Abstract
The arrangement of the proton-translocating formate dehydrogenase of the anaerobic respiratory chain of Escherichia coli within the cytoplasmic membrane was examined by direct covalent modification with non-membrane-permeant reagents. Three methods were employed, lactoperoxidase-catalysed radioiodination, labelling with diazotized [125I] di-iodosulphanilic acid and labelling with diazobenzene [35S] sulphonate. All three procedures yield consistent with the view that the two larger subunits of the enzyme, Mr 110000 and 32000, both occupy transmembranous locations within the membrane. In each case the modification of the Ca2+ or Mg2+-activated F1-ATPase was monitored, and all reagents employed correctly located this enzyme at the cytoplasmic face of the membrane. A procedure involving agglutination with specific antibodies is described which appears to fractionate membrane vesicles of mixed orientation into two populations, one with the same membrane orientation as that of spheroplasts and the other opposite orientation.
Full text
PDF




Selected References
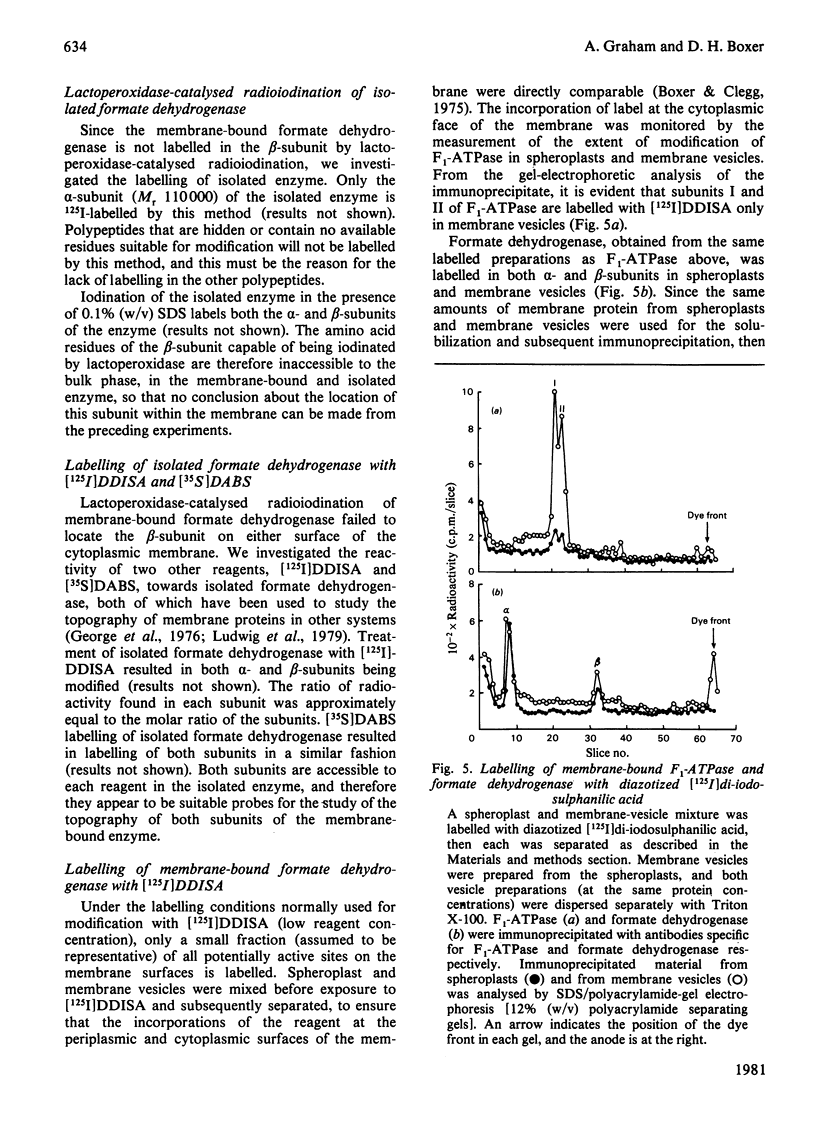
These references are in PubMed. This may not be the complete list of references from this article.
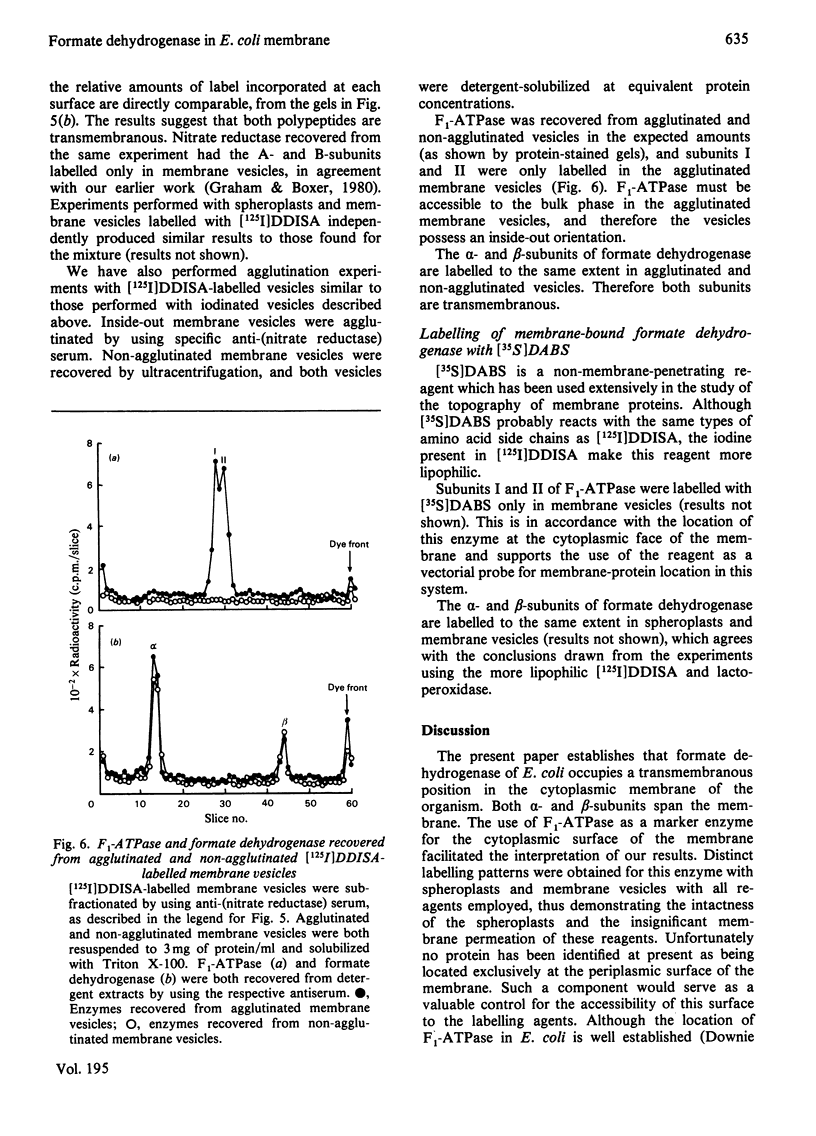
- Boxer D. H., Clegg R. A. A transmembrane location for the proton-translocating reduced ubiquinone leads to nitrate reductase segment of the respiration chain of Escherichia coli. FEBS Lett. 1975 Dec 1;60(1):54–57. doi: 10.1016/0014-5793(75)80417-0. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Hughes R. C. Surface labelling for human tumour KB cells. Iodination and fractionation of membrane glycoproteins. Biochem J. 1975 Jul;150(1):59–69. doi: 10.1042/bj1500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Potterf R. D., Lewis P. C., Sears D. A. Studies on platelet plasma membranes. I. Characterization of surface proteins of human platelets labeled with diazotized (125i)-diiodosulfanilic acid. J Lab Clin Med. 1976 Aug;88(2):232–246. [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Arrangement of respiratory nitrate reductase in the cytoplasmic membrane of Escherichia coli. Location of beta subunit. FEBS Lett. 1980 Apr 21;113(1):15–20. doi: 10.1016/0014-5793(80)80484-4. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Immunochemical localization of nitrate reductase in Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(6):1210–1211. doi: 10.1042/bst0061210. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp R. W., Sears D. A. A label for the red cell membrane: diazotized diiodosulfanilic acid. Int J Appl Radiat Isot. 1970 Nov;21(11):683–685. doi: 10.1016/0020-708x(70)90127-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Downer N. W., Capaldi R. A. Labeling of cytochrome c oxidase with [35S]diazobenzenesulfonate. Orientation of this electron transfer complex in the inner mitochondrial membrane. Biochemistry. 1979 Apr 17;18(8):1401–1407. doi: 10.1021/bi00575a002. [DOI] [PubMed] [Google Scholar]
- Mendel-Hartvig I., Nelson B. D. Lableing of complex III peptides in beef heart mitochondria and submitochondrial particles by diazonium benezene [35S]sulfonate. FEBS Lett. 1978 Aug 1;92(1):36–40. doi: 10.1016/0014-5793(78)80716-9. [DOI] [PubMed] [Google Scholar]
- Mersel M., Benenson A., Doljanski F. Lactoperoxidase-catalyzed iodination of surface membrane lipids. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1166–1171. doi: 10.1016/0006-291x(76)91025-1. [DOI] [PubMed] [Google Scholar]
- Norrild B., Bjerrum O. J., Vestergaard B. F. Polypeptide analysis of individual immunoprecipitates from crossed immunoelectrophoresis. Anal Biochem. 1977 Aug;81(2):432–441. doi: 10.1016/0003-2697(77)90714-x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Scott R. H., DeMoss J. A. Formation of the formate-nitrate electron transport pathway from inactive components in Escherichia coli. J Bacteriol. 1976 Apr;126(1):478–486. doi: 10.1128/jb.126.1.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Ragan C. I. The organization of NADH dehydrogenase polypeptides in the inner mitochondrial membrane. Biochem J. 1980 Feb 1;185(2):315–326. doi: 10.1042/bj1850315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinberg H. M., Melnick R. L., Maguire J., Packer L. Studies on mitochondrial proteins. II. Localization of components in the inner membrane: labeling with diazobenzenesulfonate, a non-penetrating probe. Biochim Biophys Acta. 1974 Apr 12;345(1):118–128. doi: 10.1016/0005-2736(74)90251-x. [DOI] [PubMed] [Google Scholar]
- Werner S. Isolation and characterisation of a mitochondrially synthesized precursor protein of cytochrome oxidase. Eur J Biochem. 1974 Mar 15;43(1):39–48. doi: 10.1111/j.1432-1033.1974.tb03382.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman B., Chapman M. L. Fractionation on lymphocyte surface antigens. I. Rapid method for eliminating labeled lipid from cell surface antigens iodinated by the lactoperoxidase catalysed reaction. J Immunol Methods. 1977;15(2):183–192. doi: 10.1016/0022-1759(77)90029-1. [DOI] [PubMed] [Google Scholar]


