Abstract
The peripheral high-affinity cyclic AMP phosphodiesterase from rat liver plasma membranes was purified to apparent homogeneity. The procedure used involved the initial purification of liver plasma membranes and the solubilization of the enzyme by using a high-ionic-strength medium. This was followed by chromatography of the enzyme on DEAE-cellulose, Affi-Gel Blue, a novel affinity column and Sephadex G-100. A 9500-fold purification of the enzyme with a 24% yield was achieved by this procedure. The purified enzyme was apparently monomeric (Mr 52000) as it exhibited identical molecular weights on analysis by gel filtration, sedimentation and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. It is suggested that the non-Michaelis kinetics exhibited by the enzyme are due to it obeying a mnemonical mechanism, where it displays Km 0.7 micrometer, Vmax. 9.1 units/mg of protein and Hill coefficient (h) 0.62. Cyclic GMP acts as a poor substrate for the enzyme, with Km 120 micrometer and Vmax. 0.4 unit/mg of protein, and also as an inhibitor of the enzyme, with I50 (concentration giving 50% inhibition) 150 micrometer when assayed at 0.4 micrometer-cyclic AMP. Inhibition by 5'-AMP is unlikely to be of physiological importance, as it is only a weak inhibitor of the enzyme (I50 47 mM assayed at 0.4 micrometer-cyclic AMP).
Full text
PDF


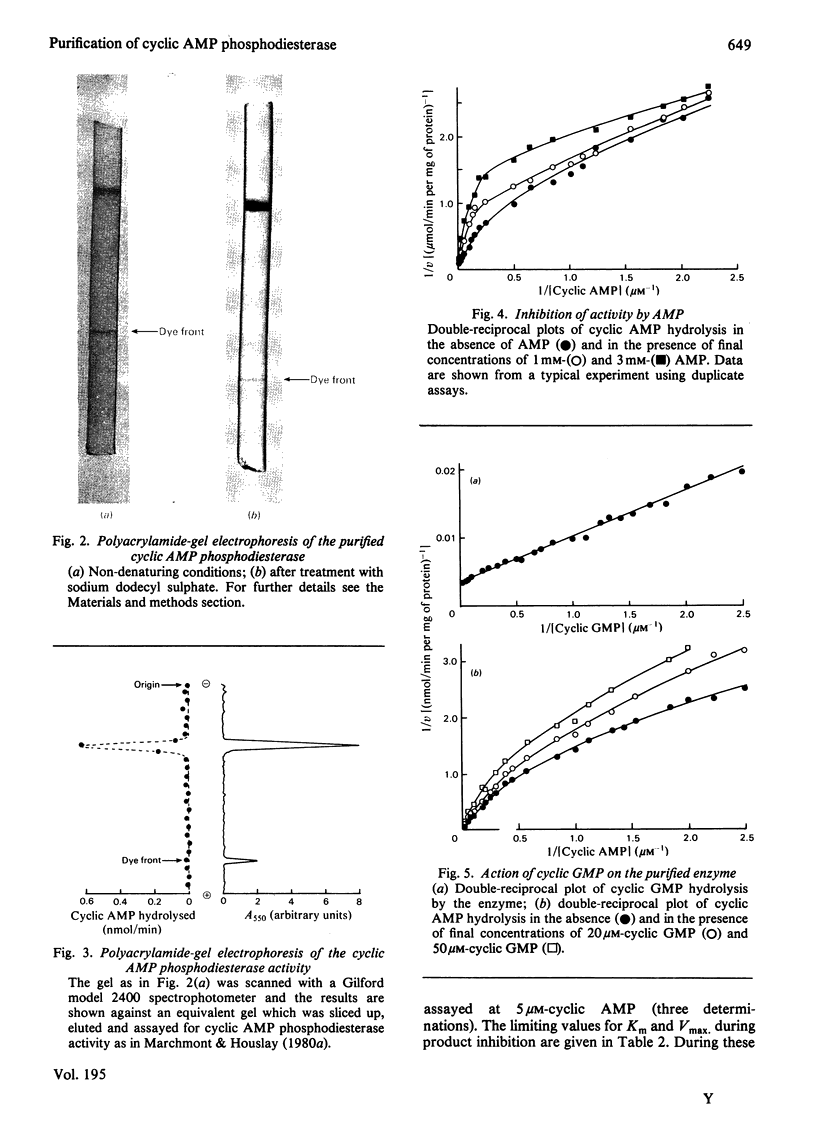



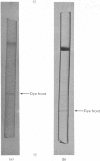
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. R., Jr, Shill J. P., Neet K. E. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J Biol Chem. 1972 Nov 10;247(21):7088–7096. [PubMed] [Google Scholar]
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Blackmore P. F., Assimacopoulos-Jeannet F., Chan T. M., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Insulin inhibition of alpha-adrenergic and glucagon actions in normal and calcium-depleted hepatocytes. J Biol Chem. 1979 Apr 25;254(8):2828–2834. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Erneux C., Boeynaems J. M., Dumont J. E. Theoretical analysis of the consequences of cyclic nucleotide phosphodiesterase negative co-operativity. Amplification and positive co-operativity of cyclic AMP accumulation. Biochem J. 1980 Oct 15;192(1):241–246. doi: 10.1042/bj1920241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D. A. Theoretical analyses of the functioning of the high- and low-Km cyclic nucleotide phosphodiesterases in the regulation of the concentration of adenosine 3',5'-cyclic monophosphate in animal cells. J Theor Biol. 1980 May 21;84(2):361–385. doi: 10.1016/s0022-5193(80)80011-7. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H. C., Wirch E., Stevens F. C., Wang J. H. Purification of a Ca2+-activatable cyclic nucleotide phosphodiesterase from bovine heart by specific interaction with its Ca2+-dependent modulator protein. J Biol Chem. 1977 Jan 10;252(1):43–50. [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Palmer R. W. Changes in the form of Arrhenius plots of the activity of glucagon-stimulated adenylate cyclase and other hamster liver plasma-membrane enzymes occurring on hibernation. Biochem J. 1978 Sep 15;174(3):909–919. doi: 10.1042/bj1740909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Subunit structure and catalytic properties of bovine brain Ca2+-dependent cyclic nucleotide phosphodiesterase. Biochemistry. 1979 Feb 20;18(4):722–729. doi: 10.1021/bi00571a026. [DOI] [PubMed] [Google Scholar]
- Lauter C. J., Solyom A., Trams E. G. Comparative studies on enzyme markers of liver plasma membranes. Biochim Biophys Acta. 1972 May 9;266(2):511–523. doi: 10.1016/0005-2736(72)90107-1. [DOI] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Characterization of the phosphorylated form of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):653–660. doi: 10.1042/bj1950653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Insulin controls the cyclic AMP-dependent phosphorylation of integral and peripheral proteins associated with the rat liver plasma membrane. FEBS Lett. 1980 Aug 25;118(1):18–24. doi: 10.1016/0014-5793(80)81209-9. [DOI] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Insulin trigger, cyclic AMP-dependent activation and phosphorylation of a plasma membrane cyclic AMP phosphodiesterase. Nature. 1980 Aug 28;286(5776):904–906. doi: 10.1038/286904a0. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Buc J., Navarro A., Ricard J. Regulatory behavior of monomeric enzymes. 2. A wheat-germ hexokinase as a mnemonical enzyme. Eur J Biochem. 1974 Nov 1;49(1):209–223. doi: 10.1111/j.1432-1033.1974.tb03826.x. [DOI] [PubMed] [Google Scholar]
- Morrill M. E., Thompson S. T., Stellwagen E. Purification of a cyclic nucleotide phosphodiesterase from bovine brain using blue dextran-Sepharose chromatography. J Biol Chem. 1979 Jun 10;254(11):4371–4374. [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Meunier J. C., Buc J. Regulatory behavior of monomeric enzymes. 1. The mnemonical enzyme concept. Eur J Biochem. 1974 Nov 1;49(1):195–208. doi: 10.1111/j.1432-1033.1974.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Wang T. H., Wirch E., Wang J. H. Purification and properties of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biol Chem. 1980 Jun 25;255(12):5916–5923. [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetic evidence for a 'mnemonical' mechanism for rat liver glucokinase. Biochem J. 1977 Jul 1;165(1):61–69. doi: 10.1042/bj1650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Toda G., Oka H., Oda T., Ikeda Y. Subfractionation of rat liver plasma membrane. Uneven distribution of plasma membrane-bound enzymes on the liver cell surface. Biochim Biophys Acta. 1975 Nov 17;413(1):52–64. doi: 10.1016/0005-2736(75)90058-9. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Hardman J. G. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1977;8:119–143. [PubMed] [Google Scholar]
- Whitehead E. The regulation of enzyme activity and allosteric transition. Prog Biophys Mol Biol. 1970;21:321–397. doi: 10.1016/0079-6107(70)90028-3. [DOI] [PubMed] [Google Scholar]