Abstract
Currently, there is a gap in understanding the neurobiological impact early adolescent toluene exposure has on subsequent actions of other drugs. Adolescent (PND 28–32) male Swiss-Webster mice (N = 210) were exposed to 0, 2000, or 4000 ppm of toluene vapor for 30 min/day for 5 days. Immediately following the last toluene exposure (PND 32; n = 15) or after a short delay (PND 35; n = 15), a subset of subjects’ brains was collected for monoamine analysis. Remaining mice were assigned to one of two abstinence periods: a short 4-day (PND 36) or long 12-day (PND 44) delay after toluene exposure. Mice were then subjected to a cumulative dose response assessment of either cocaine (0, 2.5, 5, 10, 20 mg/kg; n = 60), ethanol (0, 0.5, 1, 2, 4 g/kg; n = 60), or saline (5 control injections; n = 60). Toluene concentration-dependently increased locomotor activity during exposure. When later challenged, mice exposed previously to toluene were significantly less active after cocaine (10 and 20 mg/kg) compared to air-exposed controls. Animals were also less active at the highest dose of alcohol (4 g/kg) following prior exposure to 4000 ppm when compared to air-exposed controls. Analysis of monoamines and their metabolites using High Pressure Liquid Chromatography (HPLC) within the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), dorsal striatum (dSTR), and ventral tegmental area (VTA) revealed subtle effects on monoamine or metabolite levels following cumulative dosing that varied by drug (cocaine and ethanol) and abstinence duration. Our results suggest that early adolescent toluene exposure produces behavioral desensitization to subsequent cocaine-induced locomotor activity with subtle enhancement of ethanol’s depressive effects and less clear impacts on levels of monoamines.
Keywords: Inhalant, Polydrug, Adolescence, Gateway, Animal model
1. Introduction
Drug use during adolescence is of great concern as this period is one of substantial behavioral, social, physical, and neurochemical changes (Bava and Tapert, 2010; Doremus-Fitzwater et al., 2010; Spear, 2000a). One understudied class of drugs are the inhalants, despite their high rates of initiation and use predominantly among children and young adolescents (Cruz and Bowen, 2021; Lubman et al., 2008). There are gaps in what we know about how physiological brain changes resulting from early inhalant abuse influence subsequent neurobehavioral problems, including substance dependence (Aydin et al., 2009; Cairney et al., 2004; Kouzoupis et al., 2010; Papageorgiou et al., 2009; Woodward and Beckley, 2014). Inhalant abuse is prevalent among 8th graders in the U. S. and in 2022, 9.8% reported using inhalants at some time. Inhalant abuse ranks fourth behind alcohol (23.1%), vaping nicotine (17.0%), and marijuana (11.8%; Miech et al., 2023). Roughly 2.2 million people in the U.S. aged 12 and older reported inhalant use in 2021 (SAMHSA, 2022).
Compared to most other drug classes, inhalant abuse is higher during early adolescence than in older groups (Johnston et al., 2019). Inhalant abuse tends to decline as individuals age due to progression to other drugs of abuse, suggesting that inhalants are gateway drugs leading to other drug use (Crossin et al., 2017; Crossin et al., 2018; Miech et al., 2023). Acute or long-term inhalant abuse has been shown to influence progress to use of substances abuse (Crossin and Arunogiri, 2020; Crossin et al., 2017; Crossin et al., 2019a; Crossin et al., 2019b; Crossin et al., 2018). While progression to use and abuse of other drugs are influenced by social and individual factors, questions about the impact of earlier exposures to inhalants on behavioral and neural effects of other drugs of abuse remain.
One of the most commonly abused inhalants is toluene (Cruz and Bowen, 2021; Violante-Soria et al., 2019). Toluene is lipid soluble and readily absorbed through lung tissue and distributed into many organs throughout the body, especially brain (Ameno et al., 1992; Aydin et al., 2009; Aydin et al., 2002; Caldemeyer et al., 1996; Gospe Jr. and Calaban, 1988; Rosenberg et al., 1988; Yamanouchi et al., 1995). Toluene produces dose-dependent behavioral effects that range from initial excitation (i.e., increased locomotor activity) to motor disruption, ataxia, and loss of righting reflex, to anesthesia at concentrations similar to abuse situations (reviewed in Bowen et al., 2006; Cruz and Bowen, 2021) (Warren et al., 2000). Toluene influences glutamate and γ-amino butyric acid (GABA) by acting as an antagonist at N-methyl-d-aspartate (NMDA; now known as GluN) receptors, and as a positive allosteric modulator at GABA-A receptors (Bale et al., 2007; Callan, et al., 2017; Dick et al., 2015; Perrine et al., 2011). Through the action on glutamate and GABA, toluene affects the dopaminergic reward pathway and impacts dopamine release in the striatum (Apawu et al., 2015; Koga et al., 2007; Stengard et al., 1994; Woodward and Beckley, 2014; Zhvania et al., 2012). Both direct and indirect impact of toluene on these systems is still being explored (Apawu et al., 2020; Callan, et al., 2017; Dick et al., 2015; Nimitvilai et al., 2016; Wayman and Woodward, 2018a) and can be probed by provoking responses to other drugs, like ethanol and cocaine, which act upon amino acid transmitters and monoamine reward systems, respectively (Miech et al., 2023; SAMHSA, 2022).
Given that inhalant use tends to begin in early adolescence, it is important to explore the neurodevelopmental influences on the persistent effects of toluene on drug-related behavior and brain neurochemistry. The perturbation of the mesocorticolimbic dopaminergic pathway by inhalant use in early adolescence may influence responses to subsequent drugs of abuse and increase the likelihood of new, continued or increased drug use. For example some studies have reported greater and longer lasting ethanol sensitization after adolescent vs adult repeated ethanol exposure (Hefner and Holmes, 2007; Matthews et al., 2008; Moore et al., 2010; Pascual et al., 2009; Quoilin et al., 2012, 2014; Ristuccia and Spear, 2005; Spear and Varlinskaya, 2005), yet few studies have investigated inhalant exposure in adolescence on sensitization to other drugs. The purpose of this study was to assess in a mouse model how repeated toluene exposure during early adolescence affects subsequent responses to ethanol or cocaine in later adolescence/early adulthood. Mice were exposed during the periadolescent period to brief, repeated abuse-like concentrations of toluene (0, 2000, 4000 ppm) and observed later for their responses to cocaine (2.5–20 mg/kg) or ethanol (0.5–4 g/kg) in Cumulative Dose Response (CDR) assessments during mid or late adolescence (PND 36 or 44). Brains were collected after the initial toluene exposure, following a delay, or after the ethanol or cocaine challenge, and analyzed for monoamine levels by high pressure liquid chromatography (HPLC). It was hypothesized that early exposure to toluene would alter behavioral responses to the drug challenges and impact changes in monoamine levels after ethanol and cocaine administration.
2. Materials and methods
2.1. Subjects
Male Swiss-Webster mice (N = 210) were obtained from Envigo RMS (Indianapolis, IN, USA) on postnatal day (PND) 21 and were habituated to the vivarium until PND 27. Animals (n = 8) were housed in large static polypropylene cages (144 in2) lined with corncob bedding and fitted with steel-wire tops. Mice were allowed unrestricted access to water and Rodent Lab Diet 5001 (PMI, Nutrition International, Inc., Brentwood, MO). The certified AAALAC accredited vivarium controlled for humidity (30–60%) and temperature (20–22 °C) and maintained a 12–12-h light cycle (0600–1800 h). The Wayne State University Institutional Animal Care and Use Committee approved all procedures which were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NRS, 2011).
2.2. Chemicals
Toluene (CAS-No:108–88–3) and 190 proof ethanol (ethyl alcohol CAS–No: 64–17–5) were obtained from Sigma-Aldrich (St. Louis, MO). Cocaine hydrochloride was acquired from the National Institutes on Drug Abuse Control Supply Program (Bethesda, Maryland). Both cocaine and ethanol were dissolved or diluted in 0.9% saline prior to administration.
2.3. Static exposure system
Toluene and air exposures were 30 min, once a day (0900–1600 h) from PND 28–32 in a static solvent exposure system (for chamber diagram and stability metrics see Svenson et al., 2022) consisting of a modified chromatography jar (~27 L) fitted with a tight sealing Plexiglas lid located within a fume hood (Conti et al., 2012; Svenson et al., 2022). For each exposure, a single mouse was placed into the bottom of the chamber and then a predetermined amount of liquid toluene corresponding to the chosen concentrations (2000 or 4000 ppm was then placed onto a piece of filter paper located below the fan which was attached to the lid. The lid was then sealed shut and the fan was turned on to volatilize and distribute the toluene-laden vapor throughout the chamber. Chamber integrity and toluene concentrations were confirmed periodically using single wavelength-monitoring infrared spectrometry (Miran 1 A, Foxboro Analytical). Mean concentrations of toluene were within 3% of nominal levels ~2.5 min after toluene was added and remained within 3% of nominal throughout the 30-min exposure (see Svenson et al., 2022). For the air-only (0 ppm) control group, identical procedures were followed but without toluene. After the 30-min exposures, animals were removed, and the chambers vented and cleaned. Animals were observed for 30 min in their home cage after each exposure for any complications (e.g., seizure activity). No complications were observed, and all subjects were retained. After the observation period, animals were returned to the vivarium.
During toluene and air exposures, locomotor activity was measured within the static exposure chamber via 3 sets of photocells of 16-beam infrared (I/R) emitter/detector arrays (Med Associates, St. Albans, VT) mounted on Plexiglas® bases that bisected the exposure chambers. Interruptions of beams generated an analog signal recorded by automated activity software (Open Field Activity Software [SOF-811], Med Associates, St. Albans, VT) which quantified total beam breaks in both the vertical and horizontal planes and encoded distance traveled and ambulatory time.
2.4. Behavioral testing after toluene exposure
After repeated toluene or air exposures (PND 28–32), mice were randomly assigned to one of two experimental conditions: 1) toluene or air exposure immediate or delayed monoamine quantification or 2) short- or long-delay abstinence followed with a CDR and monoamine assessment (see Fig. 1).
Fig. 1.
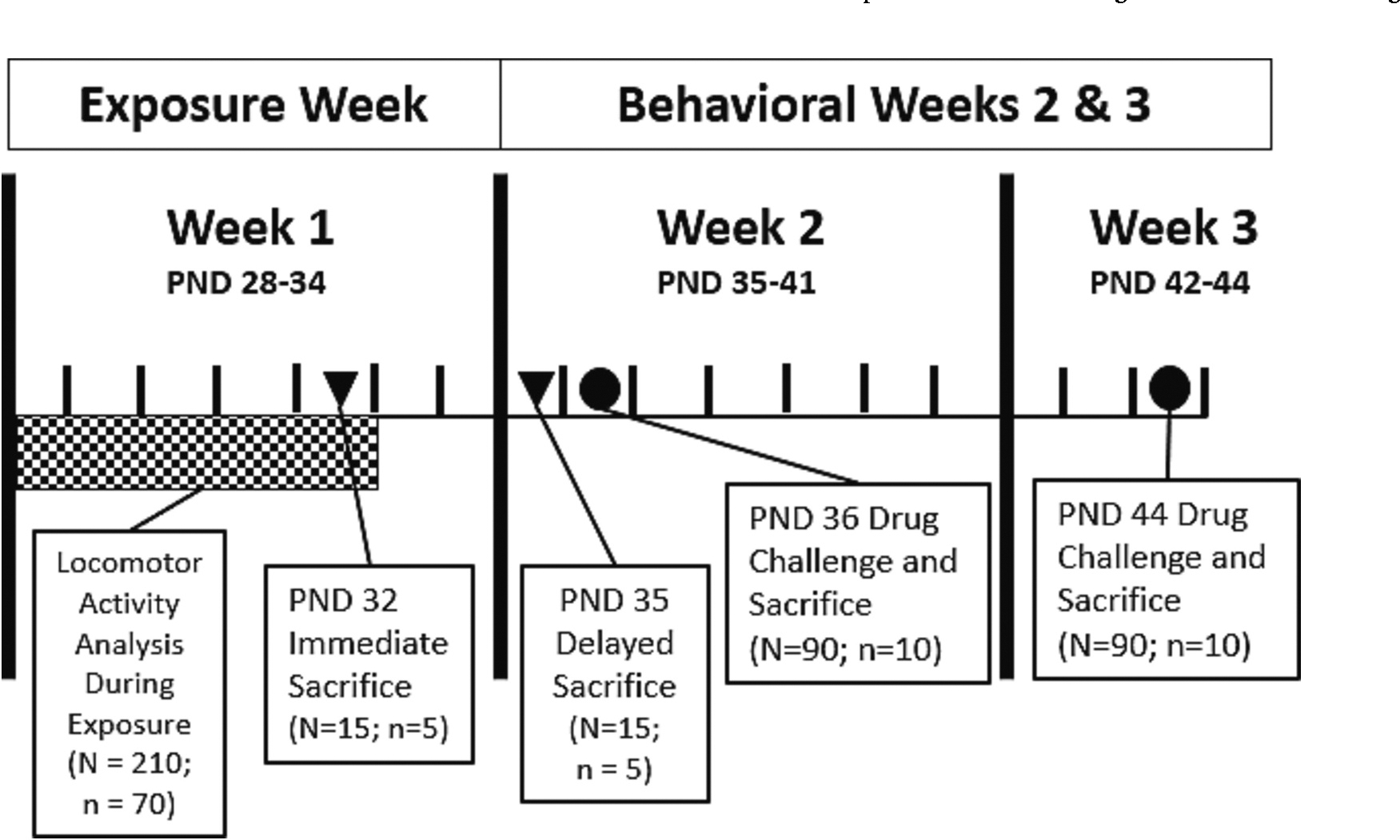
Experimental timeline.
Timeline for toluene or air exposures and behavioral assays. Experiment 1: Exposure occurred from PND 28–32 with mice euthanized on PND 32 and PND 35 (represented by inverted triangles▾) to observe immediate and delayed impact on neurochemistry. Experiment 2: A separate cohort of animals were exposed to either toluene or air using the same exposure paradigm and subsequently challenged with cumulative cocaine or ethanol on PND 36 or 44 (represented by circles●), and then immediately euthanized for HPLC analysis.
2.4.1. Toluene exposure locomotor activity and neurochemistry
All mice went through the exposure procedure and the locomotor activity measured as described above. A subgroup of mice was randomly chosen to be euthanized immediately following the last exposure (PND 32) or after a 3-day delay (PND 35) to assess dopamine (DA), serotonin (5-HT), and norepinephrine (NE) levels, as well as their metabolites 3,4-dihydorxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA), and 3-methoxy-4-hydroxyphenethylamine (3-MT). Animals were euthanized via cervical dislocation, rapid decapitation, and the brains were quickly frozen on dry ice. Samples remained at −80° Celsius (C) until processing using HPLC analysis.
2.4.2. Cumulative dose response and neurochemistry
Mice previously exposed to toluene (0, 2000, or 4000 ppm) were challenged with ethanol or cocaine, using a CDR assessment (Fig. 1) on either PND 36 or PND 44 (n = 10 per condition, n = 90 per age group, N = 180). Mice were initially injected (i.p.) with saline and immediately placed into a novel Plexiglas® open field chamber (28 cm × 28 cm ×28cm) affixed with 3 sets of photocells of 16-beam infrared (I/R) emitter/detector arrays (Med Associates, St. Albans, VT). This was done to record locomotor activity for 8 min, after which mice were removed, injected with the challenge drug (ethanol, cocaine, or saline for control), and placed without delay back into the chamber. This process was repeated every 8 mins until 5 total injections (including initial saline injection) were administered with each injection in the CDR procedure calculated to systematically increase the total dose. This timeframe was chosen to match the pharmacokinetic properties of i.p. injections of cocaine (Benuck et al., 1987; Zhu et al., 2021) & ethanol (Pastino et al., 1996) corresponding locomotor activity while still being on the ascending limb to deliver the next doses. The cumulative doses for cocaine were 0, 2.5, 5, 10, & 20 mg/kg (injected doses of 0, 2.5, 2.5, 5, & 10 mg/kg). For ethanol the cumulative doses were 0, 0.5, 1, 2, & 4 g/kg (injected doses 0, 0.5, 0.5, 1, & 2). The control group received 5 injections of volumetrically matched saline. Locomotor behavior was recorded throughout the cumulative administration (i.e., 40 min total), but the behavioral analysis focused on the last 3 min of each 8 min window to allow drug effects while limiting artifacts due to drug administration handling (Benuck et al., 1987; Frantz et al., 2007). Brain tissue was collected (as described above) immediately following the last CDR measurement for monoamine analysis.
2.5. HPLC of brain monoamines
Brains were removed from −80 °C storage and placed in a cold (~0 °C) mouse brain matrix. Cuts were made beginning at interaural 0 mm (Bregma −3.80 mm) and progressed to collect three 2-mm-thick slices between interaural ~6.02 (Bregma 2.22) and interaural ~0.00 mm (Bregma −3.80). Brain slices were then placed on ice-cold (~0 °C) glass stages covered with ice-cold dH2O-moistened filter paper, and 1.0- or 1.5-mm-dia tissue punches were taken from the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), ventral tegmental area (VTA), and dorsal striatum (dStr), respectively guided by a mouse brain atlas and defining landmarks (Franklin and Paxinos, 2007; Murnane et al., 2012). Only the mPFC and NAc were collected and assessed following toluene exposure only and all regions were collected and analyzed following cumulative dose response. Brain region tissue punches were placed into labeled centrifuge tubes and stored at −80 °C until analysis when each tissue punch was removed from storage, weighed, homogenized by sonication in 50 μl 0.2 N perchloric acid (PCA) for 3–5 s (Misonix XL-2000), visually inspected to check for uniform tissue suspension, centrifuged at 10,000 ×g for 10 min at ~4 °C, and the supernatant collected. A 20-μl aliquot of each sample was placed into a conical HPLC vial and placed in an autosampler of the Dionex Ultimate 3000 HPLC system at 5 °C (Thermo Fisher Scientific, Waltham, MA). A series of external standards for DA, 5-HT, and NE, as well as metabolites (DOPAC, HVA, 3-MT and 5-HIAA) were prepared through serial dilution corresponding to 10 ng, 5 ng, 1 ng, 0.5 ng, 0.1 ng or PCA (vehicle). The standards were processed in ascending concentrations starting with PCA and standards were run in parallel and in duplicate following the last sample of each run.
Standards and unknowns were injected (10-μl) by an autoinjector using an acetonitrile-based MD-TM mobile phase (10% acetonitrile: Thermo Scientific, CAS No. 75–5–8). The HPLC system flow rate was 0.6 ml/min and samples passed through a reverse-phase column (Hypersil™ BDS C18 column, Thermo Fisher Scientific) at 25 °C, a guard cell (ESA guard cell model 2050) set to 300 mV, and a column guard (2.1/3.0 mm ID, Thermo Scientific). An ultra-analytical dual-electrode cell (ESA model 5011 A) with a reference electrode set at −175 mV and a working electrode at 350 mV (gain = at 100 μA for both) was used for electrochemical detection. Values from the detector were captured using Chromileon 7 software (Dionex) to quantify peak height representing levels of monoamines and metabolites. A detection threshold of 3 times background (PCA) was set and samples that did not reach higher values were excluded from analysis. Standard curve equations to calculate monoamine levels as well as verify the stability of the instrument were constructed. The standard curves for all components indicated good stability for quantification in all regions (R2 ≥ 0.98). Tissue levels of monoamines and metabolites were calculated based on the linear regression equation supplied by standard curves and expressed as ng monoamine/ mg wet tissue weight.
2.6. Statistical analyses
Analyses were conducted using SPSS (version 26, IBM, Armonk, NY) or Prism (V.8.4.3; GraphPad Software, San Diego, California USA). Total locomotor activity during toluene exposure as distance traveled (cm) was summed for each day (Fig. 2) and analyzed via a 3 (TOL-0, 2000, 4000; between subjects) × 5 (RM: DAY; within subjects) repeated measures ANOVA and any significant interactions were subjected to post hoc analysis as specified. Analysis of the monoamines following toluene exposure was conducted using a 3 (TOL; between subjects) × 2 (AGE—PND 32, 35; between subjects) ANOVA separately for each area (mPFC, NAc, VTA, dStr), each component (DA, NE, & 5-HT), metabolite (DOPAC, HVA, 3-MT, 5-HIAA), and ratio (DOPAC/DA & 5-HIAA/5-HT).
Fig. 2.
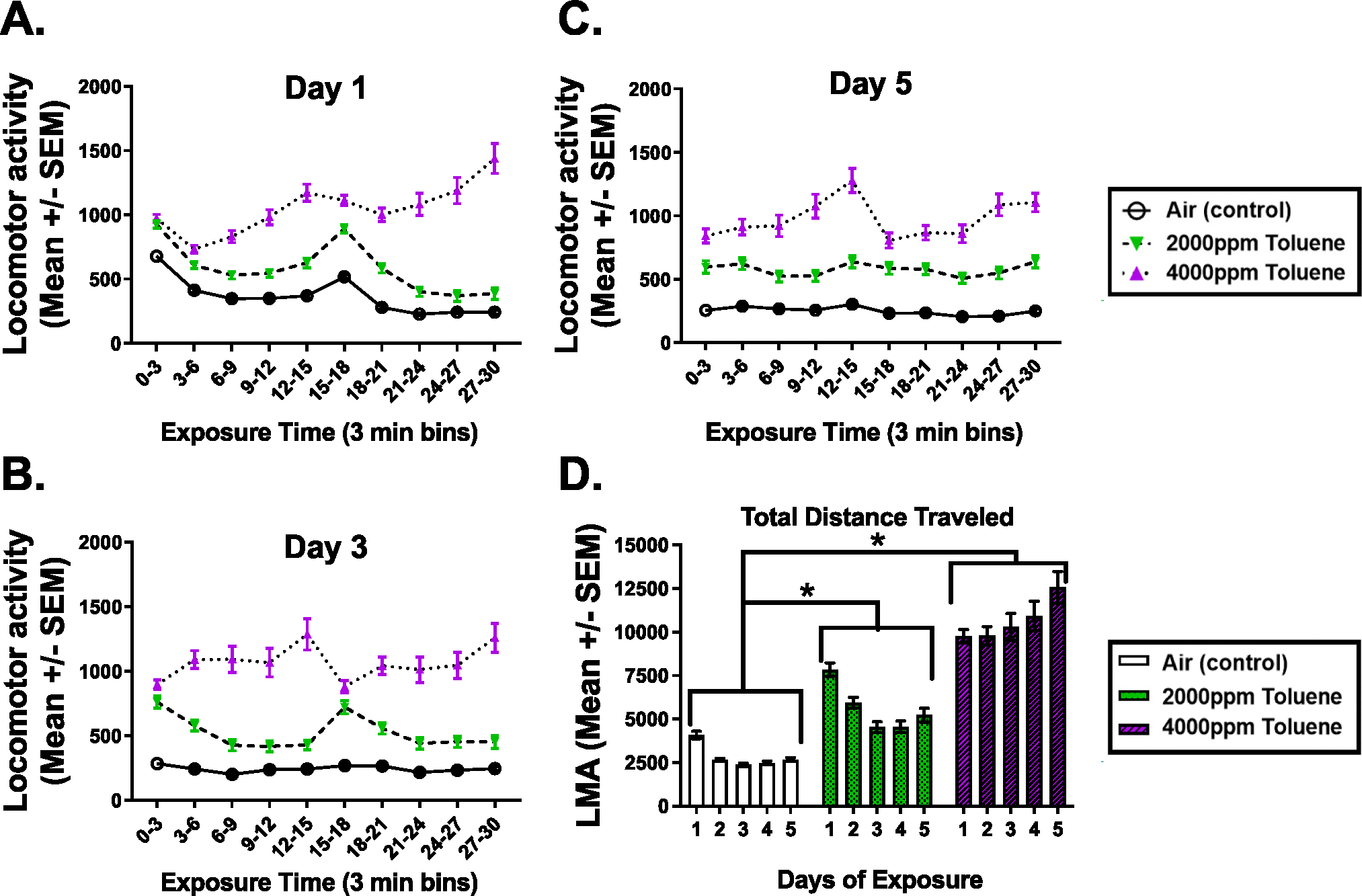
Distance traveled during repeated toluene exposure.
Experiment 1: Toluene Effects on Locomotor Activity (LMA): Shown are distance traveled during three min bins for the first, third, and fifth day of exposure to observe day-to-day changes from repeated exposures (PND 28–32; panels A-C). Additionally, the total locomotor activity each day is presented (panel D). Post-hoc analyses of the total distance traveled across the days of exposure revealed that toluene increased locomotor activity and all concentrations were significantly different from one another at each day of exposure (* p < 0.05). Analysis of the daily sessions demonstrate that the toluene groups were significantly different from control for each 3 min period every day of exposure.
Analysis of the Air (control) conditions that were subjected to CDR administration was conducted to demonstrate drug effect within the CDR procedure. Locomotor activity during the last 3 min of each injection was analyzed using separate 2 (DRUG-saline, cocaine, and ethanol) × 5 (RM DOSE) repeated measures ANOVAs at each age. This data was then incorporated with the other groups for the full analysis procedure as described below.
Locomotor activity during the CDR procedure for the last 3 min of each dose was analyzed using separate 3 (TOL-0, 2000, 4000) × 2 (DRUG-saline, ethanol or saline, cocaine) × 2 (AGE – PND36, 44) × 5 (RM DOSE) repeated measures ANOVAs comparing cocaine vs saline and ethanol vs saline. Following the CDR task, monoamines were analyzed via separate 3 (TOL-0, 2000, 4000) × 2 (DRUG- “saline vs. ethanol” or “saline vs. cocaine”) × 2 (AGE-PND36, 44) ANOVAs comparing cocaine vs saline (control) and ethanol vs saline (control) separately for each area (mPFC, NAc, VTA, dStr), each component (DA, NE, & 5-HT), metabolite (DOPAC, HVA, 3-MT, 5-HIAA), and ratio (DOPAC/DA & 5-HIAA/5-HT). The control group (saline) was the same for both analyses.
3. Results
3.1. Experiment 1 – Toluene exposure locomotor activity and neurochemistry
3.1.1. Locomotor activity testing during exposure
The assumption of Sphericity was not met and a Huynh-Feldt corrected F was used for determining statistical significance. There was a significant main effect for toluene concentration, [F (2, 345) = 95.74, p < 0.001] with the highest TOL concentration (4000 ppm) producing an approximate two-fold increase () in activity as compared to 2000 ppm TOL (), and roughly a 4-fold greater activity compared to air-control animals (; see Fig. 2). There was also a significant main effect of DAY, [F (2.02, 698.29) = 13.26, p < 0.001] with activity generally decreasing across the first three exposure days with a slight increase on the last day (Data not shown).
Additionally, there was a significant TOL × DAY interaction, [F (4.05, 698.29) = 16.37, p < 0.001] with the locomotor activity of the 4000 ppm TOL exposure groups increasing while the 2000 ppm TOL exposure groups declined across days (see Fig. 2). The exposure to 4000 ppm TOL produced significantly more locomotor activity than 2000 ppm TOL and 0 ppm (control) each day (p’s < 0.01) and 2000 ppm TOL produced significantly more locomotor activity than 0 ppm on each day of exposure (all values of p < 0.01; see Fig. 2).
3.1.2. HPLC analysis after toluene exposure
Levels of DA and DOPAC were below detection threshold for the mPFC. Within the NAc, there were no significant effects of TOL, AGE, or their interaction for DA (p ≥ 0.37), DOPAC (p ≥ 0.06), or the DOPAC/DA ratio (p ≥ 0.19; data not shown). DOPAC was trending but failed to reach significance alone or significantly impact the DOPAC/DA ratio (data not shown).
3.2. Experiment 2 – Cumulative dose response and neurochemistry
3.2.1. Cumulative dose response
3.2.1.1. Air-control only – Analysis of drug effect.
Sphericity was not assumed and a Geisser-Greenhouse correction was used where appropriate. Each age was analyzed separately using a 2-way Repeated Measures ANOVA and mice that received saline injections was used as the comparison group. The main effects for DRUG (PND36 - p < 0.001; PND44 - p < 0.001) and Injection (PND36 - p < 0.001; PND44 - p < 0.01) were significant, as well as the interaction (PND36 - p < 0.001; PND44 - p < 0.001). Analysis of multiple comparisons using an uncorrected Fishers LSD demonstrated that cocaine differed from saline injected subjects for the second through fifth injections for PND36 (p < 0.01) and for the third through fifth injections for PND44 (p < 0.05). Mice that had ethanol administered on PND36 differed from air control on the third (p < 0.05) and fifth (p < 0.01) injections with mice on PND44 only differing on the fifth injection (p < 0.05; See Fig. 3).
Fig. 3.
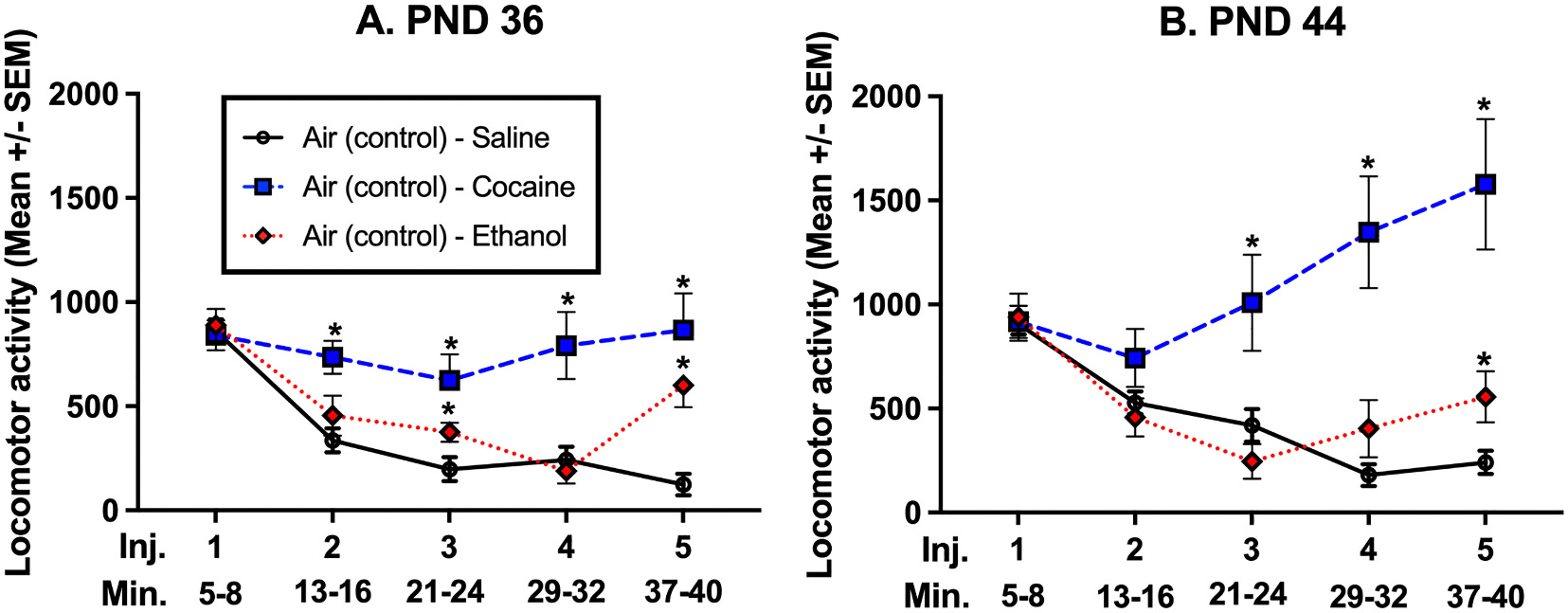
Comparison of drug effect in air-control groups only.
Experiment 2: Cumulative Dose Response Exclusively Air-control groups LMA: To show drug effects for the CDR design, only groups that were exposed to Air-control during the prior phase are included in these graphs. Panel A depicts mice tested at PN36, while Panel B depicts mice tested at PND 44. The lines represent challenge drugs with saline (black), cocaine (blue), and ethanol (red). The “*” symbol indicates a statistically significant difference (p < 0.05) from mice administered saline during the CDR. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.1.2. Cocaine vs. saline.
The assumption of sphericity was not met (χ2 (9) = 132.93, p < 0.001) and a Huynh-Feldt “F” correction was used. While there were significant main effects for DRUG (saline vs cocaine; p < 0.001) and AGE (p < 0.001), there was no main effect for TOL (p = 0.155). The TOL × DRUG interaction was significant (p < 0.05), with mice previously exposed to TOL displaying decreased locomotor activity following cocaine administration as compared to air control mice (Fig. 3B & E). All two-way interactions were significant: TOL × DOSE [F (5.23, 282.36) = 3.43, p < 0.01], DRUG (cocaine) × DOSE [F (2.61, 282.36) = 29.75, p < 0.001], and AGE × DOSE [F (2.61, 282.36) = 4.74, p < 0.01].
A Tukey’s post-hoc analysis of the TOL × DOSE (cocaine) interaction showed that mice previously exposed to 4000 ppm TOL exhibited lower cocaine induced locomotor excitation at 5 (p < 0.05), 10 (p < 0.01) and 20 (p < 0.01) mg/kg, while the previously exposed 2000 ppm TOL mice were significantly lower at 5 (p < 0.05) &10 mg/kg (p < 0.05) but not at 20 mg/kg (p = 0.270) with 5 mg/kg demonstrating a trending result (p = 0.055). The significant AGE × DOSE interaction reveals that at PND 44 mice demonstrated greater increases in locomotor activity than PND 36 mice after the higher doses of cocaine (5, 10, & 20 mg/kg; Fig. 4B vs E).
Fig. 4.
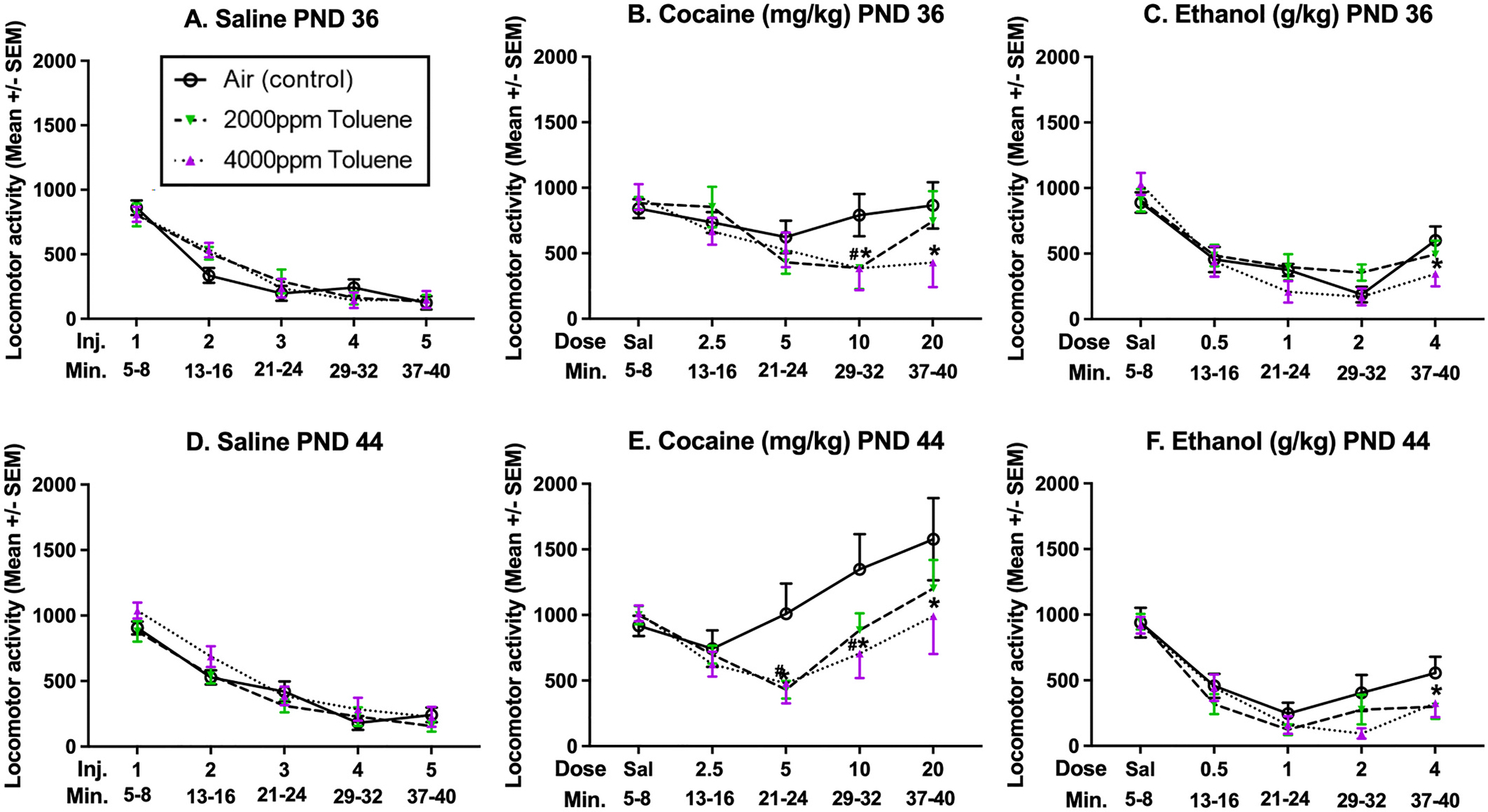
Distance traveled during the last 3 min of each administered dose in CDR.
Experiment 2: Cumulative Dose Response LMA: Shown are the distances traveled during the last 3 min of each injection during the CDR assessment. Panels A-C depict mice tested at PN36, while Panels D-F depict mice tested at PND 44. Panels A & D depict saline given at all injections; Panels B & E depict cocaine administered for injections 2–5 (mg/kg); Panels C & F depict ethanol administered for injections 2–5 (g/kg). The “#” symbol represents a significant difference between TOL 2000 ppm and Air (control; p < 0.05), while the “*” symbol represents a significant difference between TOL 4000 ppm and Air (control; p < 0.05).
While the TOL × AGE × DOSE interaction was non-significant (p = 0.59), there was a significant AGE × DRUG × DOSE interaction, [F (2.61, 282.36) = 7688, p < 0.001]. As seen in Fig. 4, PND 44 mice displayed larger locomotor increases from saline at the same age, relative to the comparison between the PND 36 challenged mice. This can be seen at the higher doses of cocaine (5, 10, & 20 mg/kg; Fig. 4B vs E). There was also a TOL × DRUG × DOSE (cocaine) interaction, [F (5.23, 282.36) = 2.30, p < 0.05], with mice previously exposed to 4000 ppm TOL showing smaller increases in cocaine-induced locomotor activity than saline mice at both ages (see Fig. 3B & E).
3.2.1.3. Ethanol vs. saline.
The assumption of Sphericity was not met (χ2 (9) = 18.60, p < 0.05) and the Huynh-Feldt corrected F was used. No significant main effects were observed for the between-subjects variables TOL (0, 2000, 4000 ppm), DRUG (saline vs ethanol), or AGE (36 vs 44; p > 0.1). There was, however, a significant DRUG × AGE interaction, [F (1, 108) = 6.69, p < 0.05] such that mice at PND 36 injected with ethanol had an overall increase in locomotor behavior compared to saline (PN36 Saline ; PN36 Ethanol ) as opposed to no difference between mice tested at PND 44 (PN44 Saline ; PN44 Ethanol ; see Fig. 4). Investigation of the within-subjects effects revealed there were significant two-way interactions between TOL × DOSE (ethanol), [F (8, 432) = 2.25, p < 0.05], and between DRUG × DOSE, [F (4, 432) = 13.75, p < 0.001].
A Tukey’s post-hoc analysis of TOL × DOSE revealed the previous TOL exposure (4000 ppm) decreased locomotor behavior to ethanol (4 g/kg) as compared to the air exposed mice (p < 0.05). At 4000 ppm, the other levels of DOSE (ethanol) did not differ from air-exposed mice (0–2 g/kg; p’s ≥ 0.09). For mice previously exposed to 2000 ppm toluene, none of the behavior at any level of DOSE was significantly different from the air-exposed mice (p’s ≥ 0.10). The significant DRUG × DOSE (ethanol) interaction showed that mice injected with ethanol, regardless of age or toluene exposure, had shifted levels of locomotor activity compared to mice injected with saline (see Fig. 4A, C, D, & F). No other 2- to 4-way interactions were statistically significant (p’s ≥ 0.20).
3.2.2. Neurochemistry following CDR
The brain tissue samples for both of the following subsections were taken immediately following the last CDR measurement, rapidly frozen using dry ice, and then remained in a − 80 °C freezer until sectioning and analysis.
3.2.2.1. Cocaine vs. saline.
TOL exposure significantly impacted DA (p < 0.01), NE (p < 0.05), and DOPAC (p < 0.01) concentrations within the dStr. The 5-HIAA/5-HT ratio within the NAc was significantly impacted by TOL (p < 0.05). TOL had trending impacts for DOPAC/DA ratio (p = 0.095) and 3MT (p = 0.079) within the dStr, and 5-HT concentrations within the NAc (p = 0.060; Data not shown).
There was a TOL × DRUG (cocaine) interaction for NE within the dStr (p < 0.05) with control and 2000 ppm TOL approaching significance (p = 0.05). There was also a trending TOL × AGE interaction for 5-HT within the VTA (p = 0.058). Finally, there were significant TOL × AGE × DRUG (cocaine) interactions for DA (p < 0.05), 5-HT (p < 0.01), and DOPAC (p < 0.05) within the dStr, as well as for DOPAC within the VTA (p < 0.05). There were significant impacts of the other main factors (drug and age) as well as interactions (2- and 3- way) that, due to this paper’s focus on toluene, are not described in the body of this manuscript. Pictorial representations of the significance value thresholds from all main effects and interactions in each neurotransmitter appear in Table 1.
Table 1.
Challenged with cocaine CDR Neurochemistry by Region.
| CDR Group Comparison | Region | Neurocomponent | Main Effects | Two-Way Interactions | Three-way Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tol | Age | Drug | Tol x Age | Tol x Drug | Age x Drug | Tol x Age x Drug | |||
| Cocaine Vs. Saline | mPFC | DA | × | × | × | × | × | × | × |
| NE | *** | ||||||||
| 5-HT | ** | ||||||||
| DOPAC | × | × | × | × | × | × | × | ||
| HVA | × | × | × | × | × | × | × | ||
| 3-MT | × | × | × | × | × | × | × | ||
| 5-HIAA | * | ||||||||
| DOPAC/DA Ratio | × | × | × | × | × | × | × | ||
| 5-HIAA/5-HT Ratio | ^ | ** | |||||||
| NAc | DA | * | # | ||||||
| NE | # | * | |||||||
| 5-HT | # | ^ | ** | ||||||
| DOPAC | *** | *** | ^ | ||||||
| HVA | * | * | # | ||||||
| 3-MT | × | × | × | × | × | × | × | ||
| 5-HIAA | ^ | * | |||||||
| DOPAC/DA Ratio | *** | *** | |||||||
| 5-HIAA/5-HT Ratio | * | *** | ^ | ||||||
| dStr | DA | ** | ** | * | |||||
| NE | * | ** | |||||||
| 5-HT | *** | ** | |||||||
| DOPAC | ** | *** | *** | * | |||||
| HVA | |||||||||
| 3-MT | ^ | ** | ** | ||||||
| 5-HIAA | ** | ||||||||
| DOPAC/DA Ratio | ^ | *** | *** | ||||||
| 5-HIAA/5-HT Ratio | * | *** | |||||||
| VTA | DA | * | |||||||
| NE | # | ||||||||
| 5-HT | # | * | |||||||
| DOPAC | *** | * | |||||||
| HVA | ** | ||||||||
| 3-MT | × | × | × | × | × | × | × | ||
| 5-HIAA | *** | ||||||||
| DOPAC/DA Ratio | ^ | *** | |||||||
| 5-HIAA/5-HT Ratio | *** | ** | |||||||
CDR Neurochemistry by Drug and Region. These tables present a representation of the significance threshold for each main effect and interaction of each neuro-component following repeated toluene and cumulative dosing. The “x” symbol represents that the component was below our detection threshold; the stars represent their typical cutoff thresholds of <0.001 (***), <0.01 (**), <0.05 (*); the “#” represents scores that fall above 0.05 but below 0.075 (e.g. 0.05 < score < 0.075); and lastly the “^” represents scores above 0.075 but <0.099 (e.g. 0.075 < score < 0.099). Table cells that don’t have a symbol in them were within the detection range but whose p-values fell above 0.1. Minimum value for inclusion was above 3 times the background (PCA) and was calculated using the curve created by a set of external standards for each component analyzed.
3.2.2.2. Ethanol vs. saline.
As a main factor, toluene produced a significant effect on HVA concentrations in the VTA [F (2, 86) = 3.82, p < 0.05], with no post hoc comparison reaching significance (adjusted p ≥ 0.275). There was a TOL × DRUG interaction for DA (p < 0.05), and trending results for HVA (p = 0.059) and DOPAC (p = 0.073) concentrations within the dStr. Within the mPFC, the 5-HIAA/5-HT ratio was trending (p ≥ 0.073). There was a significant TOL × AGE × DRUG (ethanol) interaction for DA concentrations within the dStr [F (2,88) = 3.37, p < 0.05] with trending results for NE (p = 0.07), 5-HIAA/5-HT ratio (p = 0.09), and DOPAC (p = 0.08) within the dStr and DOPAC/DA ratio (p = 0.091) in the VTA. There were significant impacts of the other main factors (drug and age) as well as interactions (2- & 3- way) that, due to this paper’s focus on toluene, are not described in the body of this manuscript. Pictorial representations of the significance value thresholds from all main effects and interactions in each neurotransmitter appear in Table 2.
Table 2.
Challenged with ethanol CDR Neurochemistry by Region.
| CDR Group Comparison | Region | Neurocomponent | Main Effects | Two-Way Interactions | Three-way Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tol | Age | Drug | Tol x Age | Tol x Drug | Age x Drug | Tol x Age x Drug | |||
| ETOH Vs. Saline | mPFC | DA | × | × | × | × | × | × | × |
| NE | *** | * | |||||||
| 5-HT | * | ||||||||
| DOPAC | × | × | × | × | × | × | × | ||
| HVA | × | × | × | × | × | × | × | ||
| 3-MT | × | × | × | × | × | × | × | ||
| 5-HIAA | *** | ||||||||
| DOPAC/DA Ratio | × | × | × | × | × | × | × | ||
| 5-HIAA/5-HT Ratio | # | # | # | ||||||
| NAc | DA | # | ^ | ||||||
| NE | ** | ||||||||
| 5-HT | ** | ||||||||
| DOPAC | * | * | |||||||
| HVA | # | ||||||||
| 3-MT | × | × | × | × | × | × | × | ||
| 5-HIAA | |||||||||
| DOPAC/DA Ratio | *** | ||||||||
| 5-HIAA/5-HT Ratio | ** | ||||||||
| dStr | DA | *** | * | * | |||||
| NE | *** | *** | ** | # | |||||
| 5-HT | ** | * | |||||||
| DOPAC | ** | # | ^ | ||||||
| HVA | ** | # | |||||||
| 3-MT | # | * | |||||||
| 5-HIAA | * | *** | * | ||||||
| DOPAC/DA Ratio | *** | ** | * | ||||||
| 5-HIAA/5-HT Ratio | * | ^ | |||||||
| VTA | DA | ||||||||
| NE | |||||||||
| 5-HT | |||||||||
| DOPAC | ^ | ||||||||
| HVA | * | ^ | |||||||
| 3-MT | × | × | × | × | × | × | × | ||
| 5-HIAA | * | ||||||||
| DOPAC/DA Ratio | ^ | ||||||||
| 5-HIAA/5-HT Ratio | * | ||||||||
CDR Neurochemistry by Drug and Region. These tables present a representation of the significance threshold for each main effect and interaction of each neuro-component following repeated toluene and cumulative dosing. The “x” symbol represents that the component was below our detection threshold; the stars represent their typical cutoff thresholds of <0.001 (***), <0.01 (**), <0.05 (*); the “#” represents scores that fall above 0.05 but below 0.075 (e.g. 0.05 < score < 0.075); and lastly the “^” represents scores above 0.075 but <0.099 (e.g. 0.075 < score < 0.099). Table cells that don’t have a symbol in them were within the detection range but whose p-values fell above 0.1. Minimum value for inclusion was above 3 times the background (PCA) and was calculated using the curve created by a set of external standards for each component analyzed.
4. Discussion
The current investigation examined whether repeated inhaled toluene (2000 or 4000 ppm) exposure during adolescence (PND 28–32) would alter the behavioral and/or monoamine responses in mice to subsequent cumulative doses of cocaine or ethanol in later adolescence (PND 36) or early adulthood (PND 44). Similar to reports from previous studies, acutely inhaled toluene concentration-dependently increased locomotor activity, while repeated toluene exposure resulted in tolerance to the lower toluene (2000 ppm) concentration and sensitization to the higher toluene (4000 ppm) concentration. When examining the effects of previous adolescent toluene exposure on other drugs of abuse, significant decreases were observed for the locomotor-stimulating effects of cocaine (“blunting”), while a significant decrease in the locomotor effects of ethanol was seen for the 4000-ppm toluene concentration at 4 g/kg EtOH as compared to Air and then EtOH exposed mice. Unexpectedly, the longer toluene “abstinence” period (12 days vs 4 days) demonstrated the greatest “blunting” effect on cocaine-induced locomotor behavior with no time differences observed for ethanol. Monoamine analysis using HPLC within the mPFC, NAc, dSTR, and VTA revealed only subtle effects that varied by drug and animal age.
4.1. Locomotor effects of toluene
The demonstration that acute 30-min inhaled toluene (2000 and 4000 ppm) exposure produced concentration-dependent increases in locomotor activity relative to air-control animals is similar to previous reports that acute toluene dose-dependently increases locomotor activity across different strains of mice (Bowen et al., 2010) and rats (Batis et al., 2010; Bowen and Balster, 1998; Riegel and French, 1999; Wiaderna and Tomas, 2002), and across a variety of developmental time points (Armenta-Resendiz et al., 2019; Batis et al., 2010; Hinman, 1984, 1987; Wood and Colotla, 1990). In comparison to acute exposures, repeated administrations of toluene (4000 ppm) over 5 days resulted in significant increases in locomotor activity across days of exposure (see Fig. 2, Panel D). These increases in locomotor activity in the present study are consistent with previous reports indicating that sensitization develops to the locomotor effects of repeated inhaled toluene exposure (Apawu et al., 2015; Batis et al., 2010; Bowen and Balster, 2006; Bowen et al., 2007; Bowen et al., 2010; Hinman, 1984, 1987). Earlier investigations have also shown that sensitization develops to toluene after repeated intraperitoneal (Riegel et al., 2003; Riegel et al., 2004; Riegel and French, 2002) or oral administration (Wiaderna and Tomas, 2000). Conversely, examination of the repeated daily 2000 ppm toluene exposure data (see Fig. 2, Panel D) showed that animals had reduced increases in locomotor activity as the days of repeated exposure continued (days 2–5), suggesting the development of tolerance to toluene’s stimulating behavioral effects at this lower concentration. This decrease or “tolerance” is similar to other investigations that reported the emergence of tolerance after repeated exposure to several abused inhalants (Apawu et al., 2015; Bowen and Balster, 2006; Rees et al., 1989; Tomaszycki et al., 2013) and appear to be concentration dependent (e.g., (de)sensitization vs sensitization). Others have shown that the appearance of behavioral tolerance in locomotor activity is dependent on the exposure patterns (Batis et al., 2010) or timeframe of observation [during and/or after exposure], sex (Bowen et al., 2007; Braunscheidel et al., 2019; Crossin and Arunogiri, 2020), or even the developmental timeframe when the subjects were exposed (Bikashvili et al., 2012; Bowen et al., 2007; Crossin et al., 2017; Wayman and Woodward, 2018b; Zhvania et al., 2010; Zhvania et al., 2012). Clearly, more research is needed to disentangle the contributions of each variable on these findings.
4.2. Toluene’s effects on behavioral responses to cocaine
Behaviorally, mice challenged with cumulative doses of cocaine at PND 36 had locomotor activity responses that were less active overall than mice challenged at PND 44, especially at the higher doses of cocaine (5–20 mg/kg; see Fig. 4). This difference is unique in that this sensitivity has been previously shown in studies demonstrating an increased sensitization, preference, and response in adolescent psychostimulant effect as compared to adult rodents (Badanich et al., 2006; Bava and Tapert, 2010; Carpenter-Hyland and Chandler, 2007; Ledesma et al., 2017; Samuel-Herter et al., 2014). Some studies indicate the ongoing development of the DA system within the prefrontal cortex and less quickly in the limbic associated regions during this period as some of the factors that define this timeframe and disposition period (Hoops and Flores, 2017; Wahlstrom et al., 2010).
While a few studies have shown that toluene has some “stimulant drug-like” effects, to our knowledge, no study has shown that previous toluene exposure could reduce or “blunt” the stimulant effects of cocaine days or weeks after the last toluene exposure. Previous investigations have shown that higher concentrations of inhaled toluene (~ > 4000 ppm) have biphasic effects on locomotor behavior, such that early in the exposure, locomotor activity is significantly increased (stimulant-like) and then decreased as the time of exposure continues (Bowen and Balster, 1998; Bowen et al., 2010). Repeated toluene exposure to higher concentrations has also been shown to result in increased sensitivity to toluene’s locomotor effects in rodents (Apawu et al., 2020; Apawu et al., 2015; Batis et al., 2010; Bowen et al., 2007; Callan, et al., 2017). Interestingly, the few studies that have explored effects on both male and female animals have demonstrated more extreme and varied responses in female subjects (Bowen et al., 2007; Braunscheidel et al., 2019; Crossin and Arunogiri, 2020).
Developmental age, sex, length/duration, and concentration/dose of exposure, are factors that influence responses to CNS stimulants in general (Badanich et al., 2006; Campbell et al., 2000; Frantz et al., 2007; Laviola et al., 1995; Nicolas et al., 2022; Spear and Brake, 1983; Torres, 2022). Acutely inhaled toluene has also been shown to substitute in drug-discrimination operant procedures for i.p. amphetamine (Bowen, 2006), while Beyer et al. (2001) reported an increase in both the locomotor stimulating effect and DA concentrations in the NAc during cocaine administration 24–96 h following repeated toluene. Although there are differences in toluene exposure (concentration and pattern), species, age, and drug challenge, the shift in direction of effect (increase in Beyer and decrease in this study) is most likely due to the fact that drug challenges in the current study were up to 12-days following toluene exposure, while Beyer et al. (2001) conducted their drug challenges between 24 and 96 h after toluene exposure and collapsed their data into a single group for analysis.
4.3. Toluene’s effects on behavioral responses to ethanol
Behaviorally, mice that were challenged with ethanol at PND 36 in the present study had locomotor activity responses that were no different from mice challenged at PND 44. These results are similar to Quoilin et al. (2012) who observed no differences between adolescent (PND 35) and adult (PND 63) female Swiss mice after acute administration of 2 or 4 g/kg ethanol. However, Quoilin et al. (2012) did report that differences between these two age groups were observed for other ethanol doses (e.g., 1.5, 2.5, 3.5 g/kg). In the current study, when mice that had been previously exposed to toluene (4000 ppm) were later challenged with cumulative doses of ethanol (0.5–4.0 g/kg), locomotor behavior was significantly decreased for the highest dose of ethanol (4 g/kg) as compared to the “air-only” mice that received the same cumulative doses of ethanol. This was observed at both 4 and 12 days (PND 36 & 44) after repeated toluene exposure (see Fig. 4) and that this was unimpacted by age/abstinence duration.
Although previous studies have shown toluene to have a depressant “drug-like” profile of effects (for review see Bowen et al., 2006; Cruz and Bowen, 2021; Evans and Balster, 1991), to our knowledge, these data demonstrating that an earlier toluene exposure can alter effects of ethanol days or weeks after exposure are novel. Indeed, acute administration of toluene, and other abused inhalants, are known to induce sedative-like behavioral effects in adult male rodents in behavioral tasks such as open-field locomotion and drug discrimination similar to CNS depressants like ethanol and barbiturates (Bowen et al., 2006; Cruz and Bowen, 2021). Toluene has also been shown to substitute in drug-discrimination operant procedures for ethanol (Bowen, 2009; Rees et al., 1987a) and pentobarbital (Rees et al., 1985; Rees et al., 1987b).
However, not all studies have shown that earlier toluene exposures will affect later behaviors. For example, Dick et al., 2014 demonstrated that adolescent toluene exposure failed to alter voluntary ethanol consumption in adulthood (Dick et al., 2014), in contrast to researcher-administered ethanol in the present study. Additionally, there was a restriction of time in the current study that may have impacted our observations. The absorption rate for ethanol is slightly slower than cocaine, however, the inter-injection timing (8 min) was kept consistent. Additionally, this testing was conducted in a novel environment without pre-assessment habituation to the chamber which likely resulted in novelty-induced activity. Additionally with a depressant effect that reduces locomotor activity, there is less room for significant change due to restriction of range in values. All of these factors combined may explain why we observe significant differences only at the final injection. Future studies should work to address these shortcomings potentially by utilizing by adjusted inter-injection intervals and recording times as well as habituate animals to the chamber prior to testing. Additionally, the use of self-administration of the subsequent drugs would allow for detection of self-directed usage rates as well as associated metrics like LMA.
4.4. Toluene’s effects on monoamine analysis
The non-significant decreases in DA from the NAc of mice in the current study are in contrast to the few preclinical toluene studies that have demonstrated decreases in NAc DA following repeated toluene exposure of DA in the NAc (Apawu et al., 2020; Riegel et al., 2004; Woodward and Beckley, 2014). Our current results showing that locomotor responses to the stimulant effects of cocaine were behaviorally “blunted” up to 12 days following cocaine exposure was not due to differences in whole tissue DA levels given our results. This suggests that the while DA system may not have fully recovered from the previous toluene exposures, the levels themselves were unchanged and the behavioral findings may be related to a number of factors (receptor trafficking, decreased firing of the activated neurons, increased number of activated neurons, change in the development of this system etc.). More research into the mechanism that underlies the reduction of locomotor behavior is warranted given the potential for impacts to drug activation and reward as well as the hedonic nature of future rewards. This blunting effect of toluene suggests that one may have to use higher concentrations of cocaine to achieve similar behavioral results.
The current model exposed mice to toluene during a “critical developmental period” for both brain and behavior, particularly for the dopaminergic system within the reward pathway (Broadwater et al., 2011; Doremus-Fitzwater et al., 2010). The ongoing development of the dopaminergic system in adolescence makes it more susceptible to disruptions than in adults (Doremus-Fitzwater et al., 2012; Spear, 2000a, 2000b). Many drugs of abuse have age-dependent interactions with and long-term impact on the dopaminergic system (Cadoni and Di Chiara, 2000; Dos Santos et al., 2018; Laviola et al., 1995; Quoilin et al., 2014; Robinson and Berridge, 2003), with comparatively few toluene investigations also having demonstrated age impacts (Bowen et al., 2007; Zhvania et al., 2010; Zhvania et al., 2012). Toluene impacts the regulation of DA in the mesolimbic pathway potentially by mPFC outputs inhibiting mesolimbic DA neurons in the midbrain through excitation of intra-VTA GABAergic interneurons and GABAergic projections from the tail of the VTA resulting in inhibition of DA activity, concurrently, enhancement of the lateral habenula further drives this inhibition as well as mPFC inputs forming synapses on GABAergic neurons that project to the NAc during acute exposure (Beckley et al., 2013; Callan et al., 2017).
Repeated exposure to higher “abuse-like” concentrations of toluene (8000 & 12,000 ppm) during a similar developmental time frame (PND 28–34) and assessment period (PND 35 & 42), produced region-dependent effects on GABA and glutamate during a period of abstinence (Perrine et al., 2011). A single binge-like exposure to toluene enhanced current-evoked firing of mPFC to NAc signaling in mid adolescence (PND 41–44), but not adulthood (PND 97–100), 24 h but not 7 d after exposure (Wayman and Woodward, 2018a; Woodward and Braunscheidel, 2023). Incorporating multiple neuro-components or methods of analysis that can better account for global changes and changes beyond whole tissue level of DA are warranted, and previous literature, current behavioral findings, and the pattern of drug progression seen in humans support further investigation into toluene polydrug progression. Future investigations should work to address a few of the limitations, first and foremost is the inclusion of female subjects. Other limitations remaining to be addressed include metrics related to the toluene exposure (daily duration, number of days, pattern of exposure, exposure concentrations used, etc.) and factors related to the subsequent drug exposure such as self-administration (contingent exposure, 3-key discrimination, etc.), changes in absorption or metabolic clearance rate of cocaine and/or ethanol, length of abstinence between toluene exposure and drug challenge, long term impact to monoamines following the challenge drug exposure following toluene, and dose ranges used. All of these factors need to be sufficiently explored to better elaborate on the observed phenomenon.
Overall, our findings indicate that abuse-like patterns of toluene exposure in the periadolescent period can influence behavioral and, to a lesser extent, neurochemical responses to subsequent administration of cocaine or ethanol in later adolescence, even twelve days after the last toluene exposure. This demonstrates persistence, if not incubation, of the toluene effect on behavior, but not in monoamine neurochemistry, when subsequently challenged with other drugs of abuse. Studies are needed to explore further the impact of early toluene exposure on subsequent drug responses, including more of the myriad drugs being consumed by teens. It is important that preclinical investigations accurately model the progression from inhalants (e.g., toluene) to other drugs of abuse which has been reported in humans. Changes in subsequent drug action following toluene may result in increased amount required for the initial use for behaviorally desired intoxication, more frequent use, longer duration of use within the lifetime and increased risk of overdose and death. For example, Acevedo et al. (2013) found correlations between initial locomotor responses and subsequent conditioned place preference and condition taste aversion for ethanol. There is also evidence in humans that prior drug history can be a contributing factor in attitudes towards and future use of drugs (Cappelli et al., 2021; Ding et al., 2009; Grant et al., 2006; Nkansah-Amankra and Minelli, 2016). Further exploration of inhalant polydrug progression may inform research and clinical practice alike through better behavioral and mechanistic understanding of these phenomena.
Acknowledgments
This work was supported in part by a Betty J. Neitzel Award to SEB, a dissertation award to CJD from the Department of Psychology and the Graduate School at Wayne State University and a NIDA-R01 award given to SAP (DA042057). Cocaine was gifted to SAP through the NIDA Drug Supply Program. The authors acknowledge contributions by Michael Naddaf for toluene exposures and behavioral testing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Cameron J. Davidson reports financial support was provided by Wayne State University (GRA). Scott E. Bowen reports financial support was provided by Wayne State University (Betty J. Neitzel Award). Shane A. Perrine reports equipment, drugs, or supplies was provided by National Institute on Drug Abuse (DA042057).
Footnotes
CRediT authorship contribution statement
Cameron J. Davidson: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. John H. Hannigan: Conceptualization, Methodology, Supervision, Writing – review & editing. Shane A. Perrine: Resources, Supervision, Writing – review & editing. Scott E. Bowen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Data availability
Data will be made available on request.
References
- Acevedo MB, Nizhnikov ME, Spear NE, Molina JC, Pautassi RM, 2013. Ethanol-induced locomotor activity in adolescent rats and the relationship with ethanol-induced conditioned place preference and conditioned taste aversion. Dev. Psychobiol. 55 (4), 429–442. 10.1002/dev.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameno K, Kiriu T, Fuke C, Ameno S, Shinohara T, Ijiri I, 1992. Regional brain distribution of toluene in rats and in a human autopsy. Arch. Toxicol. 66 (2), 153–156. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1605733. [DOI] [PubMed] [Google Scholar]
- Apawu AK, Mathews TA, Bowen SE, 2015. Striatal dopamine dynamics in mice following acute and repeated toluene exposure. Psychopharmacology 232, 173–184. 10.1007/s00213-014-3651-x. [DOI] [PubMed] [Google Scholar]
- Apawu AK, Callan SP, Mathews TA, Bowen SE, 2020. Repeated toluene exposure leads to neuroadaptation in dopamine release mechanisms within the nucleus accumbens core. Toxicol. Appl. Pharmacol. 408, 115260 10.1016/j.taap.2020.115260. [DOI] [PubMed] [Google Scholar]
- Armenta-Resendiz M, Rios-Leal E, Rivera-Garcia MT, Lopez-Rubalcava C, Cruz SL, 2019. Structure-activity study of acute neurobehavioral effects of cyclohexane, benzene, m-xylene, and toluene in rats. Toxicol. Appl. Pharmacol. 376, 38–45. 10.1016/j.taap.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Aydin K, Sencer S, Demir T, Ogel K, Tunaci A, Minareci O, 2002. Cranial MR findings in chronic toluene abuse by inhalation. AJNR Am. J. Neuroradiol. 23 (7), 1173–1179. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12169477. [PMC free article] [PubMed] [Google Scholar]
- Aydin K, Kircan S, Sarwar S, Okur O, Balaban E, 2009. Smaller gray matter volumes in frontal and parietal cortices of solvent abusers correlate with cognitive deficits. AJNR Am. J. Neuroradiol. 30 (10), 1922–1928. 10.3174/ajnr.A1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL, 2006. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur. J. Pharmacol. 550 (1–3), 95–106. 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bale AS, Jackson MD, Krantz QT, Benignus VA, Bushnell PJ, Shafer TJ, Boyes WK, 2007. Evaluating the NMDA-glutamate receptor as a site of action for toluene, in vivo. Toxicol. Sci. 98 (1), 159–166. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17420219. [DOI] [PubMed] [Google Scholar]
- Batis JC, Hannigan JH, Bowen SE, 2010. Differential effects of inhaled toluene on locomotor activity in adolescent and adult rats. Pharmacol. Biochem. Behav. 96, 438–448. 10.1016/j.pbb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF, 2010. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol. Rev. 20 (4), 398–413. 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckley JT, Evins CE, Fedarovich H, Gilstrap MJ, Woodward JJ, 2013. Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J. Neurosci. 33 (2), 804–813. 10.1523/JNEUROSCI.3729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benuck M, Lajatha A, Reith ME, 1987. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J. Pharmacol. Exp. Ther. 243 (1), 144–149. [PubMed] [Google Scholar]
- Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD, 2001. Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology 154 (2), 198–204. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11314682. [DOI] [PubMed] [Google Scholar]
- Bikashvili TZ, Chilachava LR, Gelazonia LK, Japaridze NJ, Zhvania MG, Lordkipanidze TG, Okuneva VG, 2012. Effect of chronic inhalation of toluene on behavior of rats of various age groups in multi-branched maze. Bull. Exp. Biol. Med. 152 (5), 587–589. [DOI] [PubMed] [Google Scholar]
- Bowen SE, 2006. Increases in amphetamine-like discriminative stimulus effects of the abused inhalant toluene in mice. Psychopharmacology 186 (4), 517–524. 10.1007/s00213-006-0381-8. [DOI] [PubMed] [Google Scholar]
- Bowen SE, 2009. Time course of the ethanol-like discriminative stimulus effects of abused inhalants in mice. Pharmacol. Biochem. Behav. 91 (3), 345–350. 10.1016/j.pbb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Balster RL, 1998. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp. Clin. Psychopharmacol. 6 (3), 235–247. 10.1037/1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL, 2006. Tolerance and sensitization to inhaled 1,1,1-trichloroethane in mice: results from open-field behavior and a functional observational battery. Psychopharmacology 185 (4), 405–415. 10.1007/s00213-006-0335-1. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL, 2006. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol. Teratol. 28 (6), 636–647. 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Charlesworth JD, Tokarz ME, Wright MJJ, Wiley JL, 2007. Decreased sensitivity in adolescent vs. adult rats to the locomotor activating effects of toluene. Neurotoxicol. Teratol. 29 (6), 599–606. 10.1016/j.ntt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen SE, Kimar S, Irtenkauf S, 2010. Comparison of toluene-induced locomotor activity in four mouse strains. Pharmacol. Biochem. Behav. 95 (2), 249–257. 10.1016/j.pbb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunscheidel KM, Okas MP, Hoffman M, Mulholland PJ, Floresco SB, Woodward JJ, 2019. The abused inhalant toluene impairs medial prefrontal cortex activity and risk/reward decision-making during a probabilistic discounting task. J. Neurosci. 39 (46), 9207–9220. 10.1523/JNEUROSCI.1674-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP, 2011. Different chronic ethanol exposure regimens in adolescent and adult male rats: effects on tolerance to ethanol-induced motor impairment. Behav. Brain Res. 225 (1), 358–362. 10.1016/j.bbr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G, 2000. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur. J. Pharmacol. 387 (3), R23–R25. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/10650185. [DOI] [PubMed] [Google Scholar]
- Cairney S, Maruff P, Burns CB, Currie J, Currie BJ, 2004. Neurological and cognitive impairment associated with leaded gasoline encephalopathy. Drug Alcohol Depend. 73 (2), 183–188. 10.1016/j.drugalcdep.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Caldemeyer KS, Armstrong SW, George KK, Moran CC, Pascuzzi RM, 1996. The spectrum of neuroimaging abnormalities in solvent abuse and their clinical correlation. J. Neuroimaging 6 (3), 167–173. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/8704292. [DOI] [PubMed] [Google Scholar]
- Callan SP, Apawu AK, Mathews TA, Bowen SE, 2017. Toluene’s effects on activity and extracellular dopamine in the mouse are altered by GABAA antagonism. Neurosci. Lett. 647, 67–71. 10.1016/j.neulet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP, 2000. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol. Behav. 68, 487–493. [DOI] [PubMed] [Google Scholar]
- Cappelli C, Ames SL, Xie B, Pike JR, Stacy AW, 2021. Acceptance of Drug Use Mediates Future Hard Drug Use Among At-Risk Adolescent Marijuana, Tobacco, and Alcohol Users. Prev Sci 22 (5), 545–554. 10.1007/s11121-020-01165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ, 2007. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol. Biochem. Behav. 86 (2), 200–208. 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Lowing JL, Susick LL, Bowen SE, 2012. Investigation of calcium-stimulated adenylyl cyclases 1 and 8 on toluene and ethanol neurobehavioral actions. Neurotoxicol. Teratol. 34 (5), 481–488. 10.1016/j.ntt.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Crossin R, Arunogiri S, 2020. Harms associated with inhalant misuse in adolescent females - a review of the pre-clinical and clinical evidence. Drug Alcohol Depend. 216, 108232 10.1016/j.drugalcdep.2020.108232. [DOI] [PubMed] [Google Scholar]
- Crossin R, Cairney S, Lawrence AJ, Duncan JR, 2017. Adolescent inhalant abuse leads to other drug use and impaired growth; implications for diagnosis. Aust. N. Z. J. Public Health 41 (1), 99–104. 10.1111/1753-6405.12595. [DOI] [PubMed] [Google Scholar]
- Crossin R, Scott D, Witt KG, Duncan JR, Smith K, Lubman DI, 2018. Acute harms associated with inhalant misuse: co-morbidities and trends relative to age and gender among ambulance attendees. Drug Alcohol Depend. 190, 46–53. 10.1016/j.drugalcdep.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Crossin R, Lawrence AJ, Andrews ZB, Churilov L, Duncan JR, 2019a. Growth changes after inhalant abuse and toluene exposure: A systematic review and meta-analysis of human and animal studies. Hum. Exp. Toxicol. 38 (2), 157–172. 10.1177/0960327118792064. [DOI] [PubMed] [Google Scholar]
- Crossin R, Qama A, Andrews ZB, Lawrence AJ, Duncan JR, 2019b. The effect of adolescent inhalant abuse on energy balance and growth. Pharmacol. Res. Perspect. 7 (4), e00498 10.1002/prp2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz SL, Bowen SE, 2021. The last two decades on preclinical and clinical research on inhalant effects. Neurotoxicol. Teratol. 87, 1–51 (doi:2021.106999). [DOI] [PubMed] [Google Scholar]
- Dick AL, Lawrence AJ, Duncan JR, 2014. Chronic intermittent toluene inhalation initiated during adolescence in rats does not alter voluntary consumption of ethanol in adulthood. Alcohol 48 (6), 561–569. 10.1016/j.alcohol.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Dick AL, Pooters T, Gibbs S, Giles E, Qama A, Lawrence AJ, Duncan JR, 2015. NMDA receptor binding is reduced within mesocorticolimbic regions following chronic inhalation of toluene in adolescent rats. Brain Res. 1624, 239–252. 10.1016/j.brainres.2015.07.037. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP, 2010. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 72 (1), 114–123. 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Chang GA, Southerland R, 2009. Age of inhalant first time use and its association to the use of other drugs. J Drug Educ 39 (3), 261–272. 10.2190/DE.39.3.c. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Barreto M, Spear LP, 2012. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behav. Neurosci. 126 (5), 735–741. 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos M, Cahill EN, Bo GD, Vanhoutte P, Caboche J, Giros B, Heck N, 2018. Cocaine increases dopaminergic connectivity in the nucleus accumbens. Brain Struct. Funct. 223 (2), 913–923. 10.1007/s00429-017-1532-x. [DOI] [PubMed] [Google Scholar]
- Evans EB, Balster RL, 1991. CNS depressant effects of volatile organic solvents. Neurosci. Biobehav. Rev. 15 (2), 233–241 (doi:S0149–7634(05)80003-X [pii]). [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 2007. The Mouse Brain in Stereotaxic Coordinates, Third edition.
- Frantz KJ, O’Dell LE, Parsons LH, 2007. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacol 32 (3), 625–637. 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Gospe SM Jr., Calaban MJ, 1988. Central nervous system distribution of inhaled toluene. Fundam. Appl. Toxicol. 11 (3), 540–545. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3220222. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, Bucholz KK, 2006. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med 36 (1), 109–118. 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A, 2007. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology 191 (2), 311–322. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17206494. [DOI] [PubMed] [Google Scholar]
- Hinman DJ, 1984. Tolerance and reverse tolerance to toluene inhalation: effects on open-field behavior. Pharmacol. Biochem. Behav. 21 (4), 625–631. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6542228. [DOI] [PubMed] [Google Scholar]
- Hinman DJ, 1987. Biphasic dose-response relationship for effects of toluene inhalation on locomotor activity. Pharmacol. Biochem. Behav. 26 (1), 65–69. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3562499. [DOI] [PubMed] [Google Scholar]
- Hoops D, Flores C, 2017. Making dopamine connections in adolescence. Trends Neurosci. 40 (12), 709–719. 10.1016/j.tins.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2019. Monitoring the Future National Survey Results on Drug Use, 1975–2018. University of Michigan, Ann Arbor, MI. [Google Scholar]
- Koga Y, Higashi S, Kawahara H, Ohsumi T, 2007. Toluene inhalation increases extracellular noradrenaline and dopamine in the medial prefrontal cortex and nucleus accumbens in freely-moving rats. J. Kyushu Dental Soc. 61 (1), 39–54. Retrieved from. https://www.jstage.jst.go.jp/article/kds/61/1/61_1_39/_article. [Google Scholar]
- Kouzoupis AV, Konstantakopoulos G, Panagiotis O, Kalfakis N, Papageorgiou SG, 2010. A case of severe toluene withdraw syndrome treated with clonazepam. J. Neuropsychiatr. Clin. Neurosci. 22 (1), 123.e116–123.e117. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP, 1995. Cocaine sensitization in periadolescent and adult rats. J. Pharmacol. Exp. Ther. 275 (1), 345–357. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7562570. [PubMed] [Google Scholar]
- Ledesma JC, Aguilar MA, Gimenez-Gomez P, Minarro J, Rodriguez-Arias M, 2017. Adolescent but not adult ethanol binge drinking modulates cocaine withdrawal symptoms in mice. PLoS One 12 (3), e0172956. 10.1371/journal.pone.0172956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Lawrence AJ, 2008. Inhalant abuse among adolescents: neurobiological considerations. Br. J. Pharmacol. 154 (2), 316–326. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18332858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JM, 2008. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol 42 (8), 617–621. 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, Patrick ME, O’Malley PM, Bachman JG, Schulenberg JE, 2023. Monitoring the Future National Survey Results on Drug Use, 1975–2022: Secondary School Students. Institute for Social Research, The University of Michigan, Ann Arbor. Retrieved from. http://monitoringthefuture.org/results/publications/monographs/. [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL, 2nd., 2010. Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol. Clin. Exp. Res. 34 (4), 734–742. 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Perrine SA, Finton BJ, Galloway MP, Howell LL, Fantegrossi WE, 2012. Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: correlations between behavior and neurochemistry. Psychopharmacology 220 (3), 495–508. 10.1007/s00213-011-2504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y, 2022. Sex differences in opioid and psychostimulant craving and relapse: A critical review. Pharmacol. Rev. 74 (1), 119–140. 10.1124/pharmrev.121.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, You C, Arora DS, McElvain MA, Vandegrift BJ, Brodie MS, Woodward JJ, 2016. Differential effects of toluene and ethanol on dopaminergic neurons of the ventral tegmental area. Front. Neurosci. 10, 434. 10.3389/fnins.2016.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkansah-Amankra S, Minelli M, 2016. Gateway hypothesis” and early drug use: Additional findings from tracking a population-based sample of adolescents to adulthood. Prev Med Rep 4, 134–141. 10.1016/j.pmedr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRS, 2011. Guide for the Care and Use of Laboratory Animals. National Research Council (U.S.)., Institute for Laboratory Animal Research (U.S.), National Academies Press, Washington, D.C. [Google Scholar]
- Papageorgiou SG, Karantoni E, Pandis D, Kouzoupis AV, Kalfakis N, Limouris GS, 2009. Severe dopaminergic pathways damage in a case of chronic toluene abuse. Clin. Neurol. Neurosurg. 111 (10), 864–867. 10.1016/j.clineuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C, 2009. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 108 (4), 920–931. 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pastino GM, Sultatos LG, Flynn EL, 1996. Development and application of a physiologically based pharmacokintetic model for ethanol in the mouse. Alcohol Alcohol. 31 (4), 365–374. [DOI] [PubMed] [Google Scholar]
- Perrine SA, O’Leary-Moore SK, Galloway MP, Hannigan JH, Bowen SE, 2011. Binge toluene exposure alters glutamate, glutamine and GABA in the adolescent rat brain as measured by proton magnetic resonance spectroscopy. Drug Alcohol Depend. 115 (1–2), 101–106. 10.1016/j.drugalcdep.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E, 2012. Developmental differences in ethanol-induced sensitization using postweanling, adolescent, and adult Swiss mice. Psychopharmacology 219 (4), 1165–1177. 10.1007/s00213-011-2453-7. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E, 2014. Higher long-lasting ethanol sensitization after adolescent ethanol exposure in mice. Psychopharmacology 231 (8), 1821–1829. 10.1007/s00213-013-3376-2. [DOI] [PubMed] [Google Scholar]
- Rees DC, Coggeshall E, Balster RL, 1985. Inhaled toluene produces pentobarbital-like discriminative stimulus effects in mice. Life Sci. 37 (14), 1319–1325. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/4046735. [DOI] [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Breen TJ, Balster RL, 1987a. Toluene, halothane, 1,1,1-trichloroethane and oxazepam produce ethanol-like discriminative stimulus effects in mice. J. Pharmacol. Exp. Ther. 243 (3), 931–937. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3694538. [PubMed] [Google Scholar]
- Rees DC, Knisely JS, Jordan S, Balster RL, 1987b. Discriminative stimulus properties of toluene in the mouse. Toxicol. Appl. Pharmacol. 88 (1), 97–104. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3564034. [DOI] [PubMed] [Google Scholar]
- Rees DC, Wood RW, Laties VG, 1989. Evidence of tolerance following repeated exposure to toluene in the rat. Pharmacol. Biochem. Behav. 32 (1), 283–291. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/2734339. [DOI] [PubMed] [Google Scholar]
- Riegel AC, French ED, 1999. Acute toluene induces biphasic changes in rat spontaneous locomotor activity which are blocked by remoxipride. Pharmacol. Biochem. Behav. 62 (3), 399–402. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10080229. [DOI] [PubMed] [Google Scholar]
- Riegel AC, French ED, 2002. Abused inhalants and and central reward pathways. Ann. N. Y. Acad. Sci. 965, 281–291. [PubMed] [Google Scholar]
- Riegel AC, Ali SF, French ED, 2003. Toluene-induced locomotor activity is blocked by 6-hydroxydopamine lesions of the nucleus accumbens and the mGluR2/3 agonist LY379268. Neuropsychopharmacol 28 (8), 1440–1447. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12784113. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Ali SF, Torinese S, French ED, 2004. Repeated exposure to the abused inhalant toluene alters levels of neurotransmitters and generates peroxynitrite in nigrostriatal and mesolimbic nuclei in rat. Ann. N. Y. Acad. Sci. 1025, 543–551. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15542760. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP, 2005. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol. Clin. Exp. Res. 29 (10), 1809–1820. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16269910. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2003. Addiction. Annu. Rev. Psychol. 54, 25–53. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12185211. [DOI] [PubMed] [Google Scholar]
- Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, Dreisbach JN, Hormes JT, Filley CM, 1988. Toluene abuse causes diffuse central nervous system white matter changes. Ann. Neurol. 23 (6), 611–614. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3408242. [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2022. Key substance use and mental Health indicators in the United States: results form the 2021 National Survey on Drug Use and Health. In: Center for Beahviroal Health Statistics and Quality, Substance Abuse and Mental Health Services Administration., (HHS Publication No. PEP22–07-005, HSDUH Series H-57). Retrieved from. https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report. [Google Scholar]
- Samuel-Herter SR, Slaght SL, McKay BE, 2014. Age-dependent time courses of recovery for motor functions following acute toluene intoxication in rats. Dev. Psychobiol. 56 (4), 657–673. 10.1002/dev.21134. [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000a. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24 (4), 417–463. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10817843. [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000b. Modeling adolescent development and alcohol use in animals. Alcohol Res. Health 24 (2), 115–123. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11199278. [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC, 1983. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 16 (2), 83–109. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6339302. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI, 2005. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev. Alcohol. 17, 143–159. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15789864. [PubMed] [Google Scholar]
- Stengard K, Hoglund G, Ungerstedt U, 1994. Extracellular dopamine levels within the striatum increase during inhalation exposure to toluene: a microdialysis study in awake, freely moving rats. Toxicol. Lett. 71 (3), 245–255. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8160213. [DOI] [PubMed] [Google Scholar]
- Svenson DW, Davidson CJ, Thakur C, Bowen SE, 2022. Acute exposure to abuse-like concentrations of toluene induces inflammation in mouse lungs and brain. J. Appl. Toxicol. 1–10 10.1002/jat.4285. [DOI] [PubMed] [Google Scholar]
- Tomaszycki ML, Aulerich KE, Bowen SE, 2013. Repeated toluene exposure increases c-Fos in catecholaminergic cells of the nucleus accumbens shell. Neurotoxicol. Teratol. 40C, 28–34. 10.1016/j.ntt.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Torres OV, 2022. Sex differences in psychostimulant abuse: implications for estrogen receptors and histone deacetylases. Genes (Basel) 13 (5). 10.3390/genes13050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violante-Soria V, Cruz SL, Rodriguez-Manzo G, 2019. Sexual behaviour is impaired by the abused inhalant toluene in adolescent male rats. Eur. J. Neurosci. 50 (3), 2113–2123. 10.1111/ejn.13969. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M, 2010. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci. Biobehav. Rev. 34 (5), 631–648. 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DA, Bowen SE, Jennings WB, Dallas CE, Balster RL, 2000. Biphasic effects of 1,1,1-trichloroethane on the locomotor activity of mice: relationship to blood and brain solvent concentrations. Toxicol. Sci. 56 (2), 365–373. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10910995. [DOI] [PubMed] [Google Scholar]
- Wayman WN, Woodward JJ, 2018a. Chemogenetic excitation of accumbens-projecting infralimbic cortical neurons blocks toluene-induced conditioned place preference. J. Neurosci. 38 (6), 1462–1471. 10.1523/JNEUROSCI.2503-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman WN, Woodward JJ, 2018b. Exposure to the abused inhalant toluene alters medial prefrontal cortex physiology. Neuropsychopharmacol 43 (4), 912–924. 10.1038/npp.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiaderna D, Tomas T, 2000. Effects of repeated exposure to toluene or amphetamine on locomotor activity in rats. Int. J. Occup. Med. Environ. Health 13 (4), 317–324. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11276845. [PubMed] [Google Scholar]
- Wiaderna D, Tomas T, 2002. Assessment of long-term effects of exposure to toluene based on the analysis of selected behavioral responses with particular reference to the ability to trigger behavioral hypersensitivity in rats. Int. J. Occup. Med. Environ. Health 15 (3), 239–245. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12462451. [PubMed] [Google Scholar]
- Wood RW, Colotla VA, 1990. Biphasic changes in mouse motor activity during exposure to toluene. Fundam. Appl. Toxicol. 14 (1), 6–14. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2307323. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Beckley JT, 2014. Effects of the abused inhalant toluene on the mesolimbic dopamine system. J. Drug Alcohol Res. 3, 1–8. 10.4303/jdar/235838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Braunscheidel KM, 2023. The effects of the inhalant toluene on cognitive function and behavioral flexibility: A review of recent findings. Addict. Neurosci. 5 10.1016/j.addicn.2022.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi N, Okada S, Kodama K, Hirai S, Sekine H, Murakami A, Sato T, 1995. White matter changes caused by chronic solvent abuse. AJNR Am. J. Neuroradiol. 16 (8), 1643–1649. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7502969. [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Beechinor RJ, Thompson T, Schorzman AN, Zamboni W, Crona DJ, Tarantino LM, 2021. Pharmacokinetic and pharmacodynamic analyses of cocaine and its metabolites in behaviorally divergent inbred mouse strains. Genes Brain Behav. 20 (2), e12666 10.1111/gbb.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhvania MG, Bikashvili TZ, Japaridze NJ, Gelazonia LK, Chilachava LR, Okuneva V, 2010. Effect of toluene chronic inhalation on the process of learning in adult and adolescent rats. In: Child and Adolescent Disorders and Treatment - Disorders (Clinical), S610. [Google Scholar]
- Zhvania MG, Chilachava LR, Japaridze NJ, Gelazonia LK, Lordkipanidze TG, 2012. Immediate and persisting effect of toluene chronic exposure on hippocampal cell loss in adolescent and adult rats. Brain Res. Bull. 87 (2–3), 187–192. 10.1016/j.brainresbull.2011.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


