Abstract
Introduction
Hepatic peliosis is a rare benign vascular disorder characterized by dilatation of sinusoidal blood-filled spaces within the liver. It often remains asymptomatic and is diagnosed incidentally in an autopsy. Herein, we report a case of a 1-day-old female newborn who was admitted to the neonatal resuscitation unit for abdominal distension.
Case presentation
An abdominal examination revealed a firm mass with irregular contours in the upper quadrant of the abdomen, indicating hepatomegaly. Radiological explorations confirmed the hepatomegaly, and an ultrasound-guided liver biopsy showed the presence of multiple blood cavities with sinusoidal dilation typical of hepatic peliosis.
Discussion
None of the known risk factors were found in this case except antihistaminic drug consumption during pregnancy by the mother, which could possibly have a link, although never described.
Conclusion
In this article, we report a case of neonatal hepatic peliosis likely secondary to antihistaminic consumption during pregnancy. To the best of our knowledge, this is the fifth reported case (based on the MEDLINE, Scopus, and Google Scholar databases) of neonatal hepatic peliosis.
Keywords: Peliosis hepatis, Hepatic peliosis, Hepatomegaly, Newborn, Neonatal, Case report
Highlights
-
•
Hepatic peliosis is a rare benign vascular disorder but can be fatal.
-
•
The mother's antihistamine drug use during pregnancy may be a potential risk factor.
-
•
To the best of our knowledge, this is the fifth reported case of neonatal hepatic peliosis.
1. Introduction
First described by Wagner in 1861 [1] and named by Schoenlank in 1916 [2], hepatic peliosis is a rare benign vascular disorder characterized by dilatation of sinusoidal blood-filled spaces within the liver [3].
It is a rare disease that often remains asymptomatic and is diagnosed incidentally at an autopsy. In some cases, it's revealed by a brutal hemorrhagic shock due to liver rupture [4], which makes it an uncommon pathology that should not be ignored.
It is challenging to arrive at an accurate diagnosis using imaging investigations alone, despite the importance of differential diagnosis from other hypervascular diseases, including malignancy. Thus, histological assessment is sometimes carried out in conjunction with surgery or echo-guided needle biopsy to determine the accurate diagnosis [5].
In this article, we report a case of neonatal hepatic peliosis, probably due to antihistaminic consumption during pregnancy. To the best of our knowledge, this is the fifth reported case (based on the MEDLINE, Scopus, and Google Scholar databases) of neonatal hepatic peliosis. The work has been reported in line with the SCARE 2023 criteria [6].
2. Presentation of case
A 1-day-old Moroccan female newborn was admitted to the neonatal resuscitation unit for abdominal distension. The baby was born out of a non-consanguineous marriage, at term (38 weeks old), via lower caesarean section, weighed 3.8 kg, and had an Apgar score of 10/10 at 1, 5, and 10 min of life. The mother had no history of any pathology, smoking, alcohol consumption, or illicit drug use. Standard prenatal serological tests were negative. There was no history of liver disease.
Her temperature was 37 °C, her pulse was 145 beats per minute, her blood pressure was 65/45 mmHg, her respiratory rate was 44 breaths per minute, and her oxygen saturation was 98 % in ambient air at the time of admission.
Abdominal examination showed a firm mass with irregular contours in the upper quadrant of the abdomen, palpable 9 cm below the costal margin. There was no splenomegaly and no signs of portal hypertension. The rest of the examination was normal.
Initial biological assessment was strictly normal for the age (Table 1).
Table 1.
Biological tests.
| Day 1 | Day 2 | Normal range value | |
|---|---|---|---|
| Hemoglobin (g/dL) | 12.8 | – | 12.5–20.5 |
| White blood count (x103 cells/mm3) | 16.7 | – | 5–21 |
| Platelets (x103 cells/mm3) | 357 | – | 170–500 |
| C-reactive protein (mg/L) | 15.5 | – | <5 |
| Blood glucose (g/L) | 0.87 | – | |
| AST (UI/L) | 46 | – | <35 |
| ALT (UI/L) | 8 | – | <35 |
| GGT (UI/L) | 103 | – | 100–500/mm3 |
| LDH (UI/L) | 1095 | – | <40 |
| Prothrombin time (s) | 100 | – | 70–100 |
| Na+ (mmol/L) | 142 | – | 136–145 |
| K+ (mmol/L) | 4 | – | 3.5–5.1 |
| Cl- (mmol/L) | 109 | – | 98–107 |
| HCO3− (mmol/L) | 16.7 | – | 22–29 |
| Serum protein (g/L) | 46 | – | 44–76 |
| Ca2+ (mg/L) | 87 | – | 76–104 |
| Adrenalin (μmol/L) | – | <0.02 | |
| Noradrenalin (μmol/L) | – | 0.12 | |
| Dopamine (μmol/mmol) | – | 0.41 | <2 |
| α-Fetoprotein (UI/ml) | – | 38,039 | 13,000–83,000 |
| CMV | Negative | ||
| EBV | Negative | ||
| HIV | Negative | ||
| HSV (1–2) | Negative |
Abdominal ultrasonography showed a large hyperechoic area of segments V, VI, and VII, well defined, measuring 84 × 27 mm with a small biliary tract dilatation (Fig. 1, Fig. 2).
Fig. 1.
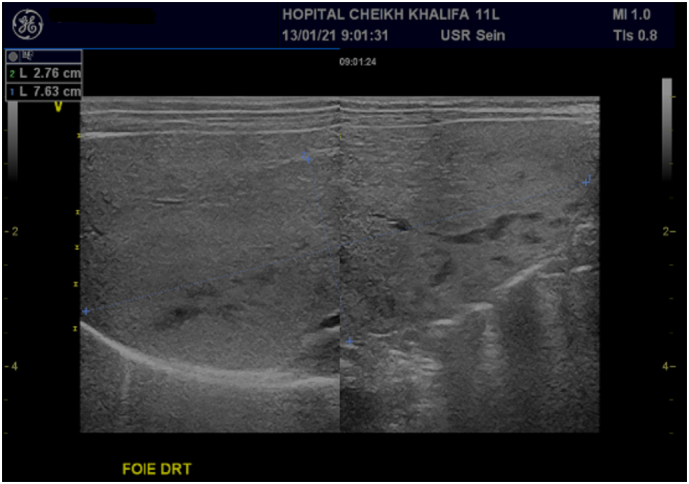
Abdominal ultrasonography showing biliary tract small dilatation.
Fig. 2.
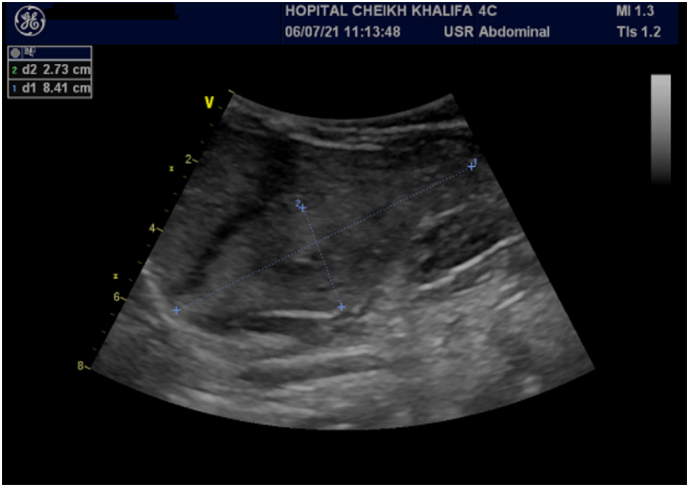
Abdominal ultrasonography showing a large hyperechoic area of segment V, VI and VII, well defined, measuring. 84 × 27 mm.
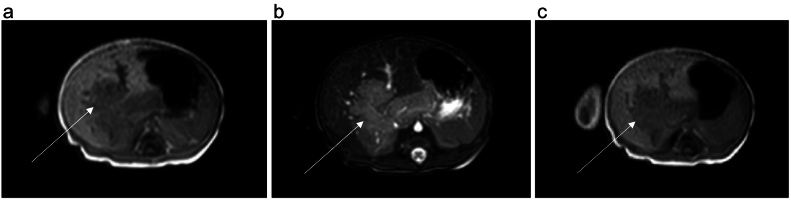
We completed the radiological investigations with a hepatic MRI, which showed an infiltrating hepatic mass poorly limited with lobulated contours in hyposignal T1, hypersignal T2 and hypersignal diffusion measuring 105 × 58 × 512 mm. It is enhanced early and intensely after injection of gadolinium (Fig. 3).
Fig. 3.
Hepatic MRI with different sequences.
A: Hepatic MRI T1 sequence which showing an infiltrating hepatic mass, poorly limited with lobulated contours in hyposignal T1.
B: Hepatic MRI T2 sequence which showing an infiltrating hepatic mass, poorly limited with lobulated contours in hypersignal T2.
C: Hepatic MRI T1-Gado which showing an enhanced early and intensely infiltrating hepatic mass after injection of gadolinium.
More investigations, including serum tumor markers (alpha-fetoprotein), viral serology for cytomegalovirus (CMV), Epstein-Barr virus (EBV), HIV, and herpes virus, were negative.
An ultrasound-guided liver biopsy was performed. The histological study showed a remodeled and congested liver parenchyma characterized by peliosis. There was the presence of multiple blood cavities with sinusoidal dilation typical of hepatic peliosis (Fig. 4). A diagnosis of hepatic peliosis was made, but the etiology remained unknown.
Fig. 4.
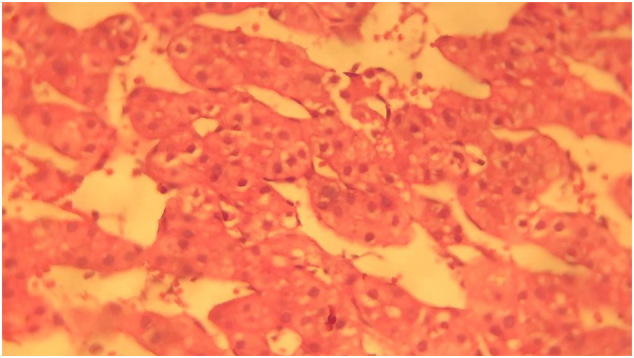
Presence of multiple blood cavities with sinusoidal dilation typical of hepatic peliosis (HE, Mx40).
Upon further questioning, we found a three-month antihistaminic drug consumption (ketotifen) during pregnancy.
Given the clinical tolerance, we pursued therapeutic abstention and close monitoring.
The patient was healthy until 3 year and 2-month-old and continued her follow-up in our hospital. The control abdominal ultrasonography found the persistence of the mass with a relatively stable appearance, well defined, measuring 90 × 34 cm, and the disappearance of the dilatation of the bile ducts currently (Fig. 5).
Fig. 5.
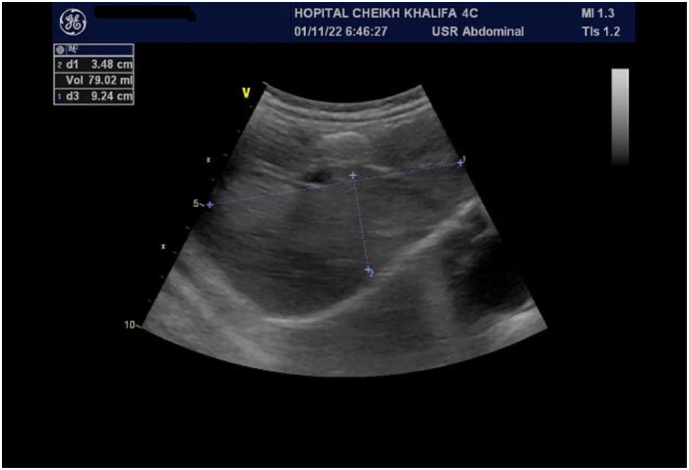
Control abdominal ultrasonography showing the persistence of the mass with relatively stable appearance measuring 90 × 34 cm and disappearance of the dilatation of the bile ducts.
3. Discussion
Hepatic peliosis (also called peliosis hepatis) is a rare vascular disorder characterized by livid purpura extravasating blood. The proliferation of the liver's sinusoids induces an engorgement of the liver's capillaries and cavities [3]. It can also affect the lungs, spleen, lymph nodes, bone marrow, parathyroid gland, and kidneys [7]. Although this condition usually emerges in adulthood, there are few reports in pediatric age group [8]. Only four neonatal cases exist in the medical literature [[8], [9], [10], [11]]. Our case is the fifth case of hepatic peliosis in a newborn.
The physiopathology of this disease remains unknown. Many risk factors have been found in case reports. We can split the etiologic factors into infectious and non-infectious causes. Infectious agents include the human immunodeficiency virus, tuberculosis, Escherichia coli, Bartonella henselae, and the Rickettsia family [8,12]. Non-infectious causes include drugs (such as androgenic steroids, tamoxifen, contraceptive steroids, and corticosteroids), malignancies (Hodgkin's lymphoma), autoimmune, and renal diseases. The etiologies of hepatic peliosis were only identified in 20–50 % of patients [5].
Hepatic peliosis is linked in the pediatric population to Escherichia coli infection, cystic fibrosis, malnutrition, Fanconi anemia, adrenal tumors, Marfan syndrome, congenital cardiopathy, myotubular myopathy, and postrenal transplant [8]. However, in the adults, it is often associated with androgenic and contraceptive steroids intake [11] (Table 2).
Table 2.
Hepatic peliosis in the newborn population.
| Author | Year | Sex | Age (day old) | Presentation | Etiology, risk factor | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Kawamato et al. [9] | 1980 | M | 1 | Hepatomegaly | – | – | – | Died |
| Bracero et al. [10] | 1995 | F | 1 | – | – | Open liver biopsy | – | – |
| Grzywacz et al. [8] | 2012 | M | 1 | Cholestasis | Consumption of green tea contaminated with pesticides during pregnancy | Surgical hepatic biopsy | Liver transplant | Alive |
| Singh et al. [11] | 2020 | 16 | Hepatomegaly | Androgen producing adrenal tumor | Autopsy in post-mortem | – | Died | |
| Lahlou et al. | 2021 | F | 1 | Hepatomegaly | Consumption of antihistaminic drugs during pregnancy | Ultrasound-guided liver biopsy | Therapeutic abstention | Alive |
Hepatic peliosis is difficult to recognize. The diagnosis is often delayed or even missed due to its rarity and imaging appearance. The radiological aspect can be more suggestive of multiple abscesses or malignant diseases. The clinical presentation is often asymptomatic. In some cases, it is revealed by hepatomegaly, ascites, portal hypertension, cholestasis, or hepatic failure. Some publications report cases of hemorrhagic shock causing death due to liver rupture [4].
The imaging features of hepatic peliosis are nonspecific. On US, it manifests as hypoechoic or hyperechoic nodules, as well as diffuse heterogeneous hepatic echogenic texture [13]. On imaging with noncontrast CT, peliosis is characterized by one or multiple low-attenuating lesions [14]. On contrast MR, the appearance depends on the age of blood products within the lacunae, with a common appearance of hyper-intensity on T2-weighted sequences and with hypo-intensity or isointensity on T1-weighted sequences [4]. Underlying hemorrhagic necrosis may result in intrinsic T1 hyper-intensity. On postconstrast CT and MRI, multiple imaging appearances have been described, including lesions appearing as hypo attenuating compared with adjacent parenchyma, as well as centrifugal enhancement [4]. Centripetal pattern of enhancement has also been described [15].
Therefore, it has been reported that it is difficult to distinguish hepatic peliosis from other liver malignancies using US, contrast-enhanced CT, and conventional MRI.
In some recent studies, it is characterized by cystic blood-filled cavities distributed randomly throughout the liver parenchyma and typically involves the entire normal liver on MRI with Gd-EOB, which is the “liver-specific” contrast material of MRI [13].
But more studies with larger samples are necessary to confirm the contribution of hepatospecific contrast agents in the characterization of peliosis hepatis lesions.
The definitive diagnosis is histological.
There is no specific treatment for hepatic peliosis. Eviction of the causative drug or toxin can resolve the disease and prevent serious complications. The treatment is primarily supportive in cases of intraperitoneal hemorrhage [16]. Many of the pediatric cases reported spontaneous resolution. Liver transplantation has been reported in some cases [8].
4. Conclusion
Although rare and difficult to diagnose, hepatic peliosis is an important pathology to consider in neonates with a right upper quadrant mass. Most of the time, it is revealed by hepatomegaly, sometimes by cholestasis, and rarely by hemorrhagic shock.
Various manifestations of hepatic peliosis may arise during imaging, which makes it challenging to diagnose. Awareness of the imaging findings of this disorder is crucial to suggest the diagnosis. It should always be considered in the differential diagnosis of atypical focal hepatic lesions in patients with an evocative clinic.
Here, we report the fifth case of neonatal hepatic peliosis (based on the MEDLINE, Scopus, and Google Scholar databases).
CRediT authorship contribution statement
Wahib Lahlou: Corresponding author and writing the manuscript.
Abderrahim Bourial: writing the manuscript.
Amal Rami: writing and investigation on the radiological part.
Abderrahmane Al Bouzidi: interpretating the histological exam.
Mouad Nejjari: investigation.
Inssaf Al Ammari: writing and validation of the manuscript.
Consent
Written informed consent was obtained from the patient's parents/legal guardian for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
No ethical approval was needed for this case report.
Guarantor
Wahib Lahlou.
Sources of funding
No sources of funding to declare.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgements
None.
Data availability
The data used and analyzed are available from the corresponding author on reasonable request.
References
- 1.Wagner E. Fall von Blutcysten der Leber. Arch Heilk. 1861;2:369–370. [Google Scholar]
- 2.Schoenlank W. Ein Fall von Peliosis hepatis. Virchows Arch Für Pathol Anat Physiol Für Klin Med. 1916;222(3):358–364. doi: 10.1007/BF02026467. [DOI] [Google Scholar]
- 3.Zak F.G. Peliosis hepatis. Am. J. Pathol. 1950;26(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- 4.Iannaccone R., Federle M.P., Brancatelli G., Matsui O., Fishman E.K., Narra V.R., Grazioli L., McCarthy S.M., Piacentini F., Maruzzelli L., Passariello R., Vilgrain V. Peliosis hepatis: spectrum of imaging findings. AJR Am. J. Roentgenol. 2006 Jul;187(1):W43–W52. doi: 10.2214/AJR.05.0167. (PMID: 16794138) [DOI] [PubMed] [Google Scholar]
- 5.Dai W., Zhong D. Peliosis hepatis mimicking cancer: a case report. Oncol. Lett. 2013;6(4):960–962. doi: 10.3892/ol.2013.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsokos M., Erbersdobler A. Pathology of peliosis. Forensic Sci. Int. 2005;149(1):25–33. doi: 10.1016/j.forsciint.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Grzywacz K., Brochu P., Beaunoyer M., Lallier M., Alvarez F. Neonatal peliosis with maternal ingestion of pesticides. J. Pediatr. Gastroenterol. Nutr. 2014;58(2) doi: 10.1097/MPG.0b013e3182716b4c. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto S., Wakabayashi T. Peliosis hepatis in a newborn infant. Arch. Pathol. Lab. Med. 1980;104(8):444–445. [PubMed] [Google Scholar]
- 10.Bracero L.A., Gambon T.B., Evans R., Beneck D. Ultrasonographic findings in a case of congenital peliosis hepatitis. J. Ultrasound Med. 1995;14(6):483–486. doi: 10.7863/jum.1995.14.6.483. [DOI] [PubMed] [Google Scholar]
- 11.Singh S., Chhikara A., Anand A., Agarwal K., Nangia S., et al. Peliosis hepatis: a rare cause of hepatomegaly in a newborn. OSP J Health Car Med. 2020;1 HCM-1-110. [Google Scholar]
- 12.Maruyama H., Takahashi K., Ishikawa N., et al. A rare case of peliosis hepatis in a patient with chronic renal failure and renal cell carcinoma. Clin. J. Gastroenterol. 2020;13(3):403–407. doi: 10.1007/s12328-019-01068-5. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida M., Utsunomiya D., Takada S., et al. The imaging findings of peliosis hepatis on gadoxetic acid enhanced MRI. Radiol Case Rep. 2020;15(8):1261–1265. doi: 10.1016/j.radcr.2020.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gouya H., Vignaux O., Legmann P., de Pigneux G., Bonnin A. Peliosis hepatis: triphasic helical CT and dynamic MRI findings. Abdom. Imaging. 2001;26(5):507–509. doi: 10.1007/s00261-001-0023-x. [DOI] [PubMed] [Google Scholar]
- 15.Steinke K., Terraciano L., Wiesner W. Unusual cross-sectional imaging findings in hepatic peliosis. Eur. Radiol. 2003;13(8):1916–1919. doi: 10.1007/s00330-002-1675-9. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu S., Sakamoto S., Fukuda A., et al. Living-donor liver transplantation for liver hemorrhaging due to peliosis hepatis in X-linked myotubular myopathy: two cases and a literature review. Am. J. Transplant. 2020;20(9):2606–2611. doi: 10.1111/ajt.15978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analyzed are available from the corresponding author on reasonable request.