Abstract
Background
The purpose of this study was to investigate the protective effect of xylosma congesta extract on kidney injury in hyperuricemic rats.
Methods
The rats were fed yeast extract and intraperitoneal injections of potassium oxonate for 3 weeks to establish the hyperuricemia model. And then the rats were treated with allopurinol and different doses of oak extract. The contents of uric acid in urine and serum, creatinine, and urea nitrogen in serum were detected by biochemical methods. TUNEL was used to detect cell apoptosis in renal tissue. The protein expression of TLR4 and NF- kappa B (NF-κB) p65 and the proportion of CD68 and CD206 positive cells in renal tissue were detected by pathological method.
Results
The xylosma congesta group showed decreased renal tubular dilatation, decreased renal interstitial inflammatory cell infiltration, decreased serum creatinine content, and decreased apoptotic cell count as compared to the model group. And positive expression of TLR4 and NF-κB decreased with each dose. Additionally, the xylosma congesta groups showed a significant rise in CD206 and a considerable decrease in CD68.
Conclusion
The extract from xylosma congesta has the ability to lower serum uric acid and creatinine levels while also providing protection against kidney damage caused by hyperuricemia.
Keywords: Hyperuricemia, Xylosma congesta, Inflammatory response, Macrophage, Kidney injury
1. Introduction
Hyperuricemia is a common metabolic disorder. Globally, the incidence and prevalence of hyperuricemia are rising gradually as a result of improving economic conditions as well as changes in food habits and lifestyles [1]. According to statistics, the prevalence rate of hyperuricemia in Chinese adults is estimated to be 11.1 % [2]. Inflammation brought on by the deposition of monosodium urate (MSU) crystals in cartilage, synovial bursa, tendon, or soft tissue is what causes the rise in blood uric acid concentration [3]. It is well known that MSU crystals can activate TLR4, and that neuroinflammation is primarily triggered by TLR4. NF- kappa B (NF-κB) can be further activated and phosphorylated by TLR4 expression. TLR4 has NF-κB as a downstream target protein. Activating NF-κB can promote the release of a series of inflammatory factors, such as TNF-α, IL-6, and IL-1β [4]. Studies have shown that the TLR4/NF-κB pathway plays an important role in hyperuricemia [5].
The two primary causes of hyperuricemia are inadequate excretion and excessive uric acid synthesis [6]. Thus, any method for reducing uric acid synthesis or increasing uric acid excretion is helpful for treating and preventing illnesses associated with hyperuricemia in addition to treating and preventing hyperuricemia [7]. The two primary efficient uric acid-lowering medications used in clinical settings are xanthine oxidase inhibitors, such as fesotan, and uric acid uric agents, such as probenecid and benzbromarone [8,9]. These medications do, however, have clear drawbacks. For instance, it causes harm to the kidneys and has poor safety and effectiveness [8,10]. Thus, the development of medications that are both effective and have fewer negative effects remains imperative.
Xylosma congesta belongs to the genus Xylosma, which is found in China, Japan, and Korea [11]. Although xylosma congesta is part of the traditional Chinese medicine system, there are few studies on its biological activity and function. The few studies in the literature show that xylosma congesta extract has a certain role in anti-inflammatory, anti-melanin, and antioxidation, and its main active components include alkaloids, terpenoids, and phenols [11].
In this study, we investigated the protective effect of xylosma congesta extract on hyperuricemia rats and preliminary explored whether the protective effect was mediated by the TLR4/NF-κB pathway.
2. Materials and methods
2.1. Preparation of xylosma congesta extract
Weigh 100 g of xylosma congesta (Bark), put it into a large beaker, and soak it in deionized water for 5 h, then boil it twice for 1.5 h each time. The extract was retained in about 300 mL, centrifuged, the dregs were discarded, and the extract was obtained and stored at −80 °C.
2.2. Animals
This study was guided and approved by the Animal Ethics Committee of Guizhou University of Traditional Chinese Medicine. Male Wistar rats (6 weeks old, Beijing Hufukang Biological, License number SCXK (Beijing) 2019-0008) were housed at 20–26 °C, humidity 40–70 %, 12/12 h light/dark, and adaptively fed for 1 week.
In this work, the hyperuricemia model was established by feeding with yeast extract and intraperitoneal injections of potassium oxonate [12]. 30 rats were randomly divided into control group (n = 5), Hyperuricemia (HUA) group (n = 5), allopurinol group (n = 5, 75 mg/kg/d), Oak-L dose group (n = 5, 1.6 g/kg/d), Oak-M dose group (n = 5, 3.2 g/kg/d), and Oak-H dose group (n = 5, 6.4 g/kg/d). With the exception of the control group, which received the same amount of normal saline, each rat was administered yeast extract at a dosage of 15 g/kg/d in feed and intraperitoneally injected with potassium oxonate at 600 mg/kg/d, respectively, for a period of three weeks to establish a hyperuricemia model. Following this, the allopurine group was administered allopurine. Rats in the Oak-L dose group, Oak-M dose group, and Oak-H dose group were given varying doses of wood pulp extract. Both the control group and the HUA group were given the same amount of normal saline. The rats were euthanized after three weeks of treatment. Prior to execution, 24-h urine samples were collected. Blood was taken before execution, and kidneys were taken after execution.
2.3. Hematoxylin-eosin (HE) staining
The renal tissue specimens were fixed with 4 % paraformaldehyde. Then treated with an anhydrous ethanol gradient. Xylene transparent, paraffin embedded, serial sections, hematoxylin-eosin staining, neutral resin-sealed sections. The morphological alterations in rats’ renal tissue were observed under a microscope (BX43, Olympus), and the images were collected.
2.4. Determination of uric acid, creatinine, and urea in serum
Serum uric acid, creatinine, and urea were determined as described above. Serum levels of uric acid, creatinine and urea are measured with the appropriate test kit according to the manufacturer's instructions.
2.5. TUNEL staining
Rat kidney tissue sections embedded in paraffin were baked, deparaffinized, and hydrated. The sections were then moved to a wet box containing citrate buffer. The reaction was then run for 30 min at 37 °C to repair antigen, and the samples were washed 3 times with PBS, each time for 5 min. After using absorbent paper to blot off the PBS surrounding the tissue, each slide was filled dropfold with an appropriate amount of TUNEL test solution and left to incubate for 1 h at 37 °C in the dark. After rinsing the excess detection solution with PBS, DAPI was dropped and allowed to incubate for 5 min in the dark. After that, a fluorescence microscope (CKX53, Olympus) was used to examine the photos in order to gather data.
2.6. Immunofluorescence
The rat kidney tissue paraffin sections were permeabilized for 10 min at room temperature using 0.5 % Triton-X-100. They were then blocked for 30 min at 37 °C using bovine serum albumin BSA (Solarbio) and treated with CD68 (DF7518, Affinity, 1:100) and CD206 (DF4149, Affinity, 1:100) and left overnight at 4 °C. Following washing, fluorescent secondary antibody cy3 (AS007, ABclonal, 1:200) was added dropfold and incubated at 37 °C for 45 min thereafter. Following DAPI counterstaining, the slides were blocked and examined using an Olympus CKX53 fluorescent microscope.
2.7. Immunohistochemistry
Rat kidney tissues were sectioned, baked, deparaffinized, hydrated, and antigen repaired with citrate buffer. After blocking with 5 % BSA, p65 (AF5006, Affinity, 1:100) was used. TLR4 (AF7017, Affinity, 1:100) was incubated with the primary antibody. After overnight at 4 °C, horseradish enzyme-labeled goat anti-rabbit IgG (H + L) (ZB-2301, Zhongshan Jinqiao, 1:100) was added, incubated at 37 °C for 30 min, and eluted thoroughly with PBS. After DAB development, hematoxylin was counterstained with reverse blue, dehydration, and transparency, and the slides were sealed and observed under a microscope (BX43, Olympus).
2.8. Statistical analysis
Graphpad 9.0 software was used for mapping and statistical analysis. Quantitative results were expressed as mean ± standard deviation (mean ± SD). One-way analysis of variance was used among multiple groups. Statistical differences were indicated by P < 0.05.
3. Results
3.1. Xylosma congesta extract improves kidney injury in hyperuricemic rats
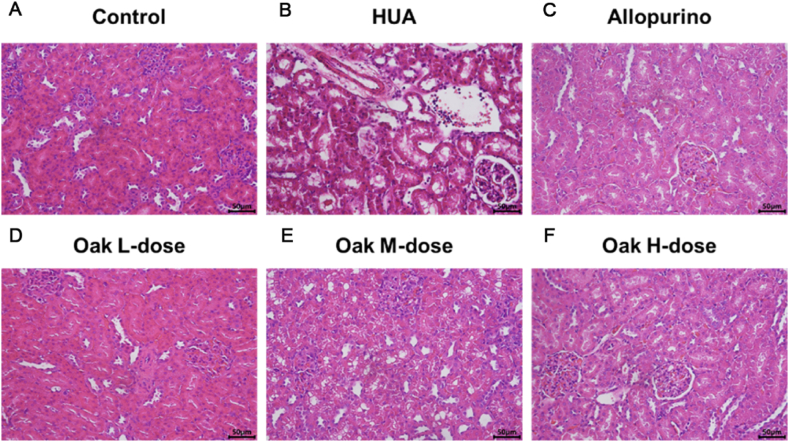
Light microscopy analysis revealed normal glomerular structure in the control group, while the HUA group exhibited renal tubular dilatation, shedding of renal tubular epithelial cells into the lumen, interstitial hemorrhage, and infiltration of inflammatory cells. Treatment with allopurinol led to improvements in renal tubular dilatation and inflammatory cell infiltration. Notably, treatment with xylosma congesta extract significantly improved interstitial hemorrhage, reduced renal tubular dilatation, and decreased inflammatory cell infiltration, with the high-dose group showing the most pronounced effects (Fig. 1A–F).
Fig. 1.
HE staining of rat kidney tissue. (A) Control group, (B) HUA group, (C) Allopurino group, (D) Oak L-dose group, (E) Oak M-dose group, (F) Oak H-dose group.
3.2. Xylosma congesta extract can reduce the uric acid and creatinine in the serum of hyperuric rats
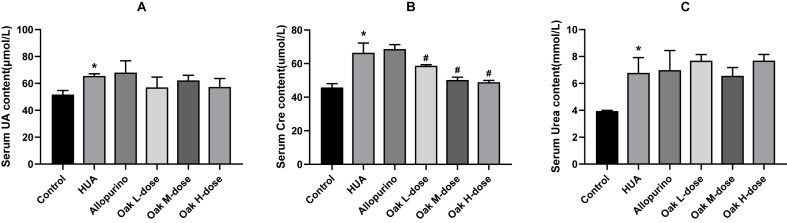
At the end of the study, serum uric acid, creatinine, and urea levels were measured across various groups. The HUA group exhibited significantly elevated serum uric acid (64.80 ± 0.84 μmol/L) compared to the control group (50.80 ± 2.28 μmol/L, P < 0.001). Treatment with xylosma congesta extract led to a reduction in uric acid levels across all dosage groups, but the decreases were not statistically significant when compared to the HUA group (Oak L-dose group vs. HUA group, P = 0.12; Oak M-dose group vs. HUA group, P = 0.17; Oak H-dose group vs. HUA group, P = 0.13). Similarly, allopurinol did not significantly reduce uric acid levels (66.40 ± 4.93 μmol/L, P = 0.45). (Fig. 2A, Supplementary Table 1).
Fig. 2.
Effects of xylosma congesta extract on serum uric acid, creatinine and urea in rats with hyperuricemia. (A) Serum uric acid, (B) creatinine, and (C) urea level in rats. ∗P < 0.05 vs Control group, #P < 0.05 vs HUA group.
For serum creatinine levels, the HUA group showed significantly higher levels (64.80 ± 4.27 μmol/L) than the control group (45.00 ± 1.22 μmol/L, P < 0.001). While allopurinol did not significantly reduce creatinine levels (P = 0.69), treatment with xylosma congesta extract led to a notable reduction in creatinine levels across all dosages, with the high-dose group showing the most substantial decrease (50.40 ± 1.51 μmol/L, P = 0.094). (Fig. 2B, Supplementary Table 1).
Serum urea levels were significantly elevated in the HUA group (6.64 ± 1.17 μmol/L) compared to the control (3.90 ± 0.07 μmol/L, P < 0.001). However, neither allopurinol nor xylosma congesta extract treatment significantly altered urea levels in comparison to the HUA group (Allopurinol group vs. HUA group, P = 0.81; Oak L-dose group vs. HUA group, P = 0.19; Oak M-dose group vs. HUA group, P = 0.64; Oak H-dose group vs. HUA group, P = 0.13). (Fig. 2C, Supplementary Table 1).
3.3. Xylosma congesta extract can inhibit kidney cells’ apoptosis in hyperuric rats
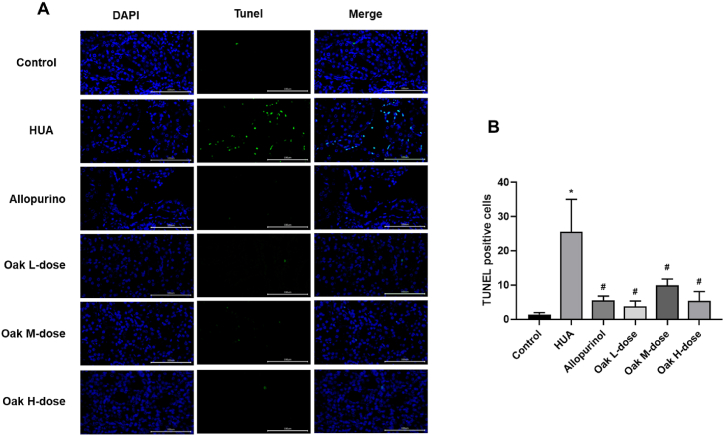
The effect of xylosma congesta extract on renal cell apoptosis was assessed using TUNEL staining. The HUA group displayed a significantly higher number of apoptotic cells (25.56 ± 9.49) compared to the control group (1.44 ± 0.53, P < 0.001). Treatment with xylosma congesta extract led to a significant reduction in apoptotic cell counts across all dosage groups, with values ranging from 3.78 ± 1.56 to 5.33 ± 2.78 (P < 0.001 for all comparisons to the HUA group). Similarly, allopurinol treatment significantly reduced apoptosis (5.56 ± 1.24, P < 0.001). These results indicate that xylosma congesta extract effectively reduces kidney cell apoptosis in hyperuricemic rats (Fig. 3A–B, Supplementary Table 2).
Fig. 3.
TUNEL staining of rat kidney tissue. (A) fluorogram, (B) Bar chart. ∗P < 0.05 vs Control group, #P < 0.05 vs HUA group.
3.4. Xylosma congesta extract can regulate the polarization of renal macrophages in hyperuric rats
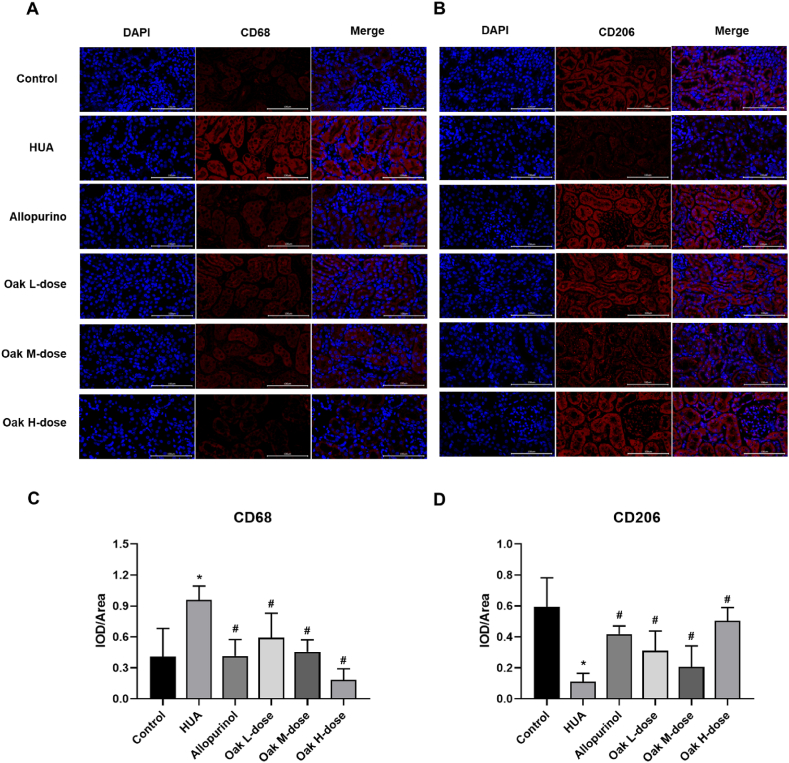
Immunofluorescence analysis was performed to assess renal macrophage polarization. The HUA group showed a significantly higher proportion of M1 macrophages (CD68+, 0.95 ± 0.13) compared to the control group (0.41 ± 0.27, P < 0.001). Treatment with xylosma congesta extract resulted in a significant reduction in M1 macrophages, with the high-dose group showing the greatest decrease (0.18 ± 0.11, P < 0.001). Similarly, allopurinol treatment also reduced M1 macrophage levels (0.41 ± 0.16, P < 0.001). (Fig. 4A, C, Supplementary Table 2).
Fig. 4.
Immunofluorescence detection of CD68 and CD206 in rat kidney tissues. (A) CD68 fluorogram, (B) CD206 fluorogram, (C) CD68 expression bar graph, (D) CD206 expression bar graph. ∗P < 0.05 vs Control group, #P < 0.05 vs HUA group.
Conversely, the proportion of M2 macrophages (CD206+) was significantly lower in the HUA group (0.11 ± 0.05) compared to the control group (0.59 ± 0.19, P < 0.001). Treatment with xylosma congesta extract significantly increased M2 macrophage levels, with the high-dose group showing the highest increase (0.50 ± 0.09, P < 0.001). Allopurinol also significantly increased M2 macrophages (0.42 ± 0.05, P < 0.001). These results suggest that xylosma congesta extract effectively suppresses M1 macrophage polarization and promotes M2 macrophage polarization, thereby modulating renal macrophage polarization in hyperuricemic rats (Fig. 4B, D, Supplementary Table 2).
3.5. Xylosma congesta extract mediates the TLR4/NF-κB p65 pathway to inhibit kidney injury in hyperuric rats
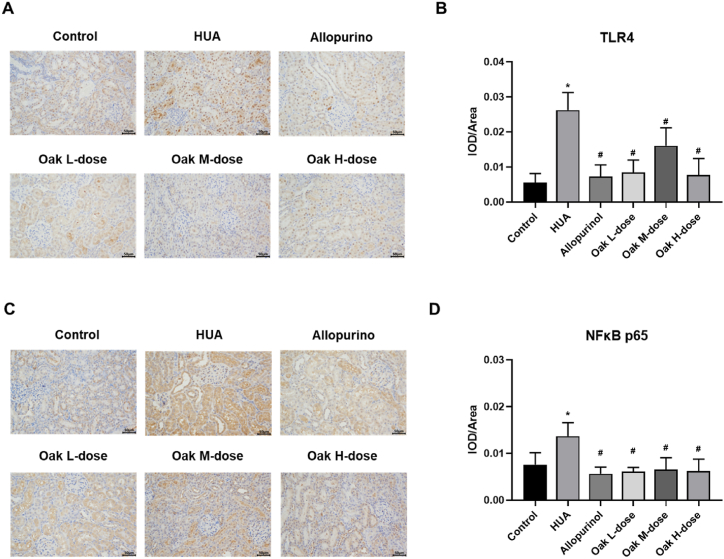
Immunohistochemistry was used to assess the expression of TLR4 and NF-κB p65 proteins in the kidneys of hyperuricemic rats. The HUA group showed significantly elevated levels of TLR4 (0.024 ± 0.006) and NF-κB p65 (0.013 ± 0.003) compared to the control group (TLR4: 0.008 ± 0.008, NF-κB p65: 0.008 ± 0.003, P < 0.001). Treatment with xylosma congesta extract significantly reduced the expression of both proteins across all dosage groups, with high-dose xylosma congesta extract showing the greatest reduction (TLR4: 0.008 ± 0.005, NF-κB p65: 0.006 ± 0.003, P < 0.001). Similarly, allopurinol also reduced protein expression levels (TLR4: 0.007 ± 0.003, NF-κB p65: 0.006 ± 0.001, P < 0.001). These results suggest that xylosma congesta extract exerts protective effects against hyperuricemia-induced kidney damage by modulating the TLR4/NF-κB p65 signaling pathway (Fig. 5A–D, Supplementary Table 2).
Fig. 5.
The protein expression of TLR4 and NF-κB p65 in kidney tissue was detected by immunohistochemistry. (A) TLR4, (B) TLR4 expression bar graph, (C) NF-κB, (D) NF-κB p65 expression bar graph. ∗P < 0.05 vs Control group, #P < 0.05 vs HUA group.
4. Discussion
In many nations, hyperuricemia has emerged as a major public health concern [13]. Hyperuricemia is an independent risk factor for the occurrence of kidney injury and is associated with mortality from renal failure [14,15]. However, there is no reliable treatment available, and the search for more effective, efficient, and safe drugs has been the subject of drug discovery. In this study, it was found that xylosma congesta extract could improve renal pathological damage, inhibit renal cell apoptosis, and reduce serum uric acid and serum creatinine in hyperuricemia rats, which revealed the potential value of xylosma congesta extract in the treatment of hyperuricemia.
An increase in uric acid reabsorption or a decrease in its excretion leads to elevated serum uric acid levels [16,17]. More and more clinical evidence definitely suggests that hyperuricemia can induce renal abnormalities by involving a variety of pathological and molecular mechanisms, especially hyperuricemia, which may be related to renal tubular injury [[18], [19], [20]]. Serum urea nitrogen and creatinine rise in response to decreased urea and creatinine clearance following renal damage [21]. Besides stimulating the synthesis of uric acid, yeast extract is rich in purine, which is considered to be the precursor of uric acid production. And prior research has demonstrated that intraperitoneal injections of potassium oxonate can raise uric acid levels [5,22]. Therefore, yeast extract combined with potassium oxonate is one of the most common and simple methods to construct a hyperuricemia model. The advantage of this modeling method is that an animal model of hyperuricemia with a stable, high uric acid level and renal injury can be obtained. Consistent with previous studies, the kidney in the HUA group showed renal tubular dilatation and tubular epithelial cell exfoliation to the lumen, renal interstitial hemorrhage, renal interstitial inflammatory cell infiltration, increased renal cell apoptosis, and increased serum uric acid, creatinine, and urea content.
A primary treatment strategy for hyperuricemia and related renal issues involves enhancing uric acid excretion or decreasing its reabsorption [23]. The most often prescribed medication for hyperuricemia is allopurinol, a xanthine oxidase inhibitor that can be progressively increased or decreased based on serum urate and creatinine clearance. However, a number of studies have been investigating for possible allopurinol alternatives in recent years due to its substantial consequences in individuals with renal insufficiency [24,25]. In this study, the allopurinol group did not show the effect of reducing serum uric acid, but only showed the effect of reducing apoptosis. However, low-dose and high-dose xylosma congesta extract groups showed a decrease in serum uric acid and serum creatinine levels at the same treatment time. Compared with allopurinol, it was suggested that xylosma congesta extract can resist hyperuricemia and reverse renal damage induced by hyperuricemia and has the potential to be used as a substitute for allopurinol.
Macrophages traditionally have the function of phagocytosis, clearing pathogens, apoptotic cells, and cell fragments [26]. Apart from their conventional function of safeguarding the host against pathogens, macrophages also modulate development, homeostasis, remodeling, and tissue restoration. Under external stimulation, macrophages polarize into different phenotypes, called M1 and M2 macrophages, and are associated with renal injury [27,28]. According to reports, uric acid has the ability to directly activate macrophage toll-like receptors (TLR), particularly TLR2/TLR4, which in turn activates the NF-κB pathway and converts macrophages into type M1 [29]. Studies have indicated that there is a drop in M2 macrophages and an increase in M1 macrophages in hyperuricemia-associated renal injury, while allopurinol can reduce the ratio of M1/M2 cells to alleviate HUA renal injury [30]. The findings demonstrated that, similar to allopurinol, the xylosma congesta extract group saw a drop in TLR4 and NF-κB p65 protein levels, as well as an increase in M2 macrophages and a decrease in M1 macrophages. Based on this, we hypothesize that the TLR4/NF-κB p65 signal pathway regulates macrophage phenotypic transformation, which may be associated to the anti-hyperuricemia effect of xylosma congesta extract.
Although this study demonstrates the protective effects of xylosma congesta extract against renal injury in hyperuricemic rats and investigates its potential mechanisms via the TLR4/NF-κB pathway, several limitations should be noted. First, this study is based primarily on animal models and has not been validated in clinical trials. Therefore, the generalizability of the results may be limited, and further clinical research is needed to confirm the efficacy and safety of xylosma congesta in humans. Second, the study used only a few dosages of xylosma congesta extract, and the effects and safety of long-term use of different dosages are not fully clear. Although significant effects were observed at higher doses, the potential toxicity or side effects of prolonged high-dose administration remain to be evaluated. Additionally, while we investigated the role of the TLR4/NF-κB pathway in the protective mechanism, other potential molecular mechanisms were not fully explored. Future research should focus on these unstudied mechanisms and conduct a thorough analysis of long-term effects of different dosages to provide a more comprehensive basis for the clinical application of xylosma congesta.
5. Conclusion
In conclusion, the xylosma congesta extract has potential value as a substitute for allopurinol. It has the ability to lower blood uric acid and creatinine levels as well as ameliorate hyperuricemia-induced kidney damage by controlling macrophage phenotypic change via the TLR4/NF-κB p65 signal pathway. This study lays the groundwork for future systematic toxicological and pharmacokinetic investigations of xylosma congesta extract as a potential alternative to allopurinol in treating hyperuricemia and associated renal injury.
CRediT authorship contribution statement
Jinjun He: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition. Weiyi Tian: Writing – original draft, Formal analysis, Data curation. Yonghui Meng: Data curation. An Yan: Visualization. Xin Lai: Visualization. Fei Wang: Visualization. Bangwei Che: Writing – review & editing.
Data availability statement
The datasets used for the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Ethics Statement
The animal study was reviewed and approval of the Animal Ethics Committee of Guizhou University of traditional Chinese Medicine (No.20230228, 2023.08.01).
Consent for publication
Not applicable.
Funding
This study was supported by the Scientific and technological Cooperation Program of Guizhou Province (Qiankehe LH word [2015] No. 7798).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40674.
Contributor Information
Jinjun He, Email: GZY_HJJ@hotmail.com.
Weiyi Tian, Email: 765196640@qq.com.
Yonghui Meng, Email: 905884370@qq.com.
An Yan, Email: 18337159@qq.com.
Xin Lai, Email: 1791183901@qq.com.
Fei Wang, Email: 3381812474@qq.com.
Bangwei Che, Email: 156645087@qq.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Johnson R.J., Bakris G.L., Borghi C., Chonchol M.B., Feldman D., Lanaspa M.A., et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am. J. Kidney Dis. 2018;71:851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M., Zhu X., Wu J., Huang Z., Zhao Z., Zhang X., et al. Prevalence of hyperuricemia among Chinese adults: findings from two nationally representative cross-sectional surveys in 2015-16 and 2018-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.791983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia P.J., Beltran L., Mejia C.C., Torres R., Tebar M.D., Pose R.A. Ultrasound in the diagnosis of asymptomatic hyperuricaemia and gout. Rev. Clin. Esp. 2016;216:445–450. doi: 10.1016/j.rce.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X., Shi Q., Li J., Quan S., Zhang X., Gu L., et al. Medicinal fungus phellinus igniarius alleviates gout in vitro by modulating tlr4/nf-kb/nlrp3 signaling. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1011406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K., Hu L., Chen J.K. Rip3-deficience attenuates potassium oxonate-induced hyperuricemia and kidney injury. Biomed. Pharmacother. 2018;101:617–626. doi: 10.1016/j.biopha.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Su J., Wei Y., Liu M., Liu T., Li J., Ji Y., et al. Anti-hyperuricemic and nephroprotective effects of rhizoma dioscoreae septemlobae extracts and its main component dioscin via regulation of moat1, murat1 and moct2 in hypertensive mice. Arch Pharm. Res. (Seoul) 2014;37:1336–1344. doi: 10.1007/s12272-014-0413-6. [DOI] [PubMed] [Google Scholar]
- 7.Ma C.H., Kang L.L., Ren H.M., Zhang D.M., Kong L.D. Simiao pill ameliorates renal glomerular injury via increasing sirt1 expression and suppressing nf-kappab/nlrp3 inflammasome activation in high fructose-fed rats. J. Ethnopharmacol. 2015;172:108–117. doi: 10.1016/j.jep.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 9.Strilchuk L., Fogacci F., Cicero A.F. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin Drug Saf. 2019;18:261–271. doi: 10.1080/14740338.2019.1594771. [DOI] [PubMed] [Google Scholar]
- 10.Hu M., Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4:1209–1220. doi: 10.2147/tcrm.s3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte-Casar R., Romero-Benavides J.C. Xylosma g. Forst. Genus: medicinal and veterinary use, phytochemical composition, and biological activity. Plants. 2022;11 doi: 10.3390/plants11091252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S., Li L., Yan H., Jiang X., Hu W., Han N., et al. Anti-gouty arthritis and anti-hyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep. 2019;20:4623–4633. doi: 10.3892/mmr.2019.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehmood A., Zhao L., Wang C., Nadeem M., Raza A., Ali N., et al. Management of hyperuricemia through dietary polyphenols as a natural medicament: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2019;59:1433–1455. doi: 10.1080/10408398.2017.1412939. [DOI] [PubMed] [Google Scholar]
- 14.Li L., Yang C., Zhao Y., Zeng X., Liu F., Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia X., Luo Q., Li B., Lin Z., Yu X., Huang F. Serum uric acid and mortality in chronic kidney disease: a systematic review and meta-analysis. Metabolism. 2016;65:1326–1341. doi: 10.1016/j.metabol.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Hyndman D., Liu S., Miner J.N. Urate handling in the human body. Curr. Rheumatol. Rep. 2016;18:34. doi: 10.1007/s11926-016-0587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puig J.G., Torres R.J., de Miguel E., Sanchez A., Bailen R., Banegas J.R. Uric acid excretion in healthy subjects: a nomogram to assess the mechanisms underlying purine metabolic disorders. Metabolism. 2012;61:512–518. doi: 10.1016/j.metabol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Wu M., Ma Y., Chen X., Liang N., Qu S., Chen H. Hyperuricemia causes kidney damage by promoting autophagy and nlrp3-mediated inflammation in rats with urate oxidase deficiency. Dis Model Mech. 2021:14. doi: 10.1242/dmm.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miake J., Hisatome I., Tomita K., Isoyama T., Sugihara S., Kuwabara M., et al. Impact of hyper- and hypo-uricemia on kidney function. Biomedicines. 2023:11. doi: 10.3390/biomedicines11051258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balakumar P., Alqahtani A., Khan N.A., Mahadevan N., Dhanaraj S.A. Mechanistic insights into hyperuricemia-associated renal abnormalities with special emphasis on epithelial-to-mesenchymal transition: pathologic implications and putative pharmacologic targets. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105209. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann D., Fuchs T.C., Henzler T., Matheis K.A., Herget T., Dekant W., et al. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology. 2010;277:49–58. doi: 10.1016/j.tox.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Zhu X., Liu F., Peng W., Zhang L., Li J. Pharmacodynamic evaluation of the xor inhibitor wn1703 in a model of chronic hyperuricemia in rats induced by yeast extract combined with potassium oxonate. Curr Res Pharmacol Drug Discov. 2022;3 doi: 10.1016/j.crphar.2022.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson A.L., Singer R.F., Walters G.D. Uric acid lowering therapies for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2017;10:D9460. doi: 10.1002/14651858.CD009460.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker M.A., Schumacher H.J., Wortmann R.L., MacDonald P.A., Eustace D., Palo W.A., et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 25.Sapankaew T., Thadanipon K., Ruenroengbun N., Chaiyakittisopon K., Ingsathit A., Numthavaj P., et al. Efficacy and safety of urate-lowering agents in asymptomatic hyperuricemia: systematic review and network meta-analysis of randomized controlled trials. BMC Nephrol. 2022;23:223. doi: 10.1186/s12882-022-02850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefater J.R., Ren S., Lang R.A., Duffield J.S. Metchnikoff's policemen: macrophages in development, homeostasis and regeneration. Trends Mol. Med. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W., Wang B.O., Hou Y.F., Fu Y., Cui S.J., Zhu J.H., et al. Jaml promotes acute kidney injury mainly through a macrophage-dependent mechanism. JCI Insight. 2022:7. doi: 10.1172/jci.insight.158571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaemi-Oskouie F., Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr. Rheumatol. Rep. 2011;13:160–166. doi: 10.1007/s11926-011-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haryono A., Nugrahaningsih D., Sari D., Romi M.M., Arfian N. Reduction of serum uric acid associated with attenuation of renal injury, inflammation and macrophages m1/m2 ratio in hyperuricemic mice model. Kobe J. Med. Sci. 2018;64:E107–E114. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.