Abstract
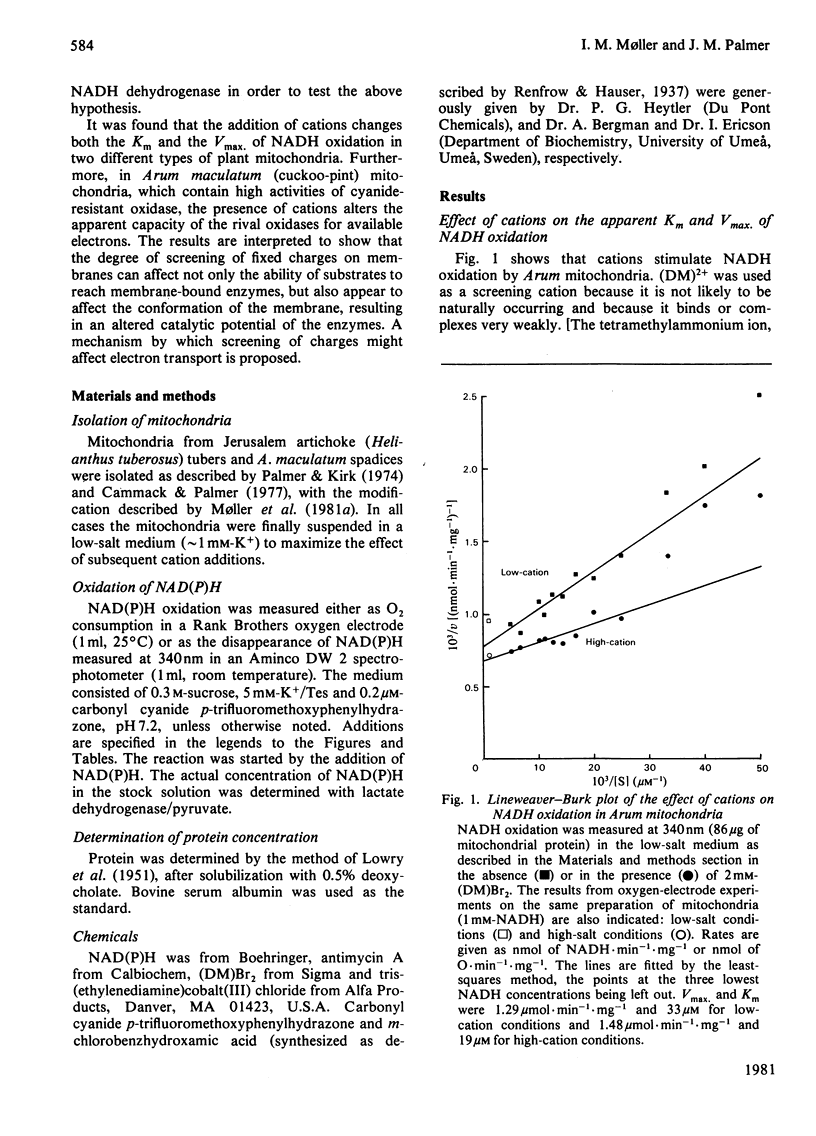
Cations caused a decrease in the apparent Km and an increase in the Vmax. for the oxidation of exogenous NADH by both Jerusalem-artichoke (Helianthus tuberosus) and Arum maculatum (cuckoo-pint) mitochondria prepared and suspended in a low-cation medium (approximately or equal to 1 mM-K+). In Arum mitochondria the addition of cations caused a much greater stimulation of the oxidation of NAD(P)H via the cytochrome oxidase pathway than via the alternative, antimycin-insensitive, pathway. This shows that cations affected a rate-limiting step in the electron-transport chain at or beyond ubiquinone, the branch-point of electron transport in plant mitochondria. The effects were only dependent on the valency of the cation (efficiency C3+ greater than C2+ greater than C+) and not on its chemical nature, which is consistent with the theory of the diffuse layer. The results are interpreted to show that the screening of fixed negative membrane changes on lipids and protein complexes causes a conformational change in the mitochondrial inner membrane, leading to a change in a rate-limiting step of NAD(P)H oxidation. More specifically, it is proposed that screening removes electrostatic restrictions on lateral diffusion and thus accelerates diffusion-limited steps in electron transport.
Full text
PDF
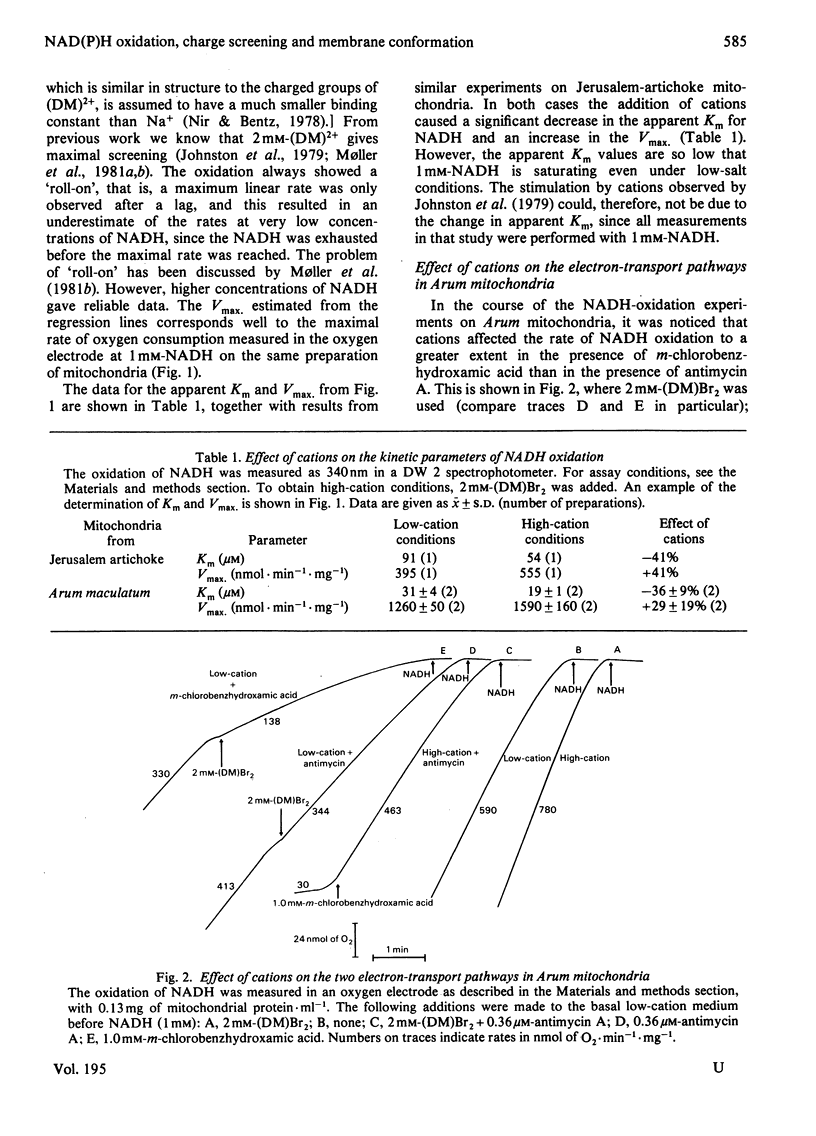



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arron G. P., Edwards G. E. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by plant mitochondria. Can J Biochem. 1979 Dec;57(12):1392–1399. doi: 10.1139/o79-185. [DOI] [PubMed] [Google Scholar]
- Cammack R., Palmer J. M. Iron-sulphur centres in mitochondria from Arum maculatum spadix with very high rates of cyanide-resistant respiration. Biochem J. 1977 Sep 15;166(3):347–355. doi: 10.1042/bj1660347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engasser J. M., Horvath C. Electrostatic effects on the kinetics of bound enzymes. Biochem J. 1975 Mar;145(3):431–435. doi: 10.1042/bj1450431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN L., LEVIN Y., KATCHALSKI E. A WATER-INSOLUBLE POLYANIONIC DERIVATIVE OF TRYPSIN. II. EFFECT OF THE POLYELECTROLYTE CARRIER ON THE KINETIC BEHAVIOR OF THE BOUND TRYPSIN. Biochemistry. 1964 Dec;3:1913–1919. doi: 10.1021/bi00900a022. [DOI] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Hornby W. E., Lilly M. D. Some changes in the reactivity of enzymes resulting from their chemical attachment to water-insoluble derivatives of cellulose. Biochem J. 1968 May;107(5):669–674. doi: 10.1042/bj1070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. P., Møller I. M., Palmer J. M. The stimulation of exogenous NADH oxidation in Jerusalem artichoke mitochondria by screening of charges on the membranes. FEBS Lett. 1979 Dec 1;108(1):28–32. doi: 10.1016/0014-5793(79)81171-0. [DOI] [PubMed] [Google Scholar]
- Koeppe D. E., Miller R. J. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by isolated corn mitochondria. Plant Physiol. 1972 Mar;49(3):353–357. doi: 10.1104/pp.49.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P., Douzou P. Catalytic implications of electrostatic potentials: the lytic activity of lysozymes as a model. J Mol Biol. 1976 Apr 5;102(2):253–264. doi: 10.1016/s0022-2836(76)80052-6. [DOI] [PubMed] [Google Scholar]
- McCarty R. E., Douce R., Benson A. A. The acyl lipids of highly purified plant mitochondria. Biochim Biophys Acta. 1973 Aug 23;316(2):266–270. doi: 10.1016/0005-2760(73)90019-2. [DOI] [PubMed] [Google Scholar]
- Møller I. M., Johnston S. P., Palmer J. M. A specific role for Ca2+ in the oxidation of exogenous NADH by Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1981 Feb 15;194(2):487–495. doi: 10.1042/bj1940487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J., Forrester J. A. Surface charges on chloroplast membranes as studied by particle electrophoresis. Biochim Biophys Acta. 1978 Oct 11;504(1):215–225. doi: 10.1016/0005-2728(78)90019-1. [DOI] [PubMed] [Google Scholar]
- Nishihara M., Yokota K., Kito M. Lipid molecular species composition of thylakoid membranes. Biochim Biophys Acta. 1980 Jan 18;617(1):12–19. doi: 10.1016/0005-2760(80)90219-2. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Kirk B. I. The influence of osmolarity on the reduction of exogenous cytochrome c and permeability of the inner membrane of Jerusalem artichoke mitochondria. Biochem J. 1974 Apr;140(1):79–86. doi: 10.1042/bj1400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Lemasters J. J., Höchli M., Hackenbrock C. R. Liposome-mitochondrial inner membrane fusion. Lateral diffusion of integral electron transfer components. J Biol Chem. 1980 Apr 25;255(8):3748–3756. [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G. F., Barber J., Mills J. D. 9-amino-acridine as a probe of the electrical double layer associated with the chloroplast thylakoid membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):413–425. doi: 10.1016/0005-2728(77)90230-4. [DOI] [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton C. W., Crook E. M., Brocklehurst K. The nature of the perturbation of the michaelis constant of the bromelain-catalysed hydrolysis of alpha-N-benzoyl-L-arginine ethyl ester consequent upon attachment of bromelain to O-(carboxymethyl)-cellulose. Eur J Biochem. 1968 Dec 5;6(4):572–578. doi: 10.1111/j.1432-1033.1968.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Wojtczak L., Nałecz M. J. Surface change of biological membranes as a possible regulator of membrane-bound enzymes. Eur J Biochem. 1979 Feb 15;94(1):99–107. doi: 10.1111/j.1432-1033.1979.tb12876.x. [DOI] [PubMed] [Google Scholar]


