Abstract
Background
Pulmonary hypertension (PH) and female have been linked to a worse survival in patients with obstructive hypertrophic cardiomyopathy (oHCM). However, female patients with PH exhibited a better prognosis than males. Herein, we investigated sex differences in the prevalence and survival of pH in oHCM following septal myectomy.
Methods
We consecutively enrolled 1491 patients diagnosed with oHCM. PH was defined as a pulmonary artery systolic pressure (PASP) > 36 mm Hg.
Results
Females were older, more likely to experience chest pain and NYHA class III/IV symptoms, and had a higher prevalence of PH (37.6 % vs. 19.9 %, p < 0.001) than males. Multivariable analysis showed that female was an independent risk for PH (OR 2.3, 95 % CI: 1.70–3.11, p < 0.001) though the PASP was comparable between males and females (44.93 ± 10.87 vs. 44.74 ± 9.72 mm Hg, p = 0.856). Over a median follow-up of 36 months [IQR 23.5–52.5 months), 28 deaths and 189 composite endpoints were observed. Kaplan-Meier analysis showed a higher cumulative incidence of death (p = 0.015) and composite endpoints (p < 0.001) in patients with PH, and Cox regression analysis revealed that PH (HR 1.78, 95 % CI: 1.30–2.45, p < 0.001) and female (HR 1.39, 95 % CI: 1.02–1.90, p = 0.038) were independently associated with composite endpoints. However, no significant survival differences were found between males and females within the PH subgroup.
Conclusions
Female was independently associated with higher prevalence but not severity of PH. Although PH and female were independently associated with worse survival, no survival difference was found between males and females in the PH subgroup.
Keywords: Hypertrophic cardiomyopathy, Pulmonary hypertension, Sex difference, Survival
1. Introduction
Hypertrophic cardiomyopathy(HCM) is a prevalent inherited heart disease characterized by myocardial hypertrophy, increased myocardial contractility, and hemodynamic disorders [1]. Approximately two-thirds of patients with HCM experience left ventricular outflow obstruction, either at rest or under provocation [2], which typically leads to elevated left atrial pressure and the subsequent development of post-capillary pulmonary hypertension (PH) [3]. PH is highly prevalent in HCM, with an estimated incidence of approximately 38 % as determined by echocardiography, and is associated with poorer survival outcomes [4], [5]. Previous studies indicated that there is a sexual dimorphism in PH with respect to disease prevalence, severity of hemodynamic alterations, right ventricular adaptation, response to therapy, and notably, clinical outcomes [6], [7]. Specifically, females are more likely to develop PH but tend to exhibit a more favorable hemodynamic profile and better survival. Conversely, among patients with obstructive HCM (oHCM), female individuals often experience more severe symptoms and worse clinical outcomes compared to their male counterparts [8], [9]. However, the impact of sex differences on the prevalence, severity, and survival of pH in patients with oHCM remains unknown. Herein, we aimed to investigate the sex differences in the prevalence and prognosis of pH in patients with oHCM following myectomy.
2. Method
2.1. Study population
This observational study involved 1491 patients diagnosed with oHCM at Fuwai Hospital from January 2015 to December 2019. The diagnosis of HCM adhered to the 2020 American Heart Association/American College of Cardiology and the 2014 European Society of Cardiology guidelines [10], [11], which primarily identify unexplained septal hypertrophy with a thickness exceeding 15 mm, or an intraventricular septum thickness of more than 13 mm accompanied by a family history of HCM, while ruling out other local or systemic causes. A left ventricular outflow tract (LVOT) gradient of 50 mm Hg or greater, either at rest or following provocation, was indicative of LVOT obstruction, necessitating surgical myectomy. All participants provided informed consent prior to their inclusion in the study, which was approved by the Ethics Committee of Fuwai Hospital and conducted in alignment with the ethical principles outlined in the Declaration of Helsinki.
2.2. Data collection
Demographic information was gathered, including variables such as age, gender, body mass index, and smoking status. Additionally, symptoms like chest pain, syncope, palpitations, and dizziness were documented. The data also encompassed family histories of HCM and sudden cardiac death, along with participants' self-reported medical backgrounds, which included conditions like hypertension, diabetes, atrial fibrillation, and any previous heart surgeries. Information about current medication use, including hypoglycemic agents, antihypertensive medications, and lipid-lowering drugs, was extracted from medical records by trained clinicians who were unaware of the study's objectives.
2.3. Echocardiography parameters
Transthoracic echocardiographic assessments were conducted utilizing a commercially available ultrasound system (E9 ultrasound system, GE Healthcare, Horten, Norway). The maximum left ventricular (LV) wall thickness was defined as the largest dimension recorded at any point within the LV chamber during end-diastole. LVOT gradients were measured using continuous Doppler ultrasound to determine the peak outflow velocities, with the estimated value derived through the simplified Bernoulli equation. Additionally, pulmonary artery systolic pressure (PASP) was ascertained from the tricuspid regurgitant jet velocity using the modified Bernoulli equation, incorporating right atrial pressure (RAP) when the tricuspid regurgitation jet was detectable. For those who could not have PASP measured via tricuspid regurgitation, mean pulmonary arterial pressure (PAP) was recorded based on the peak pulmonary regurgitation (PR) velocity. The calculation for mean PAP was derived from the formula: mean PAP = 4 (peak PR velocity)2 + RAP. Patients exhibiting a mean PAP greater than 25 mm Hg were classified as having pulmonary hypertension (PH). When PH was suspected, we assessed the PR end-velocity to estimate pulmonary arterial diastolic pressure (PADP) and subsequently applied the equation mean PAP = 2/3 PADP + 1/3 PASP to determine PASP. Right atrial pressure (RAP) estimates (5, 10, and 15 mm Hg) were made in the subcostal view, taking into account the size and collapsibility of the inferior vena cava during inspiration at rest. For further information, please refer to our earlier publication [12].
2.4. Surgical procedure
Extended septal myectomy, an evolution of the traditional Morrow procedure, was performed as previously outlined [13]. The resection spanned specific ranges: The upper limit was approximately 4 mm beneath the aortic ring, while the lower limit reached the apex of the left ventricle. From the right side, the myectomy commenced slightly to the right of the nadir of the right aortic cusp, and on the left side, resection concluded near the anterior commissure of the mitral valve. In cases where severe regurgitation or systolic anterior motion (SAM) persisted post-resection, elongated leaflets were folded to mitigate these issues. Based on preoperative assessments and intraoperative findings, additional interventions such as mitral repair or replacement, tricuspid repair, coronary artery bypass grafting, and radiofrequency ablation for atrial fibrillation were performed as necessary. Following weaning from cardiopulmonary bypass, an immediate reoperation was initiated if intraoperative transesophageal echocardiography revealed a postoperative LVOT gradient of 30 mm Hg or higher.
2.5. Follow-up
All patients underwent annual follow-ups through either clinic visits or phone interviews. Those who were lost to follow-up were censored at their last known contact date. The primary endpoint of our study was all-cause mortality, encompassing both cardiovascular and non-cardiovascular deaths. the occurrence of major adverse cardiovascular events (MACE), which was defined as a composite outcome of all-cause mortality, heart failure, new-onset atrial fibrillation, stroke, non-fatal myocardial infarction, and the implantation of a permanent pacemaker. Heart failure diagnosis was corroborated through a combination of clinical symptoms, physical signs, left ventricular ejection fraction assessments, concentrations of N-terminal prohormone of brain natriuretic peptide, and the use of diuretics. New-onset atrial fibrillation (AF) was identified in patients without prior AF who received a diagnosis through either 12-lead electrocardiography or a 24-hour Holter monitor during follow-up. Stroke was characterized by patient readmission with a diagnosis confirmed via computed tomography or magnetic resonance imaging, along with the administration of appropriate treatment. Non-fatal myocardial infarction was classified in patients experiencing angina pectoris who had angiography revealing coronary stenosis greater than 50 % or those who underwent percutaneous coronary intervention.
2.6. Statistics analysis
Baseline characteristics of participants were summarized in a sex-disaggregated table, with categorical variables as numbers and percentages, and continuous variables as means (SD) or medians (IQR). Group differences were assessed using ANOVA or chi-squared tests, accordingly. Univariable and multivariable logistic regression analyses were used to determine the correlation between sex and PH. Time to the first follow-up event was analyzed using the Kaplan–Meier survival curves and compared with log-rank test.Variables with p < 0.1 in univariable Cox analysis were included in a stepwise multivariable Cox regression to estimate adjusted hazard ratios (HR) and 95 % confidence intervals (CI). A two-tailed P value of ≤ 0.05 was considered to denote statistical significance. All statistical analyses were performed using R 4.3.3 version(https://www.R-project.org) and GraphPad Prism version 8.0 (GraphPad Software Inc., La Jolla, CA).
3. Results
3.1. Baseline characteristics
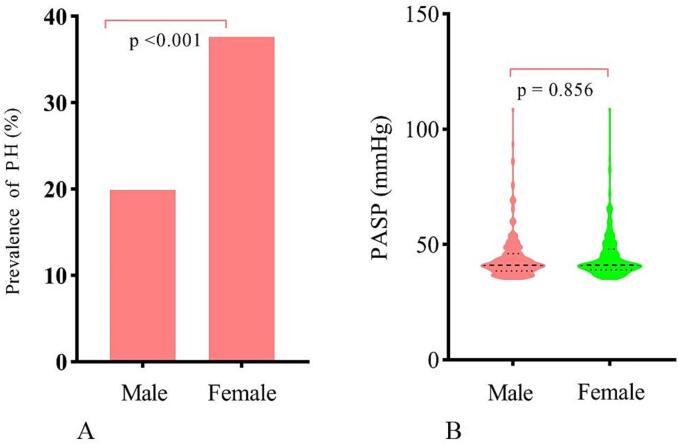
The baseline characteristics of the study patients grouped by sex are summarized in Table 1. The mean age of the participants was 49.54 ± 12.20 years, and 40.4 % of patients were female. Compared to male patients, females were much older, had a higher BMI, a higher proportion of individuals with chest pain, NYHA III/IV, and a lower prevalence of atrial fibrillation. Furthermore, females had a higher LVOT gradient and a higher proportion of individuals with moderate or severe MR, greater IVST, and lower left atrial diameter compared to their counterparts. Importantly, the prevalence of pH was significantly higher in female patients than in male patients (37.6 % vs. 19.9 %, p < 0.001, Fig. 1A). However, the PASP was comparable between males and females (44.93 ± 10.87 vs. 44.74 ± 9.72 mm Hg, p = 0.856, Fig. 1B).
Table 1.
Baseline characteristic of study population.
| Variables | Overall (N = 1491) |
Male (N = 888) |
Female (N = 603) |
p_value |
|---|---|---|---|---|
| Age (year) | 49.54 (12.20) | 47.32 (11.67) | 52.81 (12.25) | <0.001 |
| BMI (kg/m2) | 25.63 (3.46) | 25.95 (3.23) | 25.15 (3.71) | <0.001 |
| SBP (mm Hg) | 124.22 (15.72) | 125.15 (14.64) | 122.85 (17.12) | 0.005 |
| DBP (mm Hg) | 72.85 (10.09) | 73.55 (10.00) | 71.81 (10.14) | 0.001 |
| Chest pain (N,%) | 521 (34.9) | 283 (31.9) | 238 (39.5) | 0.003 |
| Syncope (N,%) | 306 (20.5) | 186 (20.9) | 120 (19.9) | 0.671 |
| Palpitation (N,%) | 403 (27.0) | 242 (27.3) | 161 (26.7) | 0.860 |
| Dizziness (N,%) | 283 (19.0) | 162 (18.2) | 121 (20.1) | 0.416 |
| AF (N,%) | 202 (13.5) | 136 (15.3) | 66 (10.9) | 0.019 |
| Diabetes (N,%) | 95 (6.4) | 58 (6.5) | 37 (6.1) | 0.842 |
| Hyperlipidemia (N,%) | 669 (44.9) | 401 (45.2) | 268 (44.4) | 0.827 |
| Hypertension (N,%) | 470 (31.5) | 272 (30.6) | 198 (32.8) | 0.399 |
| Cerebrovascular disease (N,%) | 52 (3.5) | 29 (3.3) | 23 (3.9) | 0.679 |
| Pulmonary hypertension (N,%) | 404 (27.1) | 177 (19.9) | 227 (37.6) | <0.001 |
| Smoking (N,%) | 562 (37.7) | 527 (59.3) | 35 (5.8) | <0.001 |
| FHCM (N,%) | 112 (7.5) | 65 (7.3) | 47 (7.8) | 0.809 |
| FSCD (N,%) | 48 (3.2) | 26 (2.9) | 22 (3.6) | 0.533 |
| Previous heart surgery (N,%) | 99 (6.6) | 68 (7.7) | 31 (5.1) | 0.07 |
| NYHA III/IV (N,%) | 1131 (75.9) | 647 (72.9) | 484 (80.3) | 0.001 |
| Coronary artery disease (N,%) | 209 (14.0) | 129 (14.5) | 80 (13.3) | 0.541 |
| Left atrial diameter (mm) | 44.90 (6.82) | 45.68 (7.06) | 43.75 (6.27) | <0.001 |
| IVST (mm) | 20.15 (6.76) | 20.53 (5.26) | 19.59 (8.48) | 0.009 |
| LVEDD (mm) | 42.84 (5.24) | 43.76 (5.03) | 41.48 (5.27) | <0.001 |
| EF(100 %) | 69.19 (5.83) | 69.27 (5.70) | 69.09 (6.01) | 0.555 |
| RVD (mm) | 21.35 (2.99) | 21.67 (2.77) | 20.87 (3.23) | <0.001 |
| MWT (mm) | 22.22 (4.82) | 22.81 (4.95) | 21.37 (4.48) | <0.001 |
| SAM (N, %) | 1425 (95.6) | 846 (95.3) | 579 (96.0) | 0.574 |
| LVOT gradient (mm Hg) | 83.21 (32.09) | 79.88 (30.39) | 88.12 (33.87) | <0.001 |
| Moderate or severe MR (N, %) | 788 (52.9) | 425 (47.9) | 363 (60.2) | <0.001 |
| Statin, (N, %) | 250 (16.8) | 145 (16.3) | 105 (17.4) | 0.632 |
| Hypoglycemic (N, %) | 87 (5.8) | 49 (5.5) | 38 (6.3) | 0.602 |
| β receptor Blockers (N, %) | 1424 (95.5) | 849 (95.6) | 575 (95.4) | 0.918 |
| Calcium channel blockers (N, %) | 477 (32.0) | 272 (30.6) | 205 (34.0) | 0.190 |
| ACEI (N, %) | 82 (5.5) | 45 (5.1) | 37 (6.1) | 0.440 |
| ARB (N, %) | 99 (6.6) | 60 (6.8) | 39 (6.5) | 0.909 |
BMI indicates body mass index; AF, atrial fibrillation; FHCM, family history of hypertrophic cardiomyopathy; FSCD, family history of sudden cardiac death; IVST, introventricular septal thickness; LVEDD, left ventricular end-diastolic diameter; EF, ejection fraction; RVD, right ventricular diameter; SAM, systolic anterior motion; LVOT, left ventricular outflow tract; MR, mitral regurgitation; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Fig. 1.
The prevalence of pulmonary hypertension in males and females (A). The pulmonary artery systolic pressure across the sexes within the pulmonary hypertension subgroup (B).
3.2. Logistic analysis for the risk factors of PH
The variables with a p < 0.1 in univariable analysis or previously demonstrated to be associated with PH were included in multivariable analysis and the results are presented in Table 2. Multivariable logistic regression analysis showed that female was found to be a risk factor of PH (OR 2.3, 95 % CI: 1.70–3.11, p < 0.001) after adjustment for age, BMI, smoking, atrial fibrillation, left atrial diameter, and hyperlipidemia.
Table 2.
Logistic analysis for the risk factors of pulmonary hypertension.
| Variables | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p_value | OR | 95 % CI | p_value | |
| Female | 2.43 | 1.92–3.06 | <0.001 | 2.30 | 1.70–3.11 | <0.001 |
| Age | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| BMI | 0.94 | 0.91–0.97 | <0.001 | 0.93 | 0.89–0.96 | <0.001 |
| LAD | 1.04 | 1.02–1.06 | <0.001 | 1.06 | 1.04–1.08 | <0.001 |
| AF | 1.72 | 1.26–2.35 | 0.001 | 1.52 | 1.08–2.14 | 0.020 |
| Hyperlipidemia | 0.71 | 0.56–0.9 | 0.005 | 0.66 | 0.51–0.85 | <0.001 |
| Smoking | 0.57 | 0.444–0.728 | <0.001 | 0.92 | 0.67–1.26 | 0.600 |
| ARB | 0.62 | 0.362–1.016 | 0.069 | 0.65 | 0.37–1.09 | 0.120 |
OR, odds ratio; CI, confidential interval. Other abbreviations can be found in Table 1.
3.3. Operative details
Of 1491 patients with oHCM who underwent septal myectomy, 404 patients had PH, the surgical details are presented in Supplementary Table 1. Compared to patients without PH, those with PH were more likely to receive mitral valvuloplasty (20.8 % vs. 11.6 %, p < 0.001), have a longer clamp time (74.43 ± 32.19 min vs. 67.36 ± 28.05 min, p < 0.001), and longer intensive care unit stay (62.9 ± 66.26 h vs. 50.21 ± 47.33 h, p < 0.001). Other surgical parameters were comparable between patients with and without PH.
3.4. Pulmonary hypertension and cardiovascular events
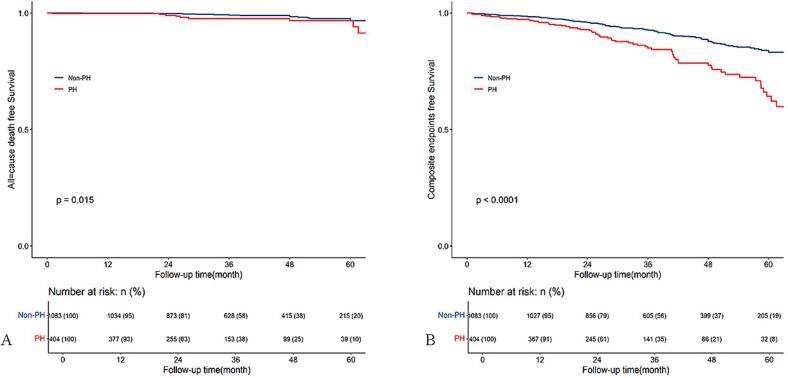
Three patients died during the operation, and 1 patient died within 30 days after discharge. Over a median follow-up of 36 months [ inter-quartile ranges(IQR) 23.5–52.5 months], 28 all-cause deaths and 189 composite endpoints (including 28 deaths, 14 S, 11 permanent pacemaker implantations, 65 new-onset atrial fibrillation, 7 coronary diseases, and 64 heart failure) were observed. The Kaplan-Meier survival curves showed that patients with PH had a higher cumulative incidence of death (Fig. 2A, log-rank test, p = 0.015) and composite endpoints (Fig. 2B, log-rank test, p < 0.001) compared to patients without PH. Univariable Cox regression analysis was used to identify the factors associated with all-cause death (Supplementary Table 2) and composite endpoints (Supplementary Table 3). Variables with p-value < 0.1 were entered into the stepwise multivariable Cox regression model, and the results were presented in Table 3. Family history of sudden cardiac death, ventilation time, and PH (HR 2.55, 95 % CI: 1.12 to 5.79, p = 0.025) were found to be independent risk factors of all-cause mortality. After adjusting for age, sex, atrial fibrillation, cerebrovascular disease, ventilation time, previous heart surgery, and preoperative NYHA III/IV, PH (HR 1.78, 95 % CI: 1.30 to 2.45, p < 0.001) was found independently associated with a higher incidence of composite endpoints.
Fig. 2.
Kaplan–Meier survival curves estimate all-cause death (A) and composite endpoints (B) in patients with obstructive hypertrophic cardiomyopathy undergoing septal myectomy, stratified by the presence or absence of pulmonary hypertension.
Table 3.
Multivariable Cox hazard risk regression analysis for clinical outcomes.
| Events | Multivariable analysis |
||
|---|---|---|---|
| HR | 95 % CI | p_value | |
| All cause death | |||
| FSCD | 4.11 | 1.23–13.8 | 0.022 |
| Pulmonary hypertension | 2.55 | 1.12–5.79 | 0.025 |
| Ventilation time | 1.01 | 1.002–1.01 | 0.006 |
| Composite endpoints | |||
| Age | 1.02 | 1.003–1.03 | 0.015 |
| Female | 1.39 | 1.02–1.90 | 0.038 |
| AF | 1.77 | 1.22–2.56 | 0.003 |
| Cerebrovascular disease | 1.88 | 1.08–3.28 | 0.026 |
| Pulmonary hypertension | 1.78 | 1.30–2.45 | <0.001 |
| Ventilation time | 1.004 | 1.001–1.01 | 0.004 |
| Previous heart surgery | 1.86 | 1.14–3.05 | 0.013 |
| Preoperative NYHA III/IV | 0.67 | 0.47–0.96 | 0.029 |
FSCD indicates family history of sudden cardiac death; HR, hazard ratio; CI, confidential interval.
3.5. Sex and cardiovascular events in the whole cohort and PH subgroup
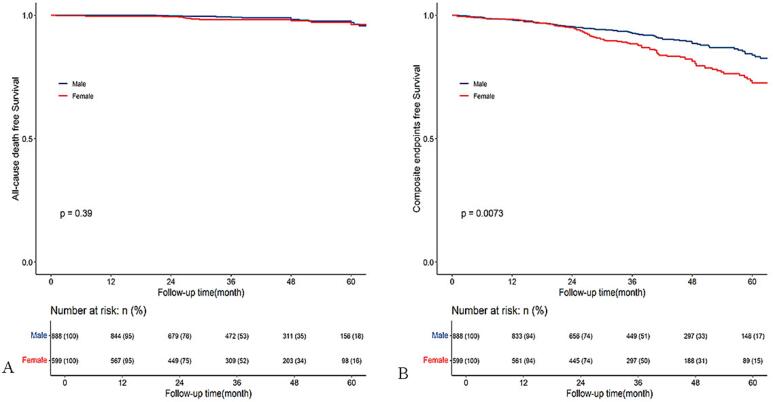
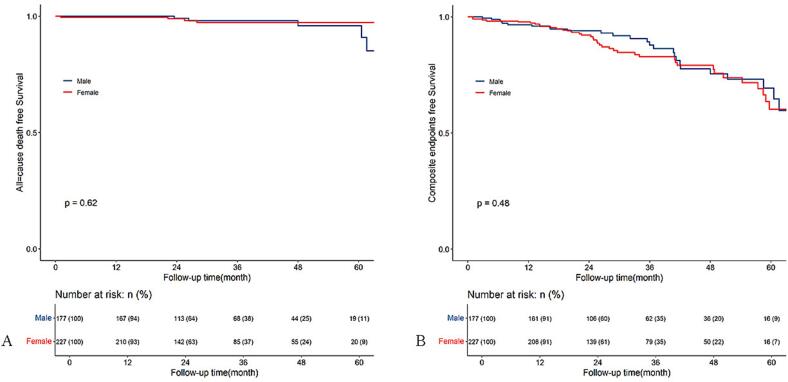
We further plotted Kaplan-Meier survival curves to show the cumulative incidence of death and composite endpoint across sex groups in the whole cohort and PH subgroup. However, the result showed no significant difference in cumulative incidence of death (Fig. 3A) but higher cumulative incidence of composite endpoints (Fig. 3B) in females than males in the whole cohort. In the PH subgroup, we found no significant difference in cumulative incidence of death (Fig. 4A) and composite endpoints (Fig. 4B) between males and females.
Fig. 3.
Kaplan–Meier survival curves estimate all-cause death (A) and composite endpoints (B) in patients with obstructive hypertrophic cardiomyopathy undergoing septal myectomy, stratified by sex (male and female).
Fig. 4.
Kaplan–Meier survival curves estimate all-cause death (A) and composite endpoints (B) of the pulmonary hypertension subgroup of patients with obstructive hypertrophic cardiomyopathy undergoing septal myectomy, stratified by sex (male and female).
4. Discussion
Our study is the first to investigate sex differences in the prevalence, severity, and prognosis of pH among patients with oHCM. Female sex emerged as a risk factor for the prognosis of patients with oHCM, while it acted as a protective factor for those with isolated PH. The impact of gender differences on the prognosis of oHCM patients with PH is particularly intriguing. Our findings indicate that female sex is an independent risk factor for PH, with a prevalence of pH in females being two-fold higher than that in males, despite comparable PASP between the sexes in the PH subgroup. Furthermore, patients with PH exhibited a higher cumulative incidence of death (log-rank test, p = 0.015) and composite endpoints (log-rank test, p < 0.001) compared to those without PH. Additionally, females demonstrated a higher incidence of composite endpoints compared to males, yet the cumulative rates of death were similar between the two groups. Conversely, no significant differences were found in the cumulative rates of death and composite endpoints between males and females within the PH subgroup.
PH is prevalent in patients with HCM, with previously reported rates ranging from 11.4 % to 53 % [4], [14], [15], [16], varying between studies attributed to differences in the definition and assessment methods of pH across studies. In our current study, we found a prevalence of 27.1 %, with female sex independently associated with a twofold higher prevalence compared to males. Notably, the PASP in the PH subgroup was similar between genders. The sex-specific prevalence of pH in our findings aligns with previous researches. A large European registry from 2007 to 2011 reported a prevalence ratio of 1.8:1 for women versus men [17]. The largest US registry [18] which began patient enrollment in 2006, documented that 80 % of idiopathic PH patients were female, while the earliest Chinese incident PH registry indicated a ratio of 2.4 females to 1 male [19]. Additionally, a comprehensive analysis of pooled patient-level data from 11 clinical trials in PH revealed that younger men exhibited higher mPAP than their female counterparts, although this difference diminished after age 45 years [20]. In both men and women with idiopathic PH, hemodynamic load tended to converge to similar levels with advancing age, resulting in a discernible sex difference in mPAP only among patients younger than 45. This phenomenon may be influenced by hormonal changes over time. In our study, the mean age of participants exceeded 45 years, which may partially account for the absence of a significant difference in PASP between males and females.
In patients with HCM, PH is predominantly secondary to LVOT obstruction, mitral regurgitation, or impaired diastolic function. These hemodynamic abnormalities predispose to elevated left atrial pressure and increased pulmonary venous pressure and congestion, ultimately leading to an increase in PAP. Over time, this increased pulmonary vascular congestion may contribute to pulmonary vascular remodeling and the development of a precapillary component alongside postcapillary PH [21], [22], [23]. Consequently, patients with elevated PASP exhibited a higher prevalence of atrial fibrillation and were independently linked to stroke and systemic embolic events [12], [15]. Additionally, elevated PASP is linked to higher risk of readmission for heart failure during both mid-term and long-term follow-ups [15]. Previously, PH has been identified as a predictor for all-cause mortality in both non-obstructive HCM and oHCM who did not undergo septal reduction therapy [4]. In those who received surgical intervention, pulmonary pressure decreased following septal myectomy, with preoperative right ventricular systolic pressure correlating with postoperative reductions in pulmonary pressure [5], [16], [22]. Although pulmonary pressure continues to decrease after surgery, preoperative pulmonary hypertension remains an independent risk factor for all-cause mortality [5]. While the underlying reasons for this finding require further investigation, our study also demonstrated that PH is independently associated with all-cause mortality and a composite outcome of heart failure, new-onset atrial fibrillation, and stroke events.
Significant sex differences were observed in both patients with HCM and patients with PH. Among HCM patients, female subjects were generally older and had a higher proportion of individuals with significant symptoms, a greater prevalence of PH, and elevated LVOT gradients at diagnosis. Moreover, in a recent meta-analysiencompassing 27 studies with a total of 42,365 HCM patients indicated that female sex was associated with an increased risk of heart failure, stroke, HCM-related death, and all-cause death, although no such association was found for atrial fibrillation or sudden cardiac death [8]. In a large cohort involving 2506 adults with oHCM following surgical myectomy [24], the unadjusted overall survival was lower in females, who exhibited a median survival that was approximately 3.9 years shorter than that of males. after controlling for baseline covariates, the relationship between sex and mortality was diminished and rendered nonsignificant. This finding aligns with our results, where unadjusted overall survival was lower in females compared to males, but no independent association between sex and all-cause mortality was detected. Furthermore, we noted a significant association between females and an increased incidence of composite outcomes, including heart failure, stroke, new-onset atrial fibrillation, and other cardiovascular events. The underlying mechanisms responsible for the observed sex differences in clinical outcomes among HCM patients remain incompletely understood. Some investigators ascribe these differences to reduced disease penetrance in female subjects, particularly among individuals with cardiac myosin-binding protein C3 gene (MYBPC3) variants. Confirmatory evidence supports that the cardiac disease penetrance of MYBPC3 mutation carriers is higher in males than in females [25]. his increased penetrance allows males to manifest the disease earlier, resulting in females being older and presenting with more severe symptoms at illness onset, which may impact their prognosis of HCM [26]. Moreover, female subjects typically exhibit a smaller left ventricular chamber, and female patients with HCM demonstrate more pronounced changes in left ventricular structure and function—such as ventricular thickness and left ventricular systolic function—compared to male patients. These factors significantly influence the risk of heart failure and LVOT obstruction across sexes. Additionally, our observations indicate that female subjects experience greater left ventricular remodeling and a more extensive degree of fibrosis than their male counterparts, which may further contribute to the higher incidence of heart failure events and mortality among females [27].
On the contrary, previous studies have identified female gender as a risk factor for developing of PH, yet males with PH exhibited poorer survival outcomes [6], [7], [28]. In our study, female sex emerged as an independent risk factor for PH; however, no significant survival difference was observed between males and females in oHCM patients. In oHCM patients, females had a smaller left ventricular chamber, higher LVOT gradient, and more severe diastolic dysfunction, leading to an elevated left atrial pressure, and more likely to develop post-capillary PH than males. Genetic alterations may also contribute to female bias for PH, such as mutations in BMPR2, in which female mutation carriers are more than twice as likely to be affected with PH as carrier men [29]. However, we did not find a sex bias in the clinical outcomes among patients with oHCM and PH. As we mentioned above, sex-difference in mPAP appeared to be modified by age; males aged > 45 years had comparable mPAP to females, which was consistent with our finding. This may explain why females and males had comparable survival in oHCM patients concomitant with PH.
5. Limitation
Our study was retrospective and therefore subject to inherent limitations, including selection bias. Additionally, the diagnosis of pulmonary hypertension (PH) relied on echocardiography rather than the right internal jugular pulmonary artery catheter, limiting PASP calculations to tricuspid regurgitant jet velocity or peak pulmonary regurgitation velocity. Furthermore, variables associated with gender differences—such as hormonal and genetic factors—are difficult to measure, which hampers our ability to provide direct evidence for the interpretation of results. Lastly, due to the relatively small sample size of patients diagnosed with PH, our findings should be cautiously extrapolated to other centers, emphasizing the need for further studies with larger populations to confirm our results.
6. Conclusion
Female sex was independently associated with higher prevalence of PH, however, the PASP was comparable between males and females in patients with oHCM. Moreover, PH and female were independently associated with a worse clinical outcomes, but no survival difference was found between males and females in PH subgroup.
Contributors
Changrong Nie, Qiulan Yang and Shuiyun Wang conceived the design of the study. Changrong Nie and Changsheng Zhu, and Minghu Xiao contributed to the data collection, analysis and interpretation. Changrong Nie and Yifeng Zhu drafted the manuscript, and Zhengyang Lu, Changsheng Zhu, and Yanhai Meng contributed significantly to the preparation. All the authors critically revised the manuscript, and gave final approval and agreed to be accountable for all aspects of the work, to ensure its integrity and accuracy.
Consent for publication
All authors have read the paper and agreed to the publication.
Ethics approval and consent to participate
This study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital (2023–2048). All patients provided written informed consent.
Availability of the data and materials
The datasets generated and/or analyzed in the current study are not publicly available due to Fuwai Hospital system, but data can be obtained from the corresponding author under reasonable request and with the permission of the Ethics Committee of Fuwai Hospital.
CRediT authorship contribution statement
Changrong Nie: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yifeng Zhu: Writing – original draft, Validation, Formal analysis. Minghu Xiao: Validation, Software, Formal analysis, Data curation. Changsheng Zhu: Validation, Investigation, Formal analysis, Data curation. Yanhai Meng: Supervision, Funding acquisition, Data curation. Zhengyang Lu: Resources, Investigation, Data curation. Qiulan Yang: Supervision, Resources, Data curation, Conceptualization. Shuiyun Wang: Supervision, Funding acquisition, Conceptualization.
Funding
This work was supported by the National High Level Hospital Clinical Research Funding (2022-GSP-GG-29 and 2023-GSP-QN-14), and CAMS Innovation Fund for Medical Sciences (2023-I2M-1–001).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101569.
Contributor Information
Qiulan Yang, Email: yqlmed@sina.com.
Shuiyun Wang, Email: wsymd@sina.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Semsarian C., Ingles J., Maron M.S., Maron B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015;65(12):1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Maron M.S., Olivotto I., Zenovich A.G., et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 3.Mitra A., Ghosh R.K., Bandyopadhyay D., Ghosh G.C., Kalra A., Lavie C.J. Significance of pulmonary hypertension in hypertrophic cardiomyopathy. Curr. Probl. Cardiol. 2020;45(6) doi: 10.1016/j.cpcardiol.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Ong K.C., Geske J.B., Hebl V.B., et al. Pulmonary hypertension is associated with worse survival in hypertrophic cardiomyopathy. Eur. Heart J. – Cardiovascular Imaging. 2016;17(6):604–610. doi: 10.1093/ehjci/jew024. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed E.A., Schaff H.V., Al-Lami H.S., et al. Prevalence and influence of pulmonary hypertension in patients with obstructive hypertrophic cardiomyopathy undergoing septal myectomy. J. Thorac. Cardiovasc. Surg. 2024;167(5):1746–1754.e7. doi: 10.1016/j.jtcvs.2022.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Ventetuolo C.E., Moutchia J., Baird G.L., et al. Baseline sex differences in pulmonary arterial hypertension randomized clinical trials. Ann. Am. Thorac. Soc. 2023;20(1):58–66. doi: 10.1513/AnnalsATS.202203-207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hester J., Ventetuolo C., Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Comprehensive Physiol. 2019:125–170. doi: 10.1002/cphy.c190011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H., Tan Z., Liu M., et al. Is there a sex difference in the prognosis of hypertrophic cardiomyopathy? A systematic review and meta-analysis. J. Am. Heart Assoc. 2023;12(11) doi: 10.1161/JAHA.122.026270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osman M., Syed M., Osman K., et al. Sex-based outcomes of surgical myectomy for hypertrophic cardiomyopathy: an analysis from the National Readmission Database. J. Thorac. Cardiovasc. Surg. 2023;166(2):504–511.e1. doi: 10.1016/j.jtcvs.2021.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Ommen S.R., Mital S., Burke M.A., et al. AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. Circulation. 2020;142(25) doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 11.Authors/Task Force m, Elliott PM, Anastasakis A, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the european society of cardiology (ESC). Eur. Heart J. 35(39) (2014) 2733-79. [DOI] [PubMed]
- 12.Nie C., Zhu C., Xiao M., et al. Risk factors of pulmonary arterial hypertension and its relationship with atrial fibrillation in patients with obstructive hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.666431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Cui H., Yu Q., et al. Excision of anomalous muscle bundles as an important addition to extended septal myectomy for treatment of left ventricular outflow tract obstruction. J. Thorac. Cardiovasc. Surg. 2016;152(2):461–468. doi: 10.1016/j.jtcvs.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Musumeci M.B., Mastromarino V., Casenghi M., et al. Pulmonary hypertension and clinical correlates in hypertrophic cardiomyopathy. Int. J. Cardiol. 2017;248:326–332. doi: 10.1016/j.ijcard.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Kanbayashi K., Minami Y., Haruki S., et al. Association of elevated pulmonary artery systolic pressure with stroke and systemic embolic events in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2017;240:320–323. doi: 10.1016/j.ijcard.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Geske J.B., Konecny T., Ommen S.R., et al. Surgical myectomy improves pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Eur. Heart J. 2013;35(30):2032–2039. doi: 10.1093/eurheartj/eht537. [DOI] [PubMed] [Google Scholar]
- 17.Hoeper M.M., Huscher D., Ghofrani H.A., et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int. J. Cardiol. 2013;168(2):871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Badesch D.B., Raskob G.E., Elliott C.G., et al. Pulmonary arterial hypertension: baseline characteristics fromthe REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 19.Jing Z.-C., Xu X.-Q., Han Z.-Y., et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132(2):373–379. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 20.Ventetuolo C.E., Praestgaard A., Palevsky H.I., Klinger J.R., Halpern S.D., Kawut S.M. Sex and haemodynamics in pulmonary arterial hypertension. Eur. Respir. J. 2013;43(2):523–530. doi: 10.1183/09031936.00027613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Ohlrich K., McGrath D.P., Cobey F., Ruopp N.F., Robich M.P. Intraoperative changes and prognostic implications of pulmonary hypertension in patients with hypertrophic obstructive cardiomyopathy undergoing surgical septal myectomy. J. Thorac. Cardiovasc. Surg. 2024;167(5):1757–1763. doi: 10.1016/j.jtcvs.2022.09.054. [DOI] [PubMed] [Google Scholar]
- 23.Maron B.A., Kleiner D.E., Arons E., et al. Evidence of advanced pulmonary vascular remodeling in obstructive hypertrophic cardiomyopathy with pulmonary hypertension. Chest. 2023;163(3):678–686. doi: 10.1016/j.chest.2022.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meghji Z., Nguyen A., Fatima B., et al. Survival differences in women and men after septal myectomy for obstructive hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4(3) doi: 10.1001/jamacardio.2019.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terauchi Y., Kubo T., Baba Y., et al. Gender differences in the clinical features of hypertrophic cardiomyopathy caused by cardiac myosin-binding protein C gene mutations. J. Cardiol. 2015;65(5):423–428. doi: 10.1016/j.jjcc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Lakdawala N.K., Olivotto I., Day S.M., et al. Associations between female sex, sarcomere variants, and clinical outcomes in hypertrophic cardiomyopathy. Circ.: Genomic Precis. Med. 2021;14(1) doi: 10.1161/CIRCGEN.120.003062. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y.-Z., Qiao S.-B., Hu F.-H., et al. Left ventricular remodeling and fibrosis: sex differences and relationship with diastolic function in hypertrophic cardiomyopathy. Eur. J. Radiol. 2015;84(8):1487–1492. doi: 10.1016/j.ejrad.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 28.DesJardin J.T., Kime N., Kolaitis N.A., et al. Investigating the “sex paradox” in pulmonary arterial hypertension: results from the Pulmonary Hypertension Association Registry (PHAR) J. Heart Lung Transplant. 2024;43(6):901–910. doi: 10.1016/j.healun.2024.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Best D.H., Sumner K.L., Smith B.P., et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest. 2017;151(4):821–828. doi: 10.1016/j.chest.2016.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.