Summary
Regardless of their cause, persistent physical symptoms are distressing somatic complaints that occur on most days for at least several months. They are common in patients with somatic diseases, functional somatic disorders, mental disorders, and undiagnosed medical conditions and are often associated with significant impairment and medical costs. Despite their prevalence and impact, persistent physical symptoms are often overlooked in medical care. This Personal View stresses the importance of recognising persistent physical symptoms as a European health issue. It advocates improvements in research, clinical management, public health, and policy. Efforts should prioritise integrating models of symptom perception and biopsychosocial perspectives into medical care and education, fostering interdisciplinary collaboration, and developing standardised guidelines to enhance patient care, reduce stigma, and improve clinical outcomes. Increased research funding can accelerate progress in understanding and effectively managing persistent physical symptoms. Addressing these priorities will support patients and healthcare professionals, ensuring adequate care and a higher quality of life for affected individuals.
Keywords: Persistent physical symptoms, Persistent somatic symptoms, Healthcare, Treatment, Public health, Education, Stigma
Search strategy and selection criteria.
We performed a literature search in PubMed, Scopus, and PsycInfo for “persistent physical symptom∗” or “persistent somatic symptom∗” combined via the Boolean AND operator with keywords related to interventions and healthcare in titles or abstracts. The search was performed from inception to September 12, 2024. Grey literature was additionally considered, as were reference lists of journal articles and book chapters. For the final reference list, we selected articles most relevant to the topics addressed in this Personal View, prioritising systematic reviews and meta-analyses, randomised controlled trials, and prospective studies over cross-sectional studies and other types of publications. Disagreements over inclusion were resolved by discussion between AT, AW, and BL.
Introduction
Persistent physical symptoms (synonymous with persistent somatic symptoms) is used as an umbrella term to describe distressing somatic complaints, regardless of their cause, that are present on most days for at least several months. Persistent physical symptoms occur in somatic diseases, functional somatic disorders, mental disorders, and undiagnosed medical conditions, making them a major health problem in Europe and beyond.1
A recent review in The Lancet by an international author group provides an evidence-driven summary of the definition, genesis, and clinical management of persistent physical symptoms. This review summarizes current findings regarding symptom perception, disease-specific and common mechanisms that lead to symptom persistence within a comprehensive biopsychosocial aetiological model and describes evidence-based treatment approaches.2
This Personal View complements the Lancet review by addressing misconceptions and unresolved challenges in the healthcare and research of persistent physical symptoms, with a particular focus on the European perspective. It is issued by the ‘European Research Network to Improve Diagnosis, Treatment, and Healthcare for Patients with Persistent Somatic Symptoms’ (EURONET-SOMA), which promotes collaboration across countries, specialties, and sectors, including European regions with fewer research and healthcare resources. EURONET-SOMA does not focus on individual symptoms such as pain or fatigue, but instead aims to conduct overarching research on a variety of persistent physical symptoms to identify commonalities and country-specific differences in their understanding and management across populations and medical specialties, learn from best practice examples, and disseminate research findings and educational material.
Alongside other working groups and consortia, EURONET-SOMA endeavoured to continuously improve the care of patients with persistent physical symptoms in recent decades. The network has advanced terminology and diagnostic criteria for persistent physical symptoms,2,3 published recommendations on core outcome domains for clinical trials,4,5 and proposed a contemporary understanding of symptom development and perception.6,7 EURONET-SOMA established an EU-funded Marie Skłodowska Curie Innovative Training Network (ETUDE) to train early career researchers,8 who contributed to key studies on epidemiology,9, 10, 11, 12 symptom perception and explanatory models,13, 14, 15 risk factors,16 collaborative healthcare models,17 barriers to diagnoses and healthcare,18 stigmatisation,19, 20, 21 and impairment.22
This Personal View highlights the progress made in recent years in the management of persistent physical symptoms, while also outlining unmet needs in care and research from an overarching perspective across symptoms and medical specialties. It calls for further progress and urges action from health authorities and policymakers.
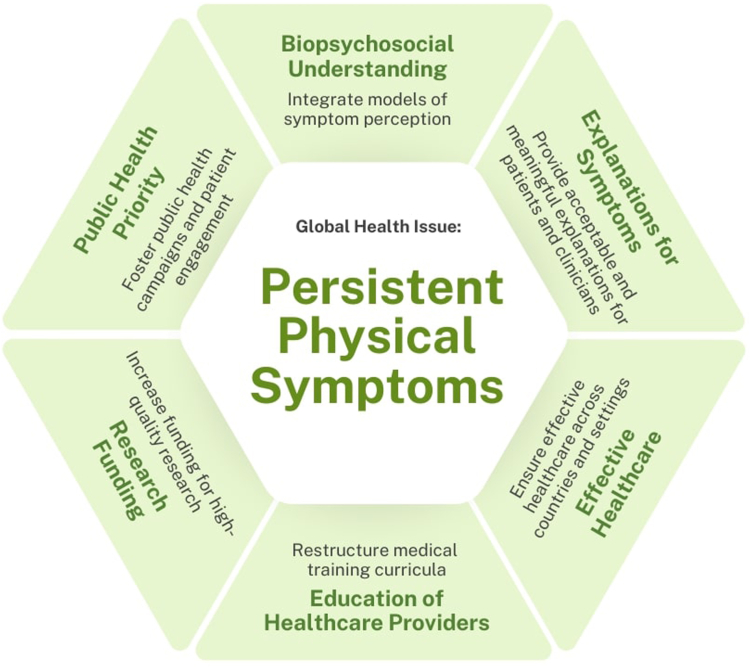
By clarifying misunderstandings, identifying challenges, and suggesting concerted actions, it aims to advance the understanding and care for patients with persistent physical symptoms across health sectors and engage in dialogue about these symptoms with specialists from various medical fields and societies, as well as with patients and their associations. Fig. 1 illustrates the key points identified in this Personal View of improving care for patients with persistent physical symptoms.
Fig. 1.
Key points to advance care for persistent somatic symptoms.
Current state and need for action
Persistent physical symptoms are a core feature of many medical conditions
Medicine has traditionally focused on diagnosing and treating diseases rather than symptoms. Until now, relatively little attention has been paid to understanding how information from disease processes in the body is processed by the brain and translated into the experience of symptoms. This is remarkable considering that symptoms such as pain, gastroenterological, cardiovascular, genito-urinary, or neurological symptoms are regularly experienced by 80% of the adult general population.23 These symptoms are most often the reason for patients to consult healthcare, they generate 30–60% of family practitioners’ workload23 and are frequent in specialised healthcare.24 Most patients experience not one but multiple symptoms, with physical and mental symptoms, such as depressive and anxiety symptoms, often co-occurring.25 In about 16–25% of the cases, the physical symptoms become persistent.26,27 Persistent physical symptoms also affect children, adolescents, and the elderly. About 25% of children and adolescents experience pain-related symptoms such as chronic abdominal pain,28 migraines or headaches,29 but also persistent fatigue,30 irritable bowel symptoms, or dyspepsia.31 In the elderly population, chronic pain affects 25–50% of those living in the community and up to 80% of those in nursing homes.32 Persistent fatigue affects 10–30%,33 sleep problems 40–70%,34 and gastrointestinal issues affect 26% of females and 16% of males aged 65 years and older.35 Persistent physical symptoms impact the quality of life of those affected and their families22 and cause great distress, work- or school-related disability, mortality, and increased risk for suicidality.36 The economic burden on healthcare systems is high.37 Despite their overwhelming prevalence and associated burden, persistent physical symptoms frequently receive little attention and are not systematically assessed or discussed with patients during medical consultations, especially if a disease diagnosis has already been established.38
Persistent physical symptoms are not a medical condition or diagnosis, but they are a core feature of many medical conditions, including somatic diseases, functional somatic disorders, mental disorders, and undiagnosed medical conditions. They should not be equated with functional symptoms, medically unexplained or somatoform symptoms. Current evidence emphasises the need for a transdiagnostic perspective on persistent physical symptoms. In this context, transdiagnostic means that different persistent physical symptoms share similar physiological and psychological processes that influence the development, course and perception of symptoms in somatic and mental illness.39 This transdiagnostic perspective not only facilitates communication between clinicians from different medical fields, but also enables an understanding of why people experience multiple and overlapping symptoms and why symptoms may persist or recur over time.40 Sometimes, physical symptoms occur as indicators of somatic disease and can persist even after the original cause has subsided, e.g., pain after an injury or post-infectious fatigue.41,42 Distressing symptoms can also accompany chronic somatic diseases (e.g., gastrointestinal complaints in ulcerative colitis2) and may not be alleviated by treatments targeting the pathophysiology or structural sequelae of the underlying disease. For instance, fatigue is a common and debilitating symptom in autoimmune liver diseases that does not respond to standard pharmacological treatment.43 Notably, one-third of patients presenting symptoms in medical care do not receive a disease diagnosis.26 Some of these cases meet the criteria of functional somatic disorders like fibromyalgia or irritable bowel syndrome.10 These disorders are characterised by persistent and troublesome symptoms associated with impairment or disability that do not have an identified underlying pathophysiology, and that occur in the absence of underlying structural abnormalities.44 Across Europe, the estimated point prevalence of functional disorders in adult populations is 8%,10 whereas prevalence rates differ considerably between individual disorders and throughout European countries.45,46 Some of these diagnoses are still made by exclusion, i.e., the presence of physical or organic disease is ruled out as a pre-requisite for making the diagnosis. However, diagnostic practices with systematic interviewing for physical and mental symptoms are gaining ground.47 A different understanding also underlies the diagnostic category of functional neurological disorders (FND),48 which are one of the most common conditions in neurological practice. The defining diagnostic criteria of FND are ‘rule in’ positive signs typically observed on clinical examination.
In psychiatry, psychosomatic medicine, and clinical psychology the former diagnostic category of somatoform and related disorders to diagnose patients with persistent and bothersome physical symptoms that cannot be fully explained by a general medical condition has been replaced by somatic symptom and related disorders in DSM-5-TR49 and bodily distress disorder in ICD-11.44 These revisions abandoned the scientifically untenable and stigmatising notion of ‘medically unexplained’ symptoms and now include ‘positive’ psychological features like anxiety, worries or preoccupation regarding the symptoms as diagnostic criteria. The new criteria significantly increased the reliability, validity, and diagnostic accuracy compared to the predecessor diagnoses of somatoform disorders.50 Although somatic symptom disorder can be diagnosed alongside a somatic disease, it is rarely used outside of psychosomatic medicine or clinical psychology. However, it should be applied to ensure appropriate care for chronically ill patients who are significantly distressed by their symptoms.2 Of note, persistent physical symptoms also occur as part of various other mental disorders, such as depressive or anxiety disorders. In the long term, it would be desirable to establish a classification for persistent physical symptoms within a neutral framework of classification systems (similar to chronic pain in ICD-1144), favouring neither somatic disease aetiology, nor mental disorder.3 Panel 1 summarizes the need to overcome current misconceptions about persistent physical symptoms.
Panel 1. Persistent physical symptoms are a core feature of many medical conditions.
In medicine, persistent physical symptoms are often overlooked in favour of diseases, despite being common across all age groups and medical specialties and significantly impacting patients' lives. To address this, misconceptions about persistent physical symptoms must be corrected, and these symptoms should receive proper attention and care in medical practice.
Common misconceptions
-
•
Persistent physical symptoms are misunderstood in the sense of a medical condition or diagnostic category.
-
•
Persistent physical symptoms are mistakenly equated with functional, medically unexplained, or somatoform symptoms, functional somatic disorders, bodily distress, or somatic symptom disorders.
Improved understanding
-
•
Persistent physical symptoms are an umbrella term to describe distressing somatic complaints, regardless of their cause, that are present on most days for at least the last several months. Persistent physical symptoms can occur in somatic diseases, functional somatic disorders, mental disorders, or undiagnosed medical conditions.
-
•
Persistent physical symptoms represent a transdiagnostic approach to symptoms, their aetiology, and their perception and experience across somatic and mental health.
Actions to be taken
-
1.Raise awareness:
-
•Highlight the prevalence and impact of persistent physical symptoms among healthcare professionals through training, and dissemination of research findings across different medical disciplines.
-
•Advocate for the inclusion of persistent physical symptoms in public health policies by working with medical associations and patient advocacy groups, submitting policy briefs to governmental bodies, and collaborating with policymakers on European-level awareness campaigns.
-
•
-
2.Enhance recognition:
-
•Implement routine assessments (e.g., standardized screening tools, targeted questions on distress) for persistent physical symptoms during medical consultations.
-
•Encourage multidisciplinary case reviews where specialists collaborate to identify and treat persistent symptoms in complex cases.
-
•Establish clear care pathways that, in addition to biomedical care, include psychosocial support, such as counseling and psychotherapy.
-
•
-
3.Enhance classification:
-
•Consider comorbid diagnoses such as functional somatic disorders, bodily distress, or somatic symptom disorders for patients with persistent physical symptoms, even when a primary disease diagnosis is present.
-
•Provide a ‘neutral space’ within disease classifications for health conditions where persistent physical symptoms are leading, favouring neither somatic disease aetiology, nor mental disorder.
-
•
A biopsychosocial understanding taking into account models of symptom perception is needed
Today’s medicine primarily follows a biomedical model of disease, focusing on separate organ systems and clinical disciplines, using objective findings from laboratory and imaging tests to classify physical complaints into defined diagnostic categories with recommended treatments.51 This view neglects modern models of symptom perception and the process of disease-related information being relayed from the body to the brain and mind.52 As symptom perception is influenced by previous experiences, prior expectations, personality factors, and contextual conditions, symptoms are not solely a direct expression of disease, but may be ‘the most human expression of clinical medicine’.53 Growing evidence supports a biopsychosocial understanding of persistent physical symptoms, identifying various predisposing, triggering, aggravating, and maintaining factors influencing their persistence in various symptoms, syndromes, and disorders.54
Predisposing factors include sociodemographics (e.g., being female,55 low socioeconomic status56), psychological factors (e.g., adverse childhood or life experiences, neuroticism),57 and biomedical aspects (e.g., prior diseases, genetic profiles, immunological factors).2,58 Triggering factors such as acute infections, injuries, medical procedures, and life events can initiate short-term somatic symptoms. Disease-specific biomedical factors (e.g., inflammation in inflammatory bowel diseases) play a role as maintaining or aggravating factors. Cognitive-perceptual mechanisms (e.g., selective attention to bodily cues, catastrophizing), emotional factors (e.g., alexithymia), and behavioural processes (e.g., learning processes, avoidance behaviour, or overuse of medical services) then contribute to symptom persistence.59 Social stressors and cultural factors,60 such as negative encounters with healthcare professionals or employers, and general population prejudice, also negatively affect symptom courses. Functioning of physiological stress systems, particularly the autonomic nervous system seem to affect how stressors are perceived and whether they contribute to the development of persistent physical symptoms.61 Disease-overarching psychobiological models postulate epigenetic alterations, dysregulations of the endocrine, immune, and autonomic nervous systems, as well as central sensitisation, as potential links between psychosocial distress and persistent physical symptoms.1,2,58,62 For instance, chronic pain may be exacerbated by stress or lack of social support, whether it arises from tissue damage or inflammation or not.63
Dysfunctional explicit and implicit expectations might contribute to the development and persistence of symptoms, influenced by a complex interplay of factors promoting a lowered threshold to the perception of physical symptoms such as emotional states or cognitive biases, social norms and culture, media information, economic conditions or political climate, among others.60,64 Persistent physical symptoms thus involve neurobiological mechanisms of perceptual dysregulation integrating patients’ knowledge, prior experience, and expectations about symptoms.6
Models of predictive processing provide a general framework of perceptual processes for persistent physical symptoms. They propose that the eventual symptom experience results from an interaction between top-down predictions by the brain about sensory input based on prior experiences and expectations, and bottom-up actual somatic input. Depending on the reliability of predictions and somatic input, the symptom experience can align more closely with either one. This means the relationship between pathophysiological mechanisms of a disease and symptom reports can vary based on the characteristics of the somatic input, the individual, and the context.65 As symptoms persist, their link to clearly identifiable pathophysiology often weakens.2 In certain cases, physical symptoms are experienced even in the absence of any somatosensory input from pathophysiological processes.52 Panel 2 summarizes the need to take a biopsychosocial perspective in the understanding of persistent physical symptoms and provides suggestions on how to achieve this.
Panel 2. A biopsychosocial understanding and acceptable explanatory models for persistent physical symptoms are needed.
Relying solely on a biomedical model often fails to address persistent physical symptoms, which result from a complex interplay of biological, psychological, and social factors. Translating aetiological knowledge into effective models is essential for patient-centred care. By adopting a biopsychosocial approach and understanding processes of symptom perception, healthcare providers can legitimize patient experiences, improve treatment effectiveness and satisfaction, and promote better health outcomes.
Common misconceptions
-
•
Persistent physical symptoms are mistakenly thought to always have a clear pathophysiological basis, hindering effective explanations when no direct cause is found.
-
•
When no pathophysiological cause is identified, it is wrongly concluded that the symptoms are psychological.
-
•
Psychological explanations for persistent physical symptoms are often rejected due to the false belief that they are less legitimate or stigmatize the patient as ‘psychologically vulnerable'.
Improved understanding
-
•
Persistent physical symptoms result from an interplay of biological, psychological, and social factors.
-
•
Symptoms are influenced top-down by brain predictions from prior experience and bottom-up by actual somatic input, meaning that perceptions can be shaped by expectations and prior knowledge.
-
•
Frameworks like the biopsychosocial model, central sensitization, perceptual dysregulation, and predictive processing help to explain persistent symptoms.
-
•
Patient experiences should be validated with individualized, co-constructed explanations tailored to their understanding and context.
Actions to be taken
-
1.Enhance research:
-
•Conduct interdisciplinary research on the combined impact of biological, psychological, and social factors on persistent symptoms.
-
•Perform longitudinal and experimental studies to identify and address modifiable risk factors that prevent acute symptoms from becoming chronic.
-
•Develop and evaluate personalized interventions targeting specific cognitive, emotional, and social factors contributing to symptom persistence.
-
•
-
2.Foster collaboration:
-
•Share knowledge and insights across medical disciplines and European countries to build an integrated understanding of persistent symptoms.
-
•Establish research consortia or partnerships across universities, healthcare institutions, and research centres to promote interdisciplinary studies.
-
•Apply for joint funding from health research bodies like the European Research Council or national funding agencies to support large-scale interdisciplinary projects.
-
•
-
3.Develop helpful materials:
-
•Create culturally sensitive, accessible patient education materials on symptom understanding and the biopsychosocial nature of symptoms for different literacy levels, in collaboration with patients, researchers, clinicians, and industry partners.
-
•
-
4.Involve patients and families:
-
•Develop explanations in collaboration with patients that align with their understanding to reduce stigma, enhance adherence, and promote optimism for recovery.
-
•Involve family members or relevant others in symptom management and explanation, especially for younger patients or those with special needs.
-
•
Acceptable, appropriate, and meaningful explanations of persistent physical symptoms for patients and clinicians are needed
Empirically validated biopsychosocial models of persistent physical symptoms, including recent symptom perception models, are essential for joint understanding, compassionate care, shared decision-making, and effective treatment in somatic and mental healthcare settings.66 Shared explanatory models can counteract stigma, provide empowerment and reassurance, and facilitate treatment adherence by fostering hope for coping with or recovering from symptoms. Patients should be recognised as authorities on their symptoms, as explanations that significantly differ from their own symptom understanding are less effective.67 For younger patients, including the family and wider network (e.g., school) in symptom explanation and management is often crucial.68
Explanatory models also promote medical professionals' understanding of persistent physical symptoms and their perceived effectiveness. By taking all dimensions of a patient's symptoms into account, explanatory models encourage multidisciplinary collaboration.69 A recent pan-European study across 16 countries identified five main explanatory approaches healthcare professionals use to explain persistent physical symptoms.18 The ‘multisystem stress approach’ explains persistent physical symptoms through physiological stress responses within a bio-psycho-social paradigm.70 ‘Sensitised alarm’ and ‘malfunctioning software’ are both approaches derived from neurosciences. Explanations related to ‘embodied experience’ are often used within integrated psychosomatic therapies. In the person-centred ‘symptoms approach’, healthcare practitioners aim for co-constructed, individualised explanations.15
Innovative consultation models for patients with persistent physical symptoms facilitate the quality of communication within the healthcare provider–patient relationship and have a positive impact on health outcomes, patient satisfaction, and therapeutic adherence.71 There is guidance for family doctors in how to address the needs of patients with persistent physical symptoms during consultations.72 The REAL (Recognition, Explanation, Action, Learning) model is a best practice example.73 Based on scientific knowledge about persistent physical symptoms, it comprises four components: Recognition seeks to validate the patient’s experience and emphasize the clinician’s recognition of the problem as legitimate. Explanation involves the negotiation of explanations for symptoms in ways that integrate brain and body without necessarily implying a psychological cause. Action comprises the clinician and the patient negotiating one or more self-management strategies (some taught by the clinician). Learning jointly reviews and modifies the preceding three components as necessary, with the ultimate aim of patient self-management. The model has been proven effective in enabling clinicians to explain persistent physical symptoms to their patients in a beneficial way.73 Panel 2 synthesises the need to provide acceptable, appropriate, and meaningful explanations for persistent physical symptoms to both patients and clinicians.
Effective healthcare for patients with persistent physical symptoms is needed across countries and healthcare settings
The treatment of persistent physical symptoms remains a complex area with evolving evidence. The primary goals are to reduce symptom burden, empower self-care and self-efficacy, improve quality of life and psychosocial wellbeing, and shift the patients’ focus towards living a value-based life despite symptoms.2 The strongest evidence for treatment includes psychological therapies like cognitive behavioural and dynamic psychotherapy, hypnosis,74, 75, 76, 77 and, in some cases, pharmacological treatments (e.g., antidepressants2). A growing body of research supports integrative, multidisciplinary and multicomponent approaches that address the psychological, physical, and social dimensions of symptoms.78 In terms of treatment pathways, care for persistent physical symptoms has typically been equated with ruling out biomedical diseases. While physical examinations and diagnostics are important, care should be person-centred along individual symptom courses.79 Early symptom management can be facilitated by identifying patients at risk for persistent symptoms based on repeated consultations, multiple or increasing symptoms, perceived distress, and impaired functioning.80 In cases with less ominous symptoms of unclear aetiology and in the absence of warning signs or red flags (e.g., unexplained weight loss, persistent fever, night sweats, unusual bleeding, lump or mass, difficulty swallowing, jaundice, etc.), a ‘watchful-waiting strategy’ can be employed as some symptoms improve without treatment.81 If symptoms persist, patients should be provided with an explanation and, where applicable, with a confident diagnosis. Management should follow a stepped-care approach, augmented by collaborative care depending on severity and comorbidity.17,82 Of course, any treatment is initially subject to managing the pathophysiological or structural cause of the persistent somatic symptoms.2 In cases where the underlying pathophysiology is unknown or available treatments are not fully effective, basic care can be enhanced with self-management and lifestyle advice, and person-centred psychological or pharmacological interventions. This may take place in specialised inpatient, day-care, or rehabilitation settings, including a combination of therapies such as medication, psychotherapy, physiotherapy, or relaxation therapies.2 For severe persistent symptoms in children and adolescents, care should involve paediatrics, child and adolescent psychiatry, psychotherapy, and collaboration with schools.83
Although existing guidelines are unanimous in these treatment steps,84, 85, 86 European countries differ significantly in guideline availability, financing of evidence-based treatments, and availability of specialised care structures for persistent physical symptoms.87 In resource-poor areas, unequal access to treatment leads to higher disability estimates.88 In Germany, psychosomatic medicine is an independent specialty integrating psychological and somatic aspects on an equal and reciprocal basis. Curricula for basic psychosomatic care have been introduced into primary care education to support this integrated approach.89 While it may not always be possible or necessary to manage persistent physical symptoms within independent specialties, collaboration across various disciplines, with each contributing their strengths, improves outcomes.90 Several European countries have established specialised treatment centres for certain subtypes of persistent somatic symptoms, such as chronic pain (e.g., pain clinics across the UK and Germany) or functional somatic disorders (e.g., Danish regional centres for functional somatic disorders) where different healthcare professionals work within multidisciplinary teams,91 while in other European countries there is currently no specialised care for persistent physical symptoms. A common problem across Europe is that patients with persistent physical symptoms often undergo numerous non-contributory diagnostic procedures and ineffective or potentially harmful treatments92 before receiving multidisciplinary symptom management.93
An online survey with healthcare professionals from four European countries highlighted that trained specialists resources, timely care, interdisciplinary collaboration, clear treatment guidelines, reimbursement practices, and education and communication on symptom management are crucial to overcoming diagnostic and treatment barriers.94 Evidence from a scoping review suggests that health outcomes in patients with persistent physical symptoms are influenced by levels of care, with the highest symptom improvement and lowest persistence rates in specialised care settings as compared to primary or secondary care settings.18 A focus group study from the Netherlands identified strategies to enhance collaborative care, including professional education, communication, care coordination, care pathways, joint consultations, funding, patient involvement, and prevention.95 Innovative treatment models from the UK and Norway to improve access to psychological therapies for patients with anxiety and depression, could also benefit patients with persistent physical symptoms.96,97 European-wide care standards could be improved by joint management guidelines that are practical, culturally sensitive, and feasible for implementation into time-restricted routine care, taking structural differences between healthcare systems into account.
Transdiagnostic interventions for different age groups including personalised e-health tools with low costs have the potential to reduce symptom severity and burden, and facilitate access to healthcare, but are still in their early stages.80,98 Particularly in medically underserved rural areas or European countries with fewer care capacities, the allocation of limited resources could be optimised by providing patients with digital health applications, where complex topics can be conveyed, with or without direct healthcare professional interaction.99,100 These applications usually focus on education and self-help interventions and aim to improve quality of life and reduce somatic symptoms.101 In some European countries, digital health applications can already be prescribed by health professionals and are covered by statutory health insurance.102 Panel 3 summarizes the need to provide effective healthcare for patients with persistent physical symptoms across countries and healthcare settings.
Panel 3. Effective healthcare for patients with persistent physical symptoms is needed across countries and healthcare settings.
Differences in European healthcare systems underscore the need for standardised, evidence-based care models and guidelines to improve outcomes for patients with persistent physical symptoms. Standardising practices and enhancing care coordination could significantly benefit patients; however, these models must also be flexible enough to account for structural differences between healthcare systems.
Shortcomings in the care for persistent physical symptoms
-
•
Early and effective multidisciplinary collaboration is often lacking in routine care.
-
•
Disparities exist in guidelines, funding for evidence-based treatments, and specialised care across European countries.
-
•
Transdiagnostic interventions and personalised e-health tools are not widely accessible or integrated.
-
•
Limited resources and funding restrict the establishment of specialised treatment centres for persistent physical symptoms.
Actions to be taken
-
1.Develop joint management guidelines:
-
•Form a task force consisting of experts from primary and specialised care, and patient advocacy groups to create care standards that are adaptable to patients with different symptoms and from different age and cultural groups.
-
•Tailor these care standards to ensure they meet the unique needs of diverse healthcare settings and systems.
-
•
-
2.Enhance stepped-care approaches and multidisciplinary collaboration:
-
•Establish evidence-based referral standards to enhance coordination across healthcare settings, and develop decision trees outlining referral criteria and care pathways based on symptom severity and complexity.
-
•Implement a stepped-care model that progressively increases treatment intensity in response to the patient’s symptom severity, complexity, and their response to initial interventions.
-
•Enhance support for primary care through formalized agreements for managing complex patients and the expansion of services from secondary care to provide more in-depth consultations.
-
•Set up joint consultations and multidisciplinary teams to collaborate on patient management, ensuring both physical and psychosomatic aspects are treated comprehensively.
-
•Adapt and evaluate innovative treatment models to expand and expedite access to effective treatment, such as psychological therapies.
-
•Advocate for policy changes that ensure healthcare systems reimburse joint consultations and multidisciplinary team meetings, recognizing the additional time required to effectively manage complex persistent physical symptoms.
-
•
-
3.Leverage digital health tools:
-
•Create e-health platforms or mobile apps that provide educational resources on symptom management, offer self-help tools (e.g., mindfulness exercises, symptom trackers), and allow patients to access telehealth services.
-
•Translate these apps into multiple languages and literacy levels to ensure accessibility for underserved populations, and customize them to address cultural nuances and local healthcare resources.
-
•Promote the use of these tools in rural and underserved areas by collaborating with local health centres, ensuring patients have access to necessary technology (e.g., tablets, internet access).
-
•
There is a need to better educate healthcare professionals in the management of persistent physical symptoms
The focus of traditional clinical medical training is on linkages between a symptom and a diagnosable and curable diseases with clear biological causes.103 Lessons on persistent physical symptoms and teaching approaches to overcome communication challenges are still largely absent from the curricula of medical training.104
Improved medical training for managing persistent physical symptoms should focus on symptom perception, biopsychosocial explanatory models, patient-centred communication, and awareness of potential biases, negative attitudes, and misconceptions in consultations with patients. Thereby, training should involve all relevant professionals, including medical students, specialists-in-training, experienced general practitioners and allied healthcare professionals.95 Education in fields like clinical psychology, nursing, physical therapy, or medical social work could further enhance care. Case-based discussions, practical communication skills, and in-service training are preferable to lecture-based teaching. Training should also incorporate intercultural competence and sensitivity to diverse patient needs.103, 104, 105 Multidisciplinary summer schools by EURONET-SOMA and ETUDE offer unique opportunities to train future healthcare professionals and researchers by disseminating the latest research into clinical practice. However, these events are sporadic and still require scientific evaluation. Digital learning opportunities, like massive open online courses (MOOCs), could enhance traditional teaching methods, providing high-quality, self-paced learning for healthcare professionals. There is a best practice example of an online, interprofessional course, which consists of six modules to teach healthcare professionals the knowledge, skills, and attitude they need to diagnose and treat persistent physical symptoms in a patient-centred manner based on the biopsychosocial model.106 Panel 4 summarizes the need for educational approaches to teach healthcare personnel about the management of persistent physical symptoms.
Panel 4. There is a need to better educate healthcare professionals in the management of persistent physical symptoms.
Persistent physical symptoms are largely absent from medical training curricula, despite their high prevalence across different medical fields. Medical education should be restructured to fill this gap, as current curricula inadequately prepare healthcare professionals to communicate, diagnose, and manage these symptoms. This neglect perpetuates a biomedical mind set, overlooking the necessary biopsychosocial approach for effective care.
Steps to take
-
1.Integrate comprehensive and ongoing learning:
-
•Revise undergraduate and postgraduate medical, nursing, and allied health curricula to incorporate modules on symptom perception, the biopsychosocial model, and interdisciplinary care for persistent physical symptoms.
-
•Develop case-based learning and simulation activities that teach students how to assess and manage persistent symptoms, emphasizing both physical and psychological aspects.
-
•Create interdisciplinary training sessions where students and health-care professionals from different healthcare fields (e.g., medicine, psychology, physiotherapy) work together to develop collaborative treatment plans, fostering a team-based approach from early in their education.
-
•Encourage continuous education through conferences, summer schools, online courses, and self-paced learning to update knowledge on diagnostics and management.
-
•Offer incentives, such as Continuing Medical Education (CME) points or certificates, to encourage participation in ongoing learning opportunities.
-
•
-
2.Enhance communication skills and attitudes:
-
•Implement competence training on cultural differences and diversity to improve care for diverse populations and address ethnic disparities.
-
•Develop and evaluate programs to address and change negative perceptions and misconceptions about persistent physical symptoms among healthcare professionals.
-
•Develop mentorship or peer support programs where experienced healthcare providers guide others in overcoming potential negative attitudes toward patients, fostering an empathetic approach to care.
-
•
Research funding is needed to enable high-quality research on persistent physical symptoms
The fact that persistent physical symptoms are a relevant global health issue2 is not reflected in corresponding research efforts. High-quality and innovative research is slowly developing and, according to the results of our search strategy, mostly conducted by research groups from (Western) Europe.18 Fewer but important research initiatives come from the US26,107 and Asia.108 Thereby, studies often focus on specific populations109 or individual symptoms.110 The research unit on persistent physical symptoms across diseases (SOMACROSS1), funded by the German Research Foundation, is an example for interdisciplinary research across patient groups and symptoms. European funding also supported the development and evaluation of targeted, patient-centred treatments,111 training measures,112 and collaborative care concepts.17
Estimating research funding for persistent physical symptoms in Europe is challenging due to a lack of centralised tracking. Major sources like the European Research Council and Horizon Europe do not specify funding for this topic. While broader funding calls and national funds for mental health and chronic conditions may indirectly support research on persistent physical symptoms, varying definitions and classifications complicate tracking. In countries where research funding bodies have distinct review boards for mental disorders and somatic diseases, interdisciplinary research projects on persistent physical symptoms may lack a designated reviewing authority. This fragmentation poses a significant disadvantage for such research, potentially resulting in the rejection of valuable projects.
Calls to action in respected medical journals have effectively highlighted the need for research and funding for conditions like low back pain and Long COVID.113,114 Scientific associations like the European Association of Psychosomatic Medicine (EAPM) and the Functional Neurological Disorder Society (FNDS) emphasize the importance of persistent physical symptoms and support research through special interest groups and conference symposia. SymPCa, an interdisciplinary European conference on symptoms in primary care, unites researchers from different disciplines and backgrounds to discuss state-of-the-art research on symptoms. Pan-European collaborations and consortia, such as Pain Alliance Europe, the European Pain Federation, INTEGRATE-pain, the Low Back Pain Phenotyping Consortium (BACPAP), the Oslo Chronic Fatigue Consortium, or the European Network for Vertigo and Balance Research (DIZZYNET) share best practices, advocate for policy changes, and support research. We urge researchers and funders to prioritize innovative interdisciplinary research for persistent physical symptoms in Europe. Panel 5 outlines the need for high-quality research and offers suggestions for achieving it.
Panel 5. Research funding is needed to enable high-quality research on persistent physical symptoms and make them a public healthcare priority.
Persistent physical symptoms are a significant global health issue, with limited research in Europe and worldwide. Efforts are needed to improve care quality across countries, particularly for aging populations. Comprehensive public health campaigns and interventions are essential to improve outcomes, enhance quality of life, reduce costs, and promote health equity.
Steps to take
-
1.Increase research funding:
-
•Allocate substantial resources for innovative and interdisciplinary research on persistent physical symptoms involving collaboration between somatic and mental medical fields.
-
•Establish grant programs to involve European regions with limited research funding.
-
•
-
2.Initiate public health campaigns:
-
•Launch targeted campaigns in Europe to raise awareness, reduce stigma, and promote a biopsychosocial view in the general public.
-
•Educate the public on risk factors, prevention, and early intervention by using multiple media channels and public events.
-
•
-
3.Foster international collaborations:
-
•Organize international conferences and working groups where researchers, patient organizations, and policymakers can share best practices, research findings, and policy recommendations.
-
•Create a European network of research institutions and advocacy groups dedicated to persistent physical symptoms to drive collaborative research efforts and policy changes.
-
•Advocate for policies at the European and national levels that ensure equitable access to the latest treatments and research findings for all patients.
-
•Engage with policymakers to support legislative changes that prioritize persistent physical symptoms and allocate resources for ongoing research and treatment development.
-
•
-
4.Enhance engagement of patient and related persons:
-
•Create patient advisory panels and involve patients in the design and execution of research studies to ensure that research questions and outcomes are relevant to those affected.
-
•Develop mechanisms for patients to participate in decision-making processes regarding their care, ensuring their perspectives are considered in treatment planning.
-
•Encourage participation in support groups and advocacy networks to strengthen the collective voices of those affected.
-
•Facilitate patient engagement, such as public speaking at conferences, to raise awareness and influence policy changes.
-
•
-
5.Disseminate information across Europe:
-
•Create a centralized database or repository for research on persistent physical symptoms, making findings readily accessible to clinicians, researchers, and policymakers.
-
•Provide accessible, verified health information for self-management through digital platforms, community health centres, and local media to ensure widespread dissemination of accurate and actionable information.
-
•
Persistent physical symptoms should become a public healthcare priority
Despite their high prevalence and impact, persistent physical symptoms have been largely ignored as a public health problem in Europe's aging population. Population-based cohorts point towards an increase in symptom burden,115 which may have been accelerated by the COVID-19 pandemic in recent years. Latest meta-analyses show the presence of post-COVID symptoms such as fatigue in 30% of patients two-years after COVID-19.116 These results provide valuable information for decision-makers in health-care planning and may serve as a target for necessary public health interventions. Public health campaigns like the Flippin’ Pain Campaign of the British Pain Society advocate for better treatment, shift public perceptions, promote multidisciplinary management, reduce stigma, and support those affected. They also help develop public health infrastructure and encourage medical advice-seeking, particularly in underserved populations. While most campaigns focus on chronic pain or specific diseases in the US, broader campaigns across Europe are needed for various persistent physical symptoms.117
There is a need for symptom-overarching patient alliances or organizations for persistent physical symptoms in Europe and better public education. Patients and the public also face misconceptions about persistent physical symptoms with a general tendency to neglect and stigmatize the psychological aspect of physical symptoms. They should be supported in adopting a broader biopsychosocial perspective, necessary to better help affected patients. Furthermore, patient involvement as research advisors is a compelling ethical rationale, assumed to lead to research findings that are more pertinent to patients' concerns and dilemmas, and it confirms that patients and the public are eager to engage in health and social care research, seeking both personal benefits and the opportunity to help others with similar conditions.118
Accessible health information, like websites, podcasts, and online courses, empower patients, relatives, and healthcare professionals. Examples include Thuisarts.nl, Bodysymptoms.org, and Neurosymptoms.org, which explain health issues and symptoms from a mind-body perspective. There is also patient information on persistent physical symptoms that frequently occur with biomedical diseases, such as fatigue in multiple sclerosis or itch in primary biliary cholangitis (e.g., Mssociety.org.uk; Pbcfoundation.org.uk). A podcast series by ETUDE researchers covers mechanisms, diagnosis, treatment, and stigma in persistent physical symptoms (Etude-itn.eu). These resources promote health literacy at low cost. It remains crucial to translate scientific findings into culturally and linguistically appropriate materials and guide people to reliable online sources, combating misinformation. This is particular important as exposure to alarming media information can provoke or aggravate physical symptoms through a nocebo response.119
Patient advocacy groups and professional organizations have influenced health policies to focus on patient-centred care, involving patients in planning and self-management. Studies show that better patient engagement improves ethical standards, outcomes, and reduces costs.120 Panel 5 highlights the need to prioritize persistent physical symptoms in national health agendas and public healthcare.
Conclusion
Recent progress has been achieved regarding the understanding, recognition, and management of persistent physical symptoms. Important challenges persist, particularly in countries where deteriorating social and economic conditions may contribute to the prevalence of these symptoms. Addressing these challenges requires recognising the need for treatment, regardless of cause, and taking critical actions to evaluate and improve care models. This includes creating specialised care structures, educating future professionals, and advising public health and policymakers for better resource allocation.
This Personal View has some limitations. It may reflect biases from EURONET-SOMA members and might not be universally applicable due to varying cultural, economic, or organizational factors in European healthcare systems. Additionally, it may not fully address practical challenges like funding constraints, policy barriers, or stakeholder resistance. There may be ethical implications in prioritising resources for patients with persistent physical symptoms over other populations. Under the light of these limitations, we hope that this Personal View will spark discussions, highlight overlooked issues, and inspire further research and action toward improving healthcare delivery and clinical outcomes for patients with persistent physical symptoms.
In conclusion, persistent physical symptoms present a global healthcare challenge, affecting individuals across various medical conditions and often enduring despite treatment efforts. This call to action emphasises the need for substantial advancements in research, clinical management, public health, and policy across Europe. Priority include integrating symptom perception and biopsychosocial models into the education of healthcare professionals, promoting interdisciplinary collaboration, and implementing standardised guidelines. Increased research funding and pan-European collaborations are needed to advance the understanding and management of these conditions. Addressing these priorities will enable prevention, early interventions, and better support for patients and caregivers. Public health initiatives should raise awareness and support a biopsychosocial perspective in the general population. Initiating a series of structured consensus processes to address specific, concrete topics derived from this Personal View could engage diverse stakeholders to reach consensus on the most pressing issues and actionable steps, ultimately helping to build a more robust evidence base for the proposed improvements. By prioritising persistent physical symptoms and fostering collaboration, Europe can enhance outcomes and quality of life for those affected, giving them hope for improvement or recovery.
Contributors
AT, AW and BL conceptualised the Personal View. AT coordinated the research activities. AT and AW conducted the literature search and analysed the literature. AT, AW and BL discussed the literature and contributed substantially to its interpretation. AT, AW and BL drafted the Personal View.
Declaration of interests
AT reports research funding (no personal honoraria) from the German Research Foundation. She has received remunerations for a printed textbook from Ernst Reinhardt Publishing. AW reports research funding (no personal honoraria) from the Werner Otto Foundation. She has received remunerations for a lecture at the Lindauer Psychotherapietage and she has been treasurer of the EAPM (unpaid) since 2021. BL reports research funding (no personal honoraria) from the German Research Foundation, the German Federal Ministry of Education and Research, the German Innovation Committee at the Joint Federal Committee, the European Commission’s Horizon 2020 Framework Programme, the European Joint Programme for Rare Diseases (EJP), the Ministry of Science, Research and Equality of the Free and Hanseatic City of Hamburg, Germany, and the Foundation Psychosomatics of Spinal Diseases, Stuttgart, Germany. He received remunerations for several scientific book articles from various book publishers, from the Norddeutscher Rundfunk (NDR) for interviews in medical knowledge programmes on public television, and as a committee member from Aarhus University, Denmark. He received travel expenses from the European Association of Psychosomatic Medicine (EAPM), and accommodation and meals from the Societatea de Medicina Biopsyhosociala, Romania, for a presentation at the EAPM Academy at the Conferința Națională de Psihosomatică, Cluj-Napoca, Romania, October 2023. He received a travel grant for a lecture on the occasion of the presentation of the Alison Creed Award at the EAPM Conference in Lausanne, 12–15 June 2024. He received remuneration and travel expenses for lecture at the Lindauer Psychotherapiewochen, April 2024. He is President of the German College of Psychosomatic Medicine (DKPM) (unpaid) since March 2024 and was a member of the Board of the European Association of Psychosomatic Medicine (EAPM) (unpaid) until 2022. He is member of the EIFFEL Study Oversight Committee (unpaid). KB reports honoraria as speaker for medical specialist meetings, she is an unpaid member of the board of directors at Unizo Limburg (Organisation for the Self-Employed and SMEs), and a founding member and shareholder of Tumi Therapeutics (interdisciplinary centre of expertise on the prevention, diagnostics and treatment of stress-related and persistent somatic symptoms). PE reports funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; GZ: EN 1376/1-1). ToH is vice-president of the North American Primary Care Research Group (NAPCRG). TH reports honoria as a lecturer in university postgradual psychotherapy training in children and adolescent psychotherapy regarding chronic (primary) pain at the universities of Cologne (AkiP), Trier, Osnabrück and Landau. She is a member of the Open Science Committee of the Institute of Psychology of University of Muenster and a board member of the “Kinder-und Jugendlichenpsychotherapie Verhaltenstherapie” (KJPVT). KH reports funding for studies on COVID and post COVID condition by Land Tirol and honoraria for lectures-at FOMF (Forum für Medizinische Fortbildung) and Hospital of Schwaz. PeHe has received remunerations as a scientific programme consultant of the Lindauer Psychotherapietage, he has received remunerations as a committee member from Aarhus University, Denmark, he has obtained remunerations for a book chapter from Oxford University Press and for a book from Springer Nature and has received payment of travel expenses and remunerations for presentations at several universities and other public hospitals in Germany (no commercial companies), he has received travel expenses and registration fees for the German Congress of Psychosomatic Medicine, and has been a board member of the EAPM (unpaid) since 2023. PH reports research funding from the Stiftung Psychosomatik der Wirbelsäulenerkrankungen (payment made to the institution), royalties for a book chapter on somatic symptom disorder by Thieme Verlag, a scholarship for attending the ICPM 2024 conference from Carus Stiftung, he operates in his own practice for psychotherapy and receives payments by the German statutory health insurances and private health insurances. KHK reports honoria as a teaching psychologist specializing in child and adolescent psychiatry about functional somatic disorders (FSD), and on a specialist course for health care professionals working with children and adolescents with the topic of etiology, communication and treatment of FSD in children and adolescents (organized by the Danish Medical Society for Functional Disorders), she was an invited speaker at the Swedish pain forum meeting—giving a lecture on specialized treatment of children and adolescents with FSD, and is a board member of the Danish Medical Society for Functional Disorders and member of the educational committee within the medical society. NL reports grants from the European Commission, Horizon 2020 Framework Programme Marie Sklodowska Curie Innovative Training Network ETUDE (project 956673), the German Federal Ministry of Education and Research (The German Centre for Mental Health), and the German Research Foundation (project LE 3754/2-1) (payments to institution), payments for article publication by Psychotherapie im Dialog and lectures at Benedictus Krankenhaus Feldafing GmbH & Co KG; ZAIM Medidays Zurich, she received Payments to attend meetings and to travel by TUM University Hospital, Munich, and is a founding member and stockholder of EyeSeeTec GmbH, Munich. AM reports honoraria for lectures and educational workshops about diagnostic assessment (Akademie für Psychologie BÖP, Vienna), characteristics and treatment of somatic symptom disorders (University of Zurich; University of Osnabrueck; The Medical Association of Lower Saxony), and travel support for EURONET SOMA group meetings (funded by own institution). CP is a board member of the European Associatoion of Psychosomatic Medicine (EAPM) and council member of the World Federation of Psychotherapy (WFP). JR reports a research grant from Krajowa Izba Lekarsko-Weterynaryjna (National Veterinarians Board) for conducting national study of work burnout of vets, consulting fees by Jansen Cilag sa and Sympomed sp z o.o as a member of the advisory board, speaker honoraria for preparation, development and delivery of author lectures on 1) Metabolic side effects in people with schizophrenia during intermediate and long-term treatment with antipsychotic drugs 2) Use of neuromodulation in the treatment of eating disorders–update 2024. WR reports research grants from the German Research Foundation DFG, the Ministry of Science and Arts Hessian (LOEWE Hessian Excellence Centre DYNAMIC), royalties for book publications (Hogrefe, Göttingen), honoraria from Boehringer Ingelheim for workshops on Long COVID, and payment for expert testimony at the Hessian Institute for Health and Care (HLfGP). MR reports grants from TrygFonden and Innovation Fund Denmark (payments were made to institution), she receives royalties for book chapters by Gyldendal, and payment for a keynote presentation at Swedish Pain Society, she is chair of the Danish Society of Functional Disorders (unpaid). JGMR reports research grants by ZonMw, EU, NOW and NIMH, she received royalties for a handbook on persistent somatic symptoms by Lannoo Publishers, she is a winner of the Wayne Katon Research Award (ACLP) (paid to university), is member of a Data Safety Monitoring Board and Advisory Board of SOMACROSS research unit (FOR 5211) (unpaid), vice-president of the European Association of Psychosomatic Medicine, EAPM (unpaid), and president of the Dutch Network Persistent Somatic Symptoms, NALK (unpaid). MS reports honoraria for lectures and presentations. RS reports honoraria as a speaker from Novartis for a lecture on psychodermatology, he is a member of the board of trustees of the Foundation Psychosomatic and Social Medicine (Ascona Foundation), coeditor of the German AWMF S3-Guidelines on Functional Complaints, and contributed to the German guidelines on irritable bowel syndrome, and on Lyme Borreliosis, he is chairman of the Basel Institute for Psychosomatic Medicine (BIPM) and founder and managing director of the Psychosomatic and Psychosocial Services GmbH, that develops and implements psychosomatic and psychosocial training and continuing education programs. StSa reports funding from the German Research Foundation (Grant number: SA 4505/3-1). MSM reports grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): Research funding for the SOMACROSS research unit (RU 5211), subproject leader (payment to institution); Deutscher Akademischer Austauschdienst (DAAD, German Academic Research Service): Funding for research exchange programme to UNSW Sydney, Australia (Payment to institution); Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): Research funding for the Transregional Research Unit Treatment Expectation (TRR 289), subproject leader (payment to institution), she reports honoraria for review of grant proposal from University of Toledo, USA, and consulting fees by Norddeutscher Rundfunk (NDR), Hamburg: Remuneration for interviews in medical knowledge programmes on public television, Hafencity-Institut für Psychotherapieausbildung (HIP), Hamburg: Honoraria for post graduate training for psychotherapy, Institut für Psychotherapieausbildung (IfP), Hamburg: Honoraria for post graduate training for psychotherapy, Institut für Psychotherapieausbildung Marburg (IPAM), Marburg: Honoraria for post graduate training for psychotherapy, she is a member of the scientific advisory board at PKD Cure e.V. (unpaid), and executive board member and vice-treasurer of the European Association of Psychosomatic Medicine (EAPM) (unpaid). JS reports royalties for articles on Functional Neurological Disorder (FND) from UpToDate, payment for expert testimony within expert witness work for UK legal firms in personal injury and negligence cases, board, he is secretary of the FND Society since 2019, on the medical advisory board of FND Hope and FND hope UK, and on the medical advisory board of FND Action, he runs neurosymptoms.org—a free self-help website for people with FND. LT reports honoraria for educational lectures on somatic symptom disorder and functional neurological disorder (paid to institution) and is (unpaid) chair of the Dutch network for Persistent Somatic Symptoms (NALK). UW reports honoraria from Lundbeck and Janssen and is a member of the scientific committees for Janssen and Teva. MW reports honoraria as speaker for several CBT training courses at different universities, reimbursement of travel expenses from the Johannes Gutenberg University of Mainz, he is member and co-chairman of the ethics committee of the Department of Psychology at the Johannes Gutenberg University Mainz, and reports royalties for different scientific book chapters and a CBT treatment manual for medically unexplained somatic symptoms. All other authors declare no conflict of interest.
Acknowledgements
This Personal View was done without external funding. BL and AT thank the Deutsche Forschungsgemeinschaft (DFG) for funding the SOMACROSS research unit (RU 5211), which investigates factors and mechanisms leading to the persistence of somatic symptoms in ten diseases. AT, AW and BL also thank the European Commission, Horizon 2020 Framework Programme for funding the Marie Sklodowska Curie Innovative Training Network ETUDE (project 956673).
We are thankful to the EURONET-SOMA group members for their critical revision of the manuscript.
EURONET-SOMA Group (in alphabetical order): Jordi Blanch, University of Barcelona, Barcelona, Spain; Katleen Bogaerts, Hasselt University, Diepenbeek, Belgium; Birgitte Boye, Oslo University Hospital, Oslo, Norway; Chris Burton, University of Sheffield, Sheffield, UK; Fiammetta Cosci, University of Florence, Florence, Italy; Petra Engelmann, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Susanne Fischer, University of Zurich, Zurich, Switzerland; Stephan Frisch, University Ulm Medical Centre, Ulm, Germany; Lisbeth Frostholm, Aarhus University Hospital, Aarhus, Denmark; Lise Kirstine Gormsen, Aarhus University Hospital, Aarhus, Denmark; Monica Greco, University of Bath, Bath, UK; Karen Hansen Kallesoe, Aarhus University Hospital, Aarhus, Denmark; Tim olde Hartman, Radboud University Medical Centre, Nijmegen, the Netherlands; Tanja Hechler, University of Münster, Münster, Germany; Severin Hennemann, Johannes Gutenberg-University Mainz, Mainz, Germany; Peter Henningsen, Technical University of Munich, Munich, Germany; Katharina Hüfner, Innsbruck Medical University, Innsbruck, Austria; Paul Hüsing, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Joram Ronel, Barmelweid Clinic AG, Barmelweid, Switzerland; Roland von Känel, University Hospital Zurich, Zurich, Switzerland; Christopher A. Kenedi, Duke University Medical Centre, Durham, USA; Ferenc Köteles, Eötvös Loránd University, Budapest, Hungary; Sebastian Kohlmann, University Medical Centre Heidelberg, Heidelberg, Germany; Willem J. Kop, Tilburg University, Tilburg, the Netherlands; Nadine Lehnen, Technical University of Munich, Munich, Germany; James Levenson, Virginia Commonwealth University, Richmond, USA; Bernd Löwe, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Kerstin Maehder, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Alexandra Martin, University of Wuppertal, Wuppertal, Germany; Christoph Pieh, University for Continuing Education Krems, Krems, Austria; Victor Pitron, Paris City University, Paris, France; Charlotte Ulrikka Rask, Aarhus University Hospital, Aarhus, Denmark; Winfried Rief, University of Marburg, Marburg, Germany; Marianne Rosendal, Aarhus University Hospital, Aarhus, Denmark; Judith Rosmalen, University of Groningen, Groningen, the Netherlands; Joanna Rymaszewska, Worclaw University of Science and Technology, Wroclaw, Poland; Markku Sainio, Helsinki University Hospital, Helsinki, Finland; Stefan Salzmann, Health and Medical University, Erfurt, Germany; Rainer Schaefert, University Hospital of Basel Basel, Switzerland; Sanna Selinheimo, Finnish Institute of Occupational Health, Helsinki, Finland; Meike Shedden-Mora, Medical School Hamburg, Hamburg, Germany; Jon Stone, University of Edinburgh, Edinburgh, Scotland; Lineke Tak, Dimence Institute for Specialised Mental Healthcare, Alkura Specialist Centre Persistent Somatic Symptoms, Deventer, the Netherlands; Anne Toussaint, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Natalie Uhlenbusch, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Omer Van den Bergh, University of Leuven, Leuven, Belgium; Lars de Vroege, Tilburg University, Tilburg, the Netherlands; Angelika Weigel, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; Ursula Werneke, Umea University, Umea, Sweden; Michael Witthöft, Johannes Gutenberg-University Mainz, Mainz, Germany.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101140.
Contributor Information
Anne Toussaint, Email: a.toussaint@uke.de.
EURONET-SOMA group:
Jordi Blanch, Katleen Bogaerts, Birgitte Boye, Chris Burton, Fiammetta Cosci, Petra Engelmann, Per Fink, Susanne Fischer, Stephan Frisch, Lisbeth Frostholm, Lise Kirstine Gormsen, Monica Greco, Karen Hansen Kallesoe, Tim olde Hartman, Tanja Hechler, Severin Hennemann, Peter Henningsen, Katharina Hüfner, Paul Hüsing, Joram Ronel, Roland von Känel, Christopher A. Kenedi, Ferenc Köteles, Sebastian Kohlmann, Willem J. Kop, Nadine Lehnen, James Levenson, Bernd Löwe, Kerstin Maehder, Alexandra Martin, Christoph Pieh, Victor Pitron, Charlotte Ulrikka Rask, Winfried Rief, Marianne Rosendal, Judith Rosmalen, Joanna Rymaszewska, Markku Sainio, Stefan Salzmann, Rainer Schaefert, Sanna Selinheimo, Meike Shedden-Mora, Jon Stone, Lineke Tak, Anne Toussaint, Natalie Uhlenbusch, Omer Van den Bergh, Lars de Vroege, Angelika Weigel, Ursula Werneke, and Michael Witthöft
Appendix ASupplementary data
References
- 1.Löwe B., Andresen V., Van den Bergh O., et al. Persistent SOMAtic symptoms ACROSS diseases - from risk factors to modification: scientific framework and overarching protocol of the interdisciplinary SOMACROSS research unit (RU 5211) BMJ Open. 2022;12(1) doi: 10.1136/bmjopen-2021-057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Löwe B., Toussaint A., Rosmalen J.G.M., et al. Persistent physical symptoms: definition, genesis, and management. Lancet. 2024;403:2649–2662. doi: 10.1016/S0140-6736(24)00623-8. [DOI] [PubMed] [Google Scholar]
- 3.Burton C., Fink P., Henningsen P., Löwe B., Rief W., EURONET-SOMA Group Functional somatic disorders: discussion paper for a new common classification for research and clinical use. BMC Med. 2020;18(1):34. doi: 10.1186/s12916-020-1505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rief W., Burton C., Frostholm L., et al. Core outcome domains for clinical trials on somatic symptom disorder, bodily distress disorder, and functional somatic syndromes: European network on somatic symptom disorders recommendations. Psychosom Med. 2017;79(9):1008–1015. doi: 10.1097/PSY.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 5.Bova G., Domenichiello A., Letzen J.E., et al. Developing consensus on core outcome sets of domains for acute, the transition from acute to chronic, recurrent/episodic, and chronic pain: results of the INTEGRATE-pain Delphi process. EClinicalMedicine. 2023;66 doi: 10.1016/j.eclinm.2023.102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henningsen P., Gündel H., Kop W.J., et al. Persistent physical symptoms as perceptual dysregulation: a neuropsychobehavioral model and its clinical implications. Psychosom Med. 2018;80(5):422–431. doi: 10.1097/PSY.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 7.Nijs J., Kosek E., Chiarotto A., et al. Nociceptive, neuropathic, or nociplastic low back pain? The low back pain phenotyping (BACPAP) consortium's international and multidisciplinary consensus recommendations. Lancet Rheumatol. 2024;6(3):e178–e188. doi: 10.1016/S2665-9913(23)00324-7. [DOI] [PubMed] [Google Scholar]
- 8.Rosmalen J.G.M., Burton C., Carson A., et al. The European Training Network ETUDE (Encompassing Training in fUnctional Disorders across Europe): a new research and training program of the EURONET-SOMA network recruiting 15 early stage researchers. J Psychosom Res. 2021;141 doi: 10.1016/j.jpsychores.2020.110345. [DOI] [PubMed] [Google Scholar]
- 9.Chaabouni A., Houwen J., Peters H., van Boven K., Schers H., Olde Hartman T.C. Symptom diagnoses in primary care: which symptoms persist? J Psychosom Res. 2022;157 doi: 10.1016/j.jpsychores.2024.111859. [DOI] [PubMed] [Google Scholar]
- 10.Rometsch C., Mansueto G., Maas Genannt Bermpohl F., Martin A., Cosci F. Prevalence of functional disorders across Europe: a systematic review and meta-analysis. Eur J Epidemiol. 2024;39(6):571–586. doi: 10.1007/s10654-024-01109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Münker L., Rimvall M.K., Frostholm L., et al. Exploring the course of functional somatic symptoms (FSS) from pre- to late adolescence and associated internalizing psychopathology - an observational cohort-study. BMC Psychiatr. 2024;24(1):495. doi: 10.1186/s12888-024-05937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabreira V., Frostholm L., McWhirter L., Stone J., Carson A. Clinical signs in functional cognitive disorders: a systematic review and diagnostic meta-analysis. J Psychosom Res. 2023;173 doi: 10.1016/j.jpsychores.2023.111447. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel K., Petzke T.M., Witthöft M. The emotion regulation process in somatic symptom disorders and related conditions—a systematic narrative review. Clin Psychol Rev. 2022;97 doi: 10.1016/j.cpr.2022.102196. [DOI] [PubMed] [Google Scholar]
- 14.Regnath F., Biersack K., Jäger N., Glasauer S., Lehnen N. Not a general, symptom-unspecific, transdiagnostic marker for functional symptoms: sensorimotor processing of head control is intact in chronic pain. Front Neurol. 2023;14 doi: 10.3389/fneur.2023.1294702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders C., Treufeldt H., Rask M.T., et al. Explanations for functional somatic symptoms across European treatment settings: a mixed methods study. J Psychosom Res. 2023;166 doi: 10.1016/j.jpsychores.2023.111155. [DOI] [PubMed] [Google Scholar]
- 16.Smakowski A., Hüsing P., Volcker S., et al. Psychological risk factors of somatic symptom disorder: a systematic review and meta-analysis of cross-sectional and longitudinal studies. J Psychosom Res. 2024;181 doi: 10.1016/j.jpsychores.2024.111608. [DOI] [PubMed] [Google Scholar]
- 17.Mamo N., van der Kludert M., Tak L., Olde Hartman T.C., Hanssen D., Rosmalen J. Characteristics of collaborative care networks in functional disorders: a systematic review. J Psychosom Res. 2023;172 doi: 10.1016/j.jpsychores.2023.111357. [DOI] [PubMed] [Google Scholar]
- 18.Kustra-Mulder A., Löwe B., Weigel A. Healthcare-related factors influencing symptom persistence, deterioration, or improvement in patients with persistent somatic symptoms: a scoping review of European studies. J Psychosom Res. 2023;174 doi: 10.1016/j.jpsychores.2023.111485. [DOI] [PubMed] [Google Scholar]
- 19.McGhie-Fraser B., Lucassen P., Ballering A., et al. Persistent somatic symptom related stigmatisation by healthcare professionals: a systematic review of questionnaire measurement instruments. J Psychosom Res. 2023;166 doi: 10.1016/j.jpsychores.2023.111161. [DOI] [PubMed] [Google Scholar]
- 20.Treufeldt H., Burton C. Stigmatisation in medical encounters for persistent physical symptoms/functional disorders: scoping review and thematic synthesis. Patient Educ Counsel. 2024;123 doi: 10.1016/j.pec.2024.108198. [DOI] [PubMed] [Google Scholar]
- 21.McLoughlin C., McWhirter L., Pisegna K., et al. Stigma in functional neurological disorder (FND) - a systematic review. Clin Psychol Rev. 2024;112 doi: 10.1016/j.cpr.2024.102460. [DOI] [PubMed] [Google Scholar]
- 22.Chaabouni A., Houwen J., Grewer G., et al. The burden of persistent symptom diagnosis in primary care patients: a cross-sectional study. Scand J Prim Health Care. 2024;42(1):112–122. doi: 10.1080/02813432.2023.2293930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elnegaard S., Andersen R.S., Pedersen A.F., et al. Self-reported symptoms and healthcare seeking in the general population--exploring “The Symptom Iceberg”. BMC Publ Health. 2015;15:685. doi: 10.1186/s12889-015-2034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosendal M., Carlsen A.H., Rask M.T. Symptoms as the main problem: a cross- sectional study of patient experience in primary care. BMC Fam Pract. 2016;17:29. doi: 10.1186/s12875-016-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohlmann S., Gierk B., Hilbert A., Brähler E., Löwe B. The overlap of somatic, anxious and depressive syndromes: a population-based analysis. J Psychosom Res. 2016;90:51–56. doi: 10.1016/j.jpsychores.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. Int J Methods Psychiatr Res. 2003;12(1):34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atasoy S., Henningsen P., Sattel H., et al. Stability and predictors of somatic symptoms in men and women over 10 years: a real-world perspective from the prospective MONICA/KORA study. J Psychosom Res. 2022;162 doi: 10.1016/j.jpsychores.2022.111022. [DOI] [PubMed] [Google Scholar]
- 28.Chitkara D.K., Rawat D.J., Talley N.J. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005;100(8):1868–1875. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- 29.Korterink J.J., Diederen K., Benninga M.A., Tabbers M.M. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijhof S.L., Maijer K., Bleijenberg G., Uiterwaal C.S., Kimpen J.L., van de Putte E.M. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. 2011;127(5):e1169–e1175. doi: 10.1542/peds.2010-1147. [DOI] [PubMed] [Google Scholar]
- 31.Boronat A.C., Ferreira-Maia A.P., Matijasevich A., Wang Y.P. Epidemiology of functional gastrointestinal disorders in children and adolescents: a systematic review. World J Gastroenterol. 2017;23(21):3915–3927. doi: 10.3748/wjg.v23.i21.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid M.C., Eccleston C., Pillemer K. Management of chronic pain in older adults. BMJ. 2015;350 doi: 10.1136/bmj.h532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avlund K., Pedersen A.N., Schroll M. Functional decline from age 80 to 85: influence of preceding changes in tiredness in daily activities. Psychosom Med. 2003;65(5):771–777. doi: 10.1097/01.psy.0000082640.61645.bf. [DOI] [PubMed] [Google Scholar]
- 34.Foley D.J., Monjan A.A., Brown S.L., Simonsick E.M., Wallace R.B., Blazer D.G. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 35.Talley N.J., Dennis E.H., Schettler-Duncan V.A., Lacy B.E., Olden K.W., Crowell M.D. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98(11):2454–2459. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 36.Torres M.E., Löwe B., Schmitz S., Pienta J.N., Van Der Feltz-Cornelis C., Fiedorowicz J.G. Suicide and suicidality in somatic symptom and related disorders: a systematic review. J Psychosom Res. 2021;140 doi: 10.1016/j.jpsychores.2020.110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konnopka A., Kaufmann C., König H.H., et al. Association of costs with somatic symptom severity in patients with medically unexplained symptoms. J Psychosom Res. 2013;75(4):370–375. doi: 10.1016/j.jpsychores.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Barry C.A., Bradley C.P., Britten N., Stevenson F.A., Barber N. Patients' unvoiced agendas in general practice consultations: qualitative study. BMJ. 2000;320(7244):1246–1250. doi: 10.1136/bmj.320.7244.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalder T., Willis C. “Lumping” and “splitting” medically unexplained symptoms: is there a role for a transdiagnostic approach? J Ment Health. 2017;26(3):187–191. doi: 10.1080/09638237.2017.1322187. [DOI] [PubMed] [Google Scholar]
- 40.Dear B.F., Scott A.J., Fogliati R., et al. The chronic conditions course: a randomised controlled trial of an internet-delivered transdiagnostic psychological intervention for people with chronic health conditions. Psychother Psychosom. 2022;91(4):265–276. doi: 10.1159/000522530. [DOI] [PubMed] [Google Scholar]
- 41.Loeser J.D., Melzack R. Pain: an overview. Lancet. 1999;353(9164):1607–1609. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- 42.O'Mahoney L.L., Routen A., Gillies C., et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2023;55 doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch E.N., Campani C., Innocenti T., et al. Understanding fatigue in primary biliary cholangitis: from pathophysiology to treatment perspectives. World J Hepatol. 2022;14(6):1111–1119. doi: 10.4254/wjh.v14.i6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization . 11th ed. 2019. International statistical classification of diseases and related health problems. [Google Scholar]
- 45.Petersen M.W., Schröder A., Jörgensen T., et al. Prevalence of functional somatic syndromes and bodily distress syndrome in the Danish population: the DanFunD study. Scand J Public Health. 2020;48(5):567–576. doi: 10.1177/1403494819868592. [DOI] [PubMed] [Google Scholar]
- 46.Joustra M.L., Janssens K.A., Bultmann U., Rosmalen J.G. Functional limitations in functional somatic syndromes and well-defined medical diseases. Results from the general population cohort LifeLines. J Psychosom Res. 2015;79(2):94–99. doi: 10.1016/j.jpsychores.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Rehman F. Schedules for clinical assessment in neuropsychiatry. BMJ. 2011;342 [Google Scholar]
- 48.Hallett M., Aybek S., Dworetzky B.A., McWhirter L., Staab J.P., Stone J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537–550. doi: 10.1016/S1474-4422(21)00422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Psychiatric Association . American Psychiatric Association; Washington, DC: 2022. Diagnostic and statistical manual of mental disorders text revision: DSM-5-TR, 2013. [Google Scholar]
- 50.Löwe B., Levenson J., Depping M., et al. Somatic symptom disorder: a scoping review on the empirical evidence of a new diagnosis. Psychol Med. 2022;52(4):632–648. doi: 10.1017/S0033291721004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fava G.A., Sonino N. The biopsychosocial model thirty years later. Psychother Psychosom. 2008;77(1):1–2. doi: 10.1159/000110052. [DOI] [PubMed] [Google Scholar]
- 52.Van den Bergh O., Witthöft M., Petersen S., Brown R.J. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev. 2017;74(Pt A):185–203. doi: 10.1016/j.neubiorev.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Kroenke K. A practical and evidence-based approach to common symptoms: a narrative review. Ann Intern Med. 2014;161(8):579–586. doi: 10.7326/M14-0461. [DOI] [PubMed] [Google Scholar]
- 54.Horwitz R.I., Singer B.H., Hayes-Conroy A., et al. Biosocial pathogenesis. Psychother Psychosom. 2022;91(2):73–77. doi: 10.1159/000521567. [DOI] [PubMed] [Google Scholar]
- 55.Leiknes K.A., Finset A., Moum T., Sandanger I. Course and predictors of medically unexplained pain symptoms in the general population. J Psychosom Res. 2007;62(2):119–128. doi: 10.1016/j.jpsychores.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Beutel M.E., Klein E.M., Henning M., et al. Somatic symptoms in the German general population from 1975 to 2013. Sci Rep. 2020;10(1):1595. doi: 10.1038/s41598-020-58602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Vroege L., Woudstra-de Jong J.E., Videler A.C., Kop W.J. Personality factors and cognitive functioning in patients with somatic symptom and related disorders. J Psychosom Res. 2022;163 doi: 10.1016/j.jpsychores.2022.111067. [DOI] [PubMed] [Google Scholar]
- 58.Creed F. The predictors of somatic symptoms in a population sample: the lifelines cohort study. Psychosom Med. 2022;84(9):1056–1066. doi: 10.1097/PSY.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 59.Barends H., Dekker J., van Dessel N.C., Twisk J.W.R., van der Horst H.E., van der Wouden J.C. Exploring maladaptive cognitions and behaviors as perpetuating factors in patients with persistent somatic symptoms: a longitudinal study. J Psychosom Res. 2023;170 doi: 10.1016/j.jpsychores.2023.111343. [DOI] [PubMed] [Google Scholar]
- 60.Kirmayer L.J., Sartorius N. Cultural models and somatic syndromes. Psychosom Med. 2007;69(9):832–840. doi: 10.1097/PSY.0b013e31815b002c. [DOI] [PubMed] [Google Scholar]
- 61.Schulz A., Larra Y.R.M.F., Vogele C., Kolsch M., Schachinger H. The relationship between self-reported chronic stress, physiological stress axis dysregulation and medically-unexplained symptoms. Biol Psychol. 2023;183 doi: 10.1016/j.biopsycho.2023.108690. [DOI] [PubMed] [Google Scholar]
- 62.Choutka J., Jansari V., Hornig M., Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28(5):911–923. doi: 10.1038/s41591-022-01810-6. [DOI] [PubMed] [Google Scholar]
- 63.Sommer C., Rittner H. Pain research in 2023: towards understanding chronic pain. Lancet Neurol. 2024;23(1):27–28. doi: 10.1016/S1474-4422(23)00446-5. [DOI] [PubMed] [Google Scholar]
- 64.Kube T., Rozenkrantz L., Rief W., Barsky A. Understanding persistent physical symptoms: conceptual integration of psychological expectation models and predictive processing accounts. Clin Psychol Rev. 2020;76 doi: 10.1016/j.cpr.2020.101829. [DOI] [PubMed] [Google Scholar]
- 65.Bräscher A.K., Sutterlin S., Scheuren R., Van den Bergh O., Witthöft M. Somatic symptom perception from a predictive processing perspective: an empirical test using the thermal grill illusion. Psychosom Med. 2020;82(7):708–714. doi: 10.1097/PSY.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 66.Weigel A., Hüsing P., Junge M., Löwe B. Helpful explanatory models for persistent somatic symptoms (HERMES): results of a three-arm randomized-controlled pilot trial. J Psychosom Res. 2023;172 doi: 10.1016/j.jpsychores.2023.111419. [DOI] [PubMed] [Google Scholar]
- 67.Mariman A., Vermeir P., Csabai M., et al. Education on medically unexplained symptoms: a systematic review with a focus on cultural diversity and migrants. Eur J Med Res. 2023;28(1):145. doi: 10.1186/s40001-023-01105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hulgaard D., Dehlholm-Lambertsen G., Rask C.U. Family-based interventions for children and adolescents with functional somatic symptoms: a systematic review. J Fam Ther. 2019;41(1):4–28. [Google Scholar]
- 69.Houwen J., Lucassen P.L., Stappers H.W., Assendelft W.J., van Dulmen S., Olde Hartman T.C. Improving GP communication in consultations on medically unexplained symptoms: a qualitative interview study with patients in primary care. Br J Gen Pract. 2017;67(663):e716–e723. doi: 10.3399/bjgp17X692537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evers A.W., Gieler U., Hasenbring M.I., van Middendorp H. Incorporating biopsychosocial characteristics into personalized healthcare: a clinical approach. Psychother Psychosom. 2014;83(3):148–157. doi: 10.1159/000358309. [DOI] [PubMed] [Google Scholar]
- 71.Aiarzaguena J.M., Grandes G., Gaminde I., Salazar A., Sanchez A., Arino J. A randomized controlled clinical trial of a psychosocial and communication intervention carried out by GPs for patients with medically unexplained symptoms. Psychol Med. 2007;37(2):283–294. doi: 10.1017/S0033291706009536. [DOI] [PubMed] [Google Scholar]
- 72.Olde Hartman T., Lam C.L., Usta J., Clarke D., Fortes S., Dowrick C. Addressing the needs of patients with medically unexplained symptoms: 10 key messages. Br J Gen Pract. 2018;68(674):442–443. doi: 10.3399/bjgp18X698813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burton C., Mooney C., Sutton L., et al. Effectiveness of a symptom-clinic intervention delivered by general practitioners with an extended role for people with multiple and persistent physical symptoms in England: the Multiple Symptoms Study 3 pragmatic, multicentre, parallel-group, individually randomised controlled trial. Lancet. 2024;403:2619–2629. doi: 10.1016/S0140-6736(24)00700-1. [DOI] [PubMed] [Google Scholar]
- 74.Kaur T., Ranjan P., Sarkar S., et al. Psychological interventions for medically unexplained physical symptoms: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2022;77:92–101. doi: 10.1016/j.genhosppsych.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Leaviss J., Davis S., Ren S., et al. Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24(46):1–490. doi: 10.3310/hta24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russell L., Abbass A., Allder S. A review of the treatment of functional neurological disorder with intensive short-term dynamic psychotherapy. Epilepsy Behav. 2022;130 doi: 10.1016/j.yebeh.2022.108657. [DOI] [PubMed] [Google Scholar]
- 77.Langlois P., Perrochon A., David R., et al. Hypnosis to manage musculoskeletal and neuropathic chronic pain: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;135 doi: 10.1016/j.neubiorev.2022.104591. [DOI] [PubMed] [Google Scholar]
- 78.Swainston K., Thursby S., Bell B., Poulter H., Dismore L., Copping L. What psychological interventions are effective for the management of persistent physical symptoms (PPS)? A systematic review and meta-analysis. Br J Health Psychol. 2023;28(1):80–97. doi: 10.1111/bjhp.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharpe M., Carson A. “Unexplained” somatic symptoms, functional syndromes, and somatization: do we need a paradigm shift? Ann Intern Med. 2001;134(9 Pt 2):926–930. doi: 10.7326/0003-4819-134-9_part_2-200105011-00018. [DOI] [PubMed] [Google Scholar]
- 80.Christensen O.R., Hedegaard L., Rask M.T., Clemensen J., Frostholm L., Rosendal M. Designing and developing an eHealth program for patients with persistent physical symptoms: usability study. JMIR Hum Factors. 2023;10 doi: 10.2196/42572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houwen J., Lucassen P., Stappers H.W., Assendelft P.J.J., van Dulmen S., Olde Hartman T.C. Medically unexplained symptoms: the person, the symptoms and the dialogue. Fam Pract. 2017;34(2):245–251. doi: 10.1093/fampra/cmw132. [DOI] [PubMed] [Google Scholar]
- 82.Simren M., Tornblom H., Palsson O.S., Whitehead W.E. Management of the multiple symptoms of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2017;2(2):112–122. doi: 10.1016/S2468-1253(16)30116-9. [DOI] [PubMed] [Google Scholar]
- 83.Sörensen N.B., Nielsen R.E., Christensen A.E., et al. Treatment provided in children and adolescents with functional seizures-A Danish nationwide cohort. Children. 2023;10(7):1218. doi: 10.3390/children10071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roenneberg C., Sattel H., Schaefert R., Henningsen P., Hausteiner-Wiehle C. Functional somatic symptoms. Dtsch Arztebl Int. 2019;116(33–34):553–560. doi: 10.3238/arztebl.2019.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olde Hartman T.C., Blankenstein A.H., Molenaar A.O., et al. 2023. Somatisch onvoldoende verklaarde lichamelijke klachten (SOLK) (M102) [NHG guideline on medically unexplained symptoms (MUS)] [Google Scholar]
- 86.Chitnis A., Dowrick C., Byng R., et al. 2011. Guidance for health professionals on medically unexplained symptoms. [Google Scholar]
- 87.Kohlmann S., Löwe B., Shedden-Mora M.C. Health care for persistent somatic symptoms across Europe: a qualitative evaluation of the EURONET-SOMA expert discussion. Front Psychiatry. 2018;9:646. doi: 10.3389/fpsyt.2018.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y., Wulf Hanson S., Culbreth G., et al. Assessing the impact of health-care access on the severity of low back pain by country: a case study within the GBD framework. Lancet Rheumatol. 2024;6(9):e598–e606. doi: 10.1016/S2665-9913(24)00151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scheidt C.E. Psychosomatic medicine in Germany. Int J Body Mind Cult. 2017;4(2):78–86. [Google Scholar]
- 90.McCarty C.A., Zatzick D.F., Marcynyszyn L.A., et al. Effect of collaborative care on persistent postconcussive symptoms in adolescents: a randomized clinical trial. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brostrom S. Improving care for patients with functional disorders in Denmark. J Psychosom Res. 2019;116:22–24. doi: 10.1016/j.jpsychores.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Schneider A., Donnachie E., Zipfel S., Enck P. Patients with somatoform disorders are prone to expensive and potentially harmful medical procedures-results of a retrospective cohort study over 15 years. Dtsch Arztebl Int. 2021;118(25):425–431. doi: 10.3238/arztebl.m2021.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roditi D., Robinson M.E. The role of psychological interventions in the management of patients with chronic pain. Psychol Res Behav Manag. 2011;4:41–49. doi: 10.2147/PRBM.S15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kustra-Mulder A., Liebau M., Grewer G., et al. Healthcare professionals' views on factors influencing persistent somatic symptoms - ARISE-HCP online survey across countries. J Psychosom Res. 2024;183 doi: 10.1016/j.jpsychores.2024.111695. [DOI] [PubMed] [Google Scholar]
- 95.Mamo N., Tak L.M., Olde Hartman T.C., Rosmalen J.G.M., Hanssen D.J.C. Strategies to improve implementation of collaborative care for functional disorders and persistent somatic symptoms: a qualitative study using a Research World Cafe design. J Psychosom Res. 2024;181 doi: 10.1016/j.jpsychores.2024.111665. [DOI] [PubMed] [Google Scholar]
- 96.Knapstad M., Lervik L.V., Saether S.M.M., Aaro L.E., Smith O.R.F. Effectiveness of prompt mental health care, the Norwegian version of improving access to psychological therapies: a randomized controlled trial. Psychother Psychosom. 2020;89(2):90–105. doi: 10.1159/000504453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Community and Mental Health Team . Annual report on the use of IAPT services, England 2017-2018. NHS Digital; London: 2018. Psychological therapies. [Google Scholar]
- 98.Rosmalen J.G.M., van Gils A., Acevedo Mesa M.A., Schoevers R.A., Monden R., Hanssen D.J.C. Development of Grip self-help: an online patient-tailored self-help intervention for functional somatic symptoms in primary care. Internet Interv. 2020;19 doi: 10.1016/j.invent.2019.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lalouni M., Ljotsson B., Bonnert M., et al. Clinical and cost effectiveness of online cognitive behavioral therapy in children with functional abdominal pain disorders. Clin Gastroenterol Hepatol. 2019;17(11):2236–2244.e11. doi: 10.1016/j.cgh.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 100.Jones A.S.K., Harding S., Seaton N., et al. A real-world longitudinal study implementing digital screening and treatment for distress in inflammatory bowel disease (IBD): the COMPASS-IBD study protocol. Contemp Clin Trials. 2024;145 doi: 10.1016/j.cct.2024.107658. [DOI] [PubMed] [Google Scholar]
- 101.Rask M.T., Frostholm L., Hansen S.H., Petersen M.W., Ornbol E., Rosendal M. Self-help interventions for persistent physical symptoms: a systematic review of behaviour change components and their potential effects. Health Psychol Rev. 2024;18(1):75–116. doi: 10.1080/17437199.2022.2163917. [DOI] [PubMed] [Google Scholar]
- 102.Dahlhausen F., Zinner M., Bieske L., Ehlers J.P., Boehme P., Fehring L. Physicians' attitudes toward prescribable mHealth apps and implications for adoption in Germany: mixed methods study. JMIR Mhealth Uhealth. 2021;9(11) doi: 10.2196/33012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johansen M.L., Risor M.B. What is the problem with medically unexplained symptoms for GPs? A meta-synthesis of qualitative studies. Patient Educ Counsel. 2017;100(4):647–654. doi: 10.1016/j.pec.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 104.Nagel C., Queenan C., Burton C. What are medical students taught about persistent physical symptoms? A scoping review of the literature. BMC Med Educ. 2024;24(1):618. doi: 10.1186/s12909-024-05610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marcum J.A. Biomechanical and phenomenological models of the body, the meaning of illness and quality of care. Med Health Care Philos. 2004;7(3):311–320. doi: 10.1007/s11019-004-9033-0. [DOI] [PubMed] [Google Scholar]
- 106.van Gils A., Tak L.M., Sattel H., Rosmalen J.G.M. Development and user experiences of a biopsychosocial interprofessional online course on persistent somatic symptoms. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.725546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barsky A.J., Silbersweig D.A. The amplification of symptoms in the medically ill. J Gen Intern Med. 2023;38(1):195–202. doi: 10.1007/s11606-022-07699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seto H., Nakao M. Relationships between catastrophic thought, bodily sensations and physical symptoms. Biopsychosoc Med. 2017;11:28. doi: 10.1186/s13030-017-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helmer D.A., Markowitz S., Quigley K. Interrelationships between symptom burden and health functioning and health care utilization among veterans with persistent physical symptoms. BMC Fam Pract. 2020;21:124. doi: 10.1186/s12875-020-01193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Staab J.P. Persistent postural-perceptual dizziness: review and update on key mechanisms of the most common functional neuro-otologic disorder. Neurol Clin. 2023;41(4):647–664. doi: 10.1016/j.ncl.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 111.Everitt H.A., Landau S., O'Reilly G., et al. Cognitive behavioural therapy for irritable bowel syndrome: 24-month follow-up of participants in the ACTIB randomised trial. Lancet Gastroenterol Hepatol. 2019;4(11):863–872. doi: 10.1016/S2468-1253(19)30243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patel M., James K., Moss-Morris R., et al. Persistent physical symptoms reduction intervention: a system change and evaluation (PRINCE)-integrated GP care for persistent physical symptoms: protocol for a feasibility and cluster randomised waiting list, controlled trial. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2018-025513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buchbinder R., Underwood M., Hartvigsen J., Maher C.G. The Lancet Series call to action to reduce low value care for low back pain: an update. Pain. 2020;161(Suppl 1):S57–S64. doi: 10.1097/j.pain.0000000000001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kluge H.H.P., Muscat N.A., Contribution f, et al. Call for action: health services in the European region must adopt integrated care models to manage Post-Covid-19 Condition. Lancet Reg Health Eur. 2022;18 doi: 10.1016/j.lanepe.2022.100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raasthoj I., Jarbol D.E., Rasmussen S., et al. Multiple physical symptoms and coping strategies over the last decade - knowledge from two Danish population-based cross-sectional studies in 2012 and 2022. J Psychosom Res. 2024;184 doi: 10.1016/j.jpsychores.2024.111832. [DOI] [PubMed] [Google Scholar]
- 116.Shen Q., Joyce E.E., Ebrahimi O.V., et al. COVID-19 illness severity and 2-year prevalence of physical symptoms: an observational study in Iceland, Sweden, Norway and Denmark. Lancet Reg Health Eur. 2023;35 doi: 10.1016/j.lanepe.2023.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Institute of Medicine (US) Committee on Pain, Disability, and Chronic Illness Behavior . National Academies Press (US); 2011. Pain as a public health challenge. [Google Scholar]
- 118.Domecq J.P., Prutsky G., Elraiyah T., et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matta J., Wiernik E., Robineau O., et al. Trust in sources of information on COVID-19 at the beginning of the pandemic's first wave and incident persistent symptoms in the population-based CONSTANCES cohort: a prospective study. J Psychosom Res. 2023;169 doi: 10.1016/j.jpsychores.2023.111326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maurer M., Dardess P., Rouse T.B. In: Patient engagement organizational behaviour in healthcare. Pomey M., Denis J.L., Dumez V., editors. Palgrave Macmillan; Cham: 2019. Patient and family engagement in the United States: a social movement from patient to advocate to partner. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.