) | 1.59 (0.95–2.67) | 1.53 (0.84–2.79) |
| Length of hospital stay, days | 13 (6–24) | 10.5 (4–25) | – | – |
| Length of ICU stay, days | 7 (4–15) | 5 (2–11.5) | – | – |
OR, odds ratio; CI, confidence intervals; PIH, post-intubation hypotension; ICU, intensive care unit.
PIHα was defined as 1) sBP of ≥90 mmHg before intubation and sBP of <90 mmHg after intubation, or 2) sBP of ≥90 mmHg before intubation and sBP of ≥90 mmHg with the initiation of any vasopressor.
PIHβ was defined as sBP of ≥90 mmHg before intubation and sBP of <90 mmHg after intubation.
Data are presented as medians (interquartile range) for continuous variables and n (%) for categorical variables.
Adjusted with age, sex, shock index >0.8 before intubation, use of non-depolarizing neuromuscular blocking agents, use of sedative agents, chronic renal disease, and intubation for respiratory failure.
Although in-hospital mortality did not differ (31.9 % [36/113] vs. 22.7 % [45/198], OR 1.53, 95 % CI 0.84–2.79), metabolic acidosis was significantly associated with longer length of hospital stay (13 [IQR: 6–24] days vs. 10 [IQR: 4–25] days, p = 0.04) and longer length of ICU stay (7 [IQR: 4–15] days vs. 5 [IQR: 2–11.5] days, p = 0.04).
In sensitivity analysis for various definitions of PIH, multivariable logistic regression analysis showed that metabolic acidosis was significantly associated with PIH, regardless of the definition (PIHα: 42.5 % [48/113] vs. 19.7 % [39/198], OR 2.98, 95 % CI 1.67–5.33, PIHβ: 27.4 % [31/113] vs.13.6 % [27/198], OR 2.50, 95 % CI 1.30–4.80).
3.3. Association between pre-intubation BE and PIH
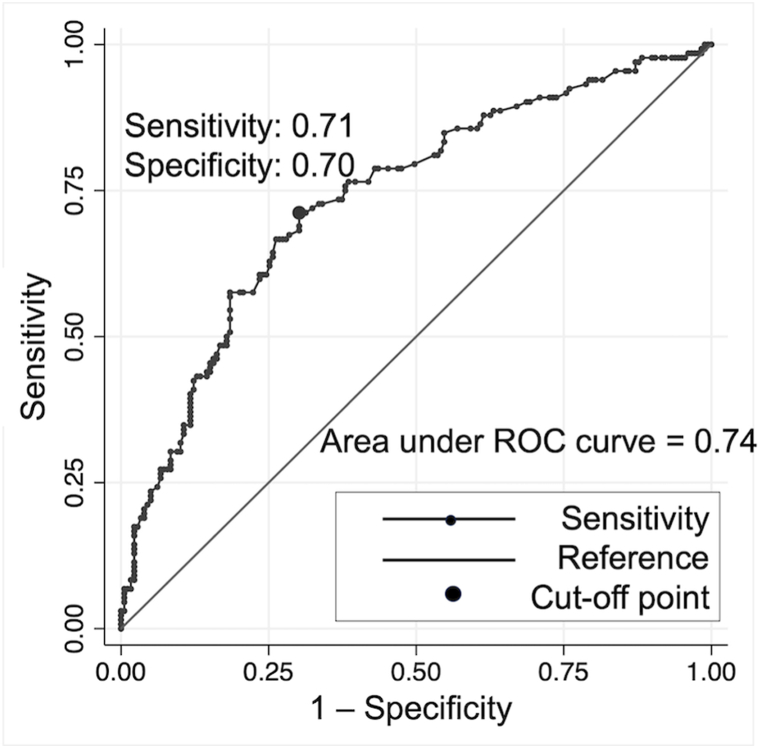
The ROC curve of pre-intubation BE as a predictor of PIH is shown in Fig. 3. The optimal cut-off point for BE was −4.1. BE of −4.1 or lower predicted PIH with 71 % sensitivity and 70 % specificity. The AUC value was 0.74.
Fig. 3.
ROC curve analysis to examine the optimal pre-intubation BE cut-off for PIH (n = 311).
ROC, receiver operating characteristic; BE, base excess; PIH, post-intubation hypotension; AUC, area under the ROC curve. The optimal cut-off point for base excess was −4.1 (sensitivity = 71 %, specificity = 70 %). The AUC value was 0.74.
3.4. Subgroup analyses according to intubation indication
The association between pre-intubation metabolic acidosis and PIH during ETI was also evaluated in subgroups. In both trauma and medical patients, metabolic acidosis was associated with PIH (trauma patients; OR: 4.69, 95%CI: 1.52–14.50, medical patients; OR: 4.47, 95%CI: 2.22–8.99) (Table 4).
Table 4.
Subgroup analyses according to intubation indication.
| Adjusteda | |||
|---|---|---|---|
| Subgroup | Total | PIH (%) | OR (95 % CI) |
| Indication of ETI | |||
| Trauma | 108 | 26 (24.1) | 4.69 (1.52–14.50) |
| Medical | 190 | 89 (46.8) | 4.47 (2.22–8.99) |
OR, odds ratio; CI, confidence intervals; PIH, post-intubation hypotension; ETI, endotracheal intubation.
Data are presented as n (%) for categorical variables.
Adjusted with age, sex, shock index >0.8 before intubation, use of non-depolarizing neuromuscular blocking agents, use of sedative agents, chronic renal disease, and intubation for respiratory failure.
4. Discussion
In this study, we assessed the association between pre-intubation metabolic acidosis and PIH. Although previous studies suggested pre-intubation metabolic acidosis was likely to cause hemodynamic collapse, the direct association between pre-intubation metabolic acidosis and PIH has yet to be evaluated [12,18]. Our results were consistent with those earlier studies. The sensitivity analysis of PIH performed by applying various definitions demonstrated that the results were consistent, regardless of the definitions. In the ROC curve analysis, the cutoff value for pre-intubation BE was −4.1, which was consistent with the general definition of metabolic acidosis of BE (BE < −4 mmol/L) [20]. These findings support the validity of our study and underscore the association between pre-intubation metabolic acidosis and PIH.
The mechanism underlying the development of PIH in the presence of pre-intubation metabolic acidosis has not been thoroughly elucidated. Severe acidemia, characterized by plasma pH values below 7.2, represents a potentially life-threatening condition and is likely to have detrimental effects on myocardial contractility, resulting in pulmonary vasoconstriction, reduced responsiveness to catecholamines, and systemic vasodilation [16,21]. In critically ill patients with severely low pH levels, respiratory alkalosis accompanied by alveolar hyperventilation plays a pivotal role in maintaining acid-base balance [22]. The extended apneic period during ETI procedures, coupled with muscle paralysis, can contribute to decompensated metabolic acidosis and a sudden decline in pH, resulting in hemodynamic collapse [23,24].
Our findings have significant practical implications for the prevention of PIH. While intravenous administration of fluid boluses, sodium bicarbonate or vasopressors before ETI has been considered as preventive measures [20,[25], [26], [27], [28]], a reliable prophylactic approach has yet to be established. Considering the mechanism of PIH described above, it is plausible that interventions targeting metabolic acidosis could effectively prevent PIH. Of note, unlike many other laboratory data, metabolic acidosis can be promptly detected through blood gas analysis and corrected prior to urgent ETI. Ideally, it is recommended to address underlying causes before proceeding with intubation. Additionally, minimizing the duration of apnea during intubation and maintaining appropriate ventilation levels both before and after intubation represent practical and reasonable interventions for managing pre-intubation metabolic acidosis [23]. Further investigations are warranted to explore the efficacy of these interventions targeting metabolic acidosis in preventing PIH among patients undergoing ETI.
In addition, most studies on risk factors for PIH have included heterogeneous study populations and have not sufficiently examined each population separately. Although our study included heterogeneous patients, we performed subgroup analyses to focus on more specific populations. As a result, the subgroup analysis showed similar results for both trauma and medical patients. Given the wide variety of causes of metabolic acidosis and intubation, future studies are needed to explore more specific subgroups of patients that may exhibit different outcomes.
Our study has several limitations. First, the generalizability of our findings may be restricted because this study was conducted at a single center. Notably, the proficiency level of ETI, as well as the selection of intubation techniques and sedative medications, can vary across healthcare facilities. Second, we excluded as many as 63.7 % of patients. Most of the reason for exclusion was CA before intubation (n = 394, 46.0 %), however, this population was not suitable for our study objective. Furthermore, patients missing arterial blood gas analysis data or blood pressure measurements, or who were administered vasopressors or inotropes were excluded, although the number of these patients was small. These missing data could result in biased estimates. Third, this was a retrospective study, which may cause information bias. The decision to perform ETI was made at the discretion of the attending physicians. Nonetheless, we believe that these practices reflect real-world emergency department procedures. Moreover, multivariable logistic regression analysis was used to adjust for potential confounders, though there may still be unmeasured confounding factors outside the scope of our study. Finally, data on peri-intubation ventilation parameters and blood gas analysis were not obtained. As a result, it remains unclear whether hypoventilation-induced mixed acidosis was responsible for PIH.
5. Conclusions
In conclusion, our study demonstrates an association between pre-intubation metabolic acidosis and the incidence of PIH in the ED. This finding underscores the importance of clinical vigilance when patients with metabolic acidosis require urgent ETI in the ED. Clinicians should be aware of the potential risk of PIH in patients with metabolic acidosis.
CRediT authorship contribution statement
Masafumi Suga: Writing – original draft, Methodology, Investigation, Conceptualization. Takeshi Nishimura: Writing – review & editing, Methodology, Conceptualization. Tatsuya Ochi: Writing – review & editing, Investigation. Takashi Hongo: Writing – review & editing, Investigation. Tetsuya Yumoto: Writing – review & editing. Atsunori Nakao: Writing – review & editing. Satoshi Ishihara: Writing – review & editing. Hiromichi Naito: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank Christine Burr for editing the manuscript.
References
- 1.Sakles J.C., Laurin E.G., Rantapaa A.A., Panacek E.A. Airway management in the emergency department: a one-year study of 610 tracheal intubations. Ann. Emerg. Med. Mar 1998;31(3):325–332. doi: 10.1016/s0196-0644(98)70342-7. [DOI] [PubMed] [Google Scholar]
- 2.Russotto V., Myatra S.N., Laffey J.G., Tassistro E., Antolini L., Bauer P., et al. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325(12):1164–1172. doi: 10.1001/jama.2021.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mort T.C. Complications of emergency tracheal intubation: hemodynamic alterations--part I. J. Intensive Care Med. 2007;22(3):157–165. doi: 10.1177/0885066607299525. [DOI] [PubMed] [Google Scholar]
- 4.Émond M., Lachance-Perreault D., Boucher V., Carmichael P.H., Turgeon J., Brousseau A.A., et al. The impact of post-intubation hypotension on length of stay and mortality in adult and geriatric patients: a cohort study. CJEM. Aug 2022;24(5):509–514. doi: 10.1007/s43678-022-00305-0. [DOI] [PubMed] [Google Scholar]
- 5.Russotto V., Tassistro E., Myatra S.N., Parotto M., Antolini L., Bauer P., et al. Peri-intubation cardiovascular collapse in patients who are critically ill: insights from the INTUBE study. Am. J. Respir. Crit. Care Med. Aug 2022;(4):449–458. doi: 10.1164/rccm.202111-2575OC. [DOI] [PubMed] [Google Scholar]
- 6.Heffner A.C., Swords D.S., Nussbaum M.L., Kline J.A., Jones A.E. Predictors of the complication of postintubation hypotension during emergency airway management. J. Crit. Care. Dec 2012;27(6):587–593. doi: 10.1016/j.jcrc.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Dubée V., Hariri G., Joffre J., Hagry J., Raia L., Bonny V., et al. Peripheral tissue hypoperfusion predicts post intubation hemodynamic instability. Ann. Intensive Care. Jul 18 2022;12(1):68. doi: 10.1186/s13613-022-01043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishimaru T., Goto T., Takahashi J., Okamoto H., Hagiwara Y., Watase H., et al. Association of ketamine use with lower risks of post-intubation hypotension in hemodynamically-unstable patients in the emergency department. Sci. Rep. Nov 21 2019;9(1) doi: 10.1038/s41598-019-53360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halliday S.J., Casey J.D., Rice T.W., Semler M.W., Janz D.R., Russell D.W., et al. Risk factors for cardiovascular collapse during tracheal intubation of critically III adults. Ann Am Thorac Soc. 2020 Aug;17(8):1021–1024. doi: 10.1513/AnnalsATS.201912-894RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K., Jang J.S., Kim J., Suh Y.J. Age shock index, shock index, and modified shock index for predicting postintubation hypotension in the emergency department. Am. J. Emerg. Med. May 2020;38(5):911–915. doi: 10.1016/j.ajem.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Tamsett Z., Douglas N., King C., Johnston T., Bentley C., Hao B., et al. Does the choice of induction agent in rapid sequence intubation in the emergency department influence the incidence of post-induction hypotension? Emerg. Med. Australasia (EMA) 2023 Nov 29 doi: 10.1111/1742-6723.14355. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.M., Shin T.G., Hwang S.Y., Yoon H., Cha W.C., Sim M.S., et al. Sedative dose and patient variable impacts on postintubation hypotension in emergency airway management. Am. J. Emerg. Med. Jul 2019;37(7):1248–1253. doi: 10.1016/j.ajem.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Smischney N.J., Kashyap R., Khanna A.K., Brauer E., Morrow L.E., Seisa M.O., et al. Risk factors for and prediction of post-intubation hypotension in critically ill adults: a multicenter prospective cohort study. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0233852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orchard C.H., Hamilton D.L., Astles P., McCall E., Jewell B.R. The effect of acidosis on the relationship between Ca2+ and force in isolated ferret cardiac muscle. J. Physiol. May 1991;436:559–578. doi: 10.1113/jphysiol.1991.sp018567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drapeau P., Nachshen D.A. Effects of lowering extracellular and cytosolic pH on calcium fluxes, cytosolic calcium levels, and transmitter release in presynaptic nerve terminals isolated from rat brain. J. Gen. Physiol. Feb 1988;91(2):305–315. doi: 10.1085/jgp.91.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh J.D., Margolis T.I., Kim D. Mechanism of diminished contractile response to catecholamines during acidosis. Am. J. Physiol. Jan 1988;254(1 Pt 2):H20–H27. doi: 10.1152/ajpheart.1988.254.1.H20. [DOI] [PubMed] [Google Scholar]
- 17.Mosier J.M., Joshi R., Hypes C., Pacheco G., Valenzuela T., Sakles J.C. The physiologically difficult airway. West. J. Emerg. Med. Dec 2015;16(7):1109–1117. doi: 10.5811/westjem.2015.8.27467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T.H., Chen K.F., Gao S.Y., Lin C.C. Risk factors associated with peri-intubation cardiac arrest in the emergency department. Am. J. Emerg. Med. Aug 2022;58:229–234. doi: 10.1016/j.ajem.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Green R.S., Erdogan M. Are outcomes worse in patients who develop post-intubation hypotension? CJEM. Aug 2022;24(5):465–466. doi: 10.1007/s43678-022-00340-x. [DOI] [PubMed] [Google Scholar]
- 20.Fujii T., Udy A.A., Nichol A., Bellomo R., Deane A.M., El-Khawas K., et al. Incidence and management of metabolic acidosis with sodium bicarbonate in the ICU: an international observational study. Crit. Care. Feb 02 2021;25(1):45. doi: 10.1186/s13054-020-03431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung B., Rimmele T., Le Goff C., Chanques G., Corne P., Jonquet O., et al. Severe metabolic or mixed acidemia on intensive care unit admission: incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit. Care. 2011;15(5):R238. doi: 10.1186/cc10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manthous C.A. Avoiding circulatory complications during endotracheal intubation and initiation of positive pressure ventilation. J. Emerg. Med. Jun 2010;38(5):622–631. doi: 10.1016/j.jemermed.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Lentz S., Grossman A., Koyfman A., Long B. High-risk airway management in the emergency department. Part I: diseases and approaches. J. Emerg. Med. Jul 2020;59(1):84–95. doi: 10.1016/j.jemermed.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West J.R., Scoccimarro A., Kramer C., Caputo N.D. The effect of the apneic period on the respiratory physiology of patients undergoing intubation in the ED. Am. J. Emerg. Med. 2017;35(9):1320–1323. doi: 10.1016/j.ajem.2017.03.076. Epub 2017 Apr 2Sep. [DOI] [PubMed] [Google Scholar]
- 25.Panchal A.R., Satyanarayan A., Bahadir J.D., Hays D., Mosier J. Efficacy of bolus-dose phenylephrine for peri-intubation hypotension. J. Emerg. Med. Oct 2015;49(4):488–494. doi: 10.1016/j.jemermed.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A., Azim A. Difficult tracheal intubation in critically ill. J Intensive Care. 2018;6:49. doi: 10.1186/s40560-018-0318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell D.W., Casey J.D., Gibbs K.W., Ghamande S., Dargin J.M., Vonderhaar D.J., et al. Effect of fluid bolus administration on cardiovascular collapse among critically ill patients undergoing tracheal intubation: a randomized clinical trial. JAMA. Jul 19 2022;328(3):270–279. doi: 10.1001/jama.2022.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z., Zhu C., Mo L., Hong Y. Effectiveness of sodium bicarbonate infusion on mortality in septic patients with metabolic acidosis. Intensive Care Med. Nov 2018;44(11):1888–1895. doi: 10.1007/s00134-018-5379-2. [DOI] [PubMed] [Google Scholar]