Abstract
Intermediate uveitis (IU) may be associated with multiple sclerosis (MS), with both conditions possibly sharing pathogenic mechanisms. Two patients presented with bilateral IU. Despite targeted uveitis treatment with corticosteroids and methotrexate, both had ongoing disease activity with symptoms, and fluorescein angiographic abnormalities. Both were subsequently identified to have radiologically isolated MS in the absence of clinical demyelination. Treatment with natalizumab in isolation, led to rapid and sustained resolution of uveitis, enabling discontinuation of other immunosuppression. This case series adds evidence supporting use of alpha-4 integrins in the treatment of MS-associated uveitis, in addition to its known high-efficacy in MS.
Keywords: Uveitis, multiple sclerosis, natalizumab, demyelination
Introduction
Uveitis is an important cause of visual impairment globally. Intermediate uveitis (IU) predominantly involves the vitreous humour, with frequent retinal vasculitis involving the peripheral retinal veins. 1 IU can be associated with multiple sclerosis (MS). 1 Patients with IU may present with an insidious onset of floaters, blurred vision, photophobia and a red eye. Further features which may differentiate IU from ON include the presence of vitreous opacities, exudates, or haemorrhage; and the absence of a relative afferent pupillary defect in IU. 1 Optic neuritis may present with loss of visual acuity, pain with eye movement, and colour desaturation. The prevalence of MS among patients with uveitis, and conversely of uveitis in patients with MS, is around 1%. Although the exact pathogenesis of uveitis in MS is unknown, both conditions may rely on shared mechanisms such as the controlled migration of activated lymphocytes into target tissues.
A unified treatment for both MS and uveitis without exposure to additional immunosuppression is of value. We describe two patients with treatment-resistant IU and MS, who had a favourable response to natalizumab as primary treatment for uveitis, while facilitating radiological remission of MS. Ethics approval was granted (2019/ETH06041, 2020/STE05763), and patients provided informed written consent. Deidentified clinical data can be provided to requesting researchers upon reasonable request, with data sharing agreements in place.
Results
Case 1
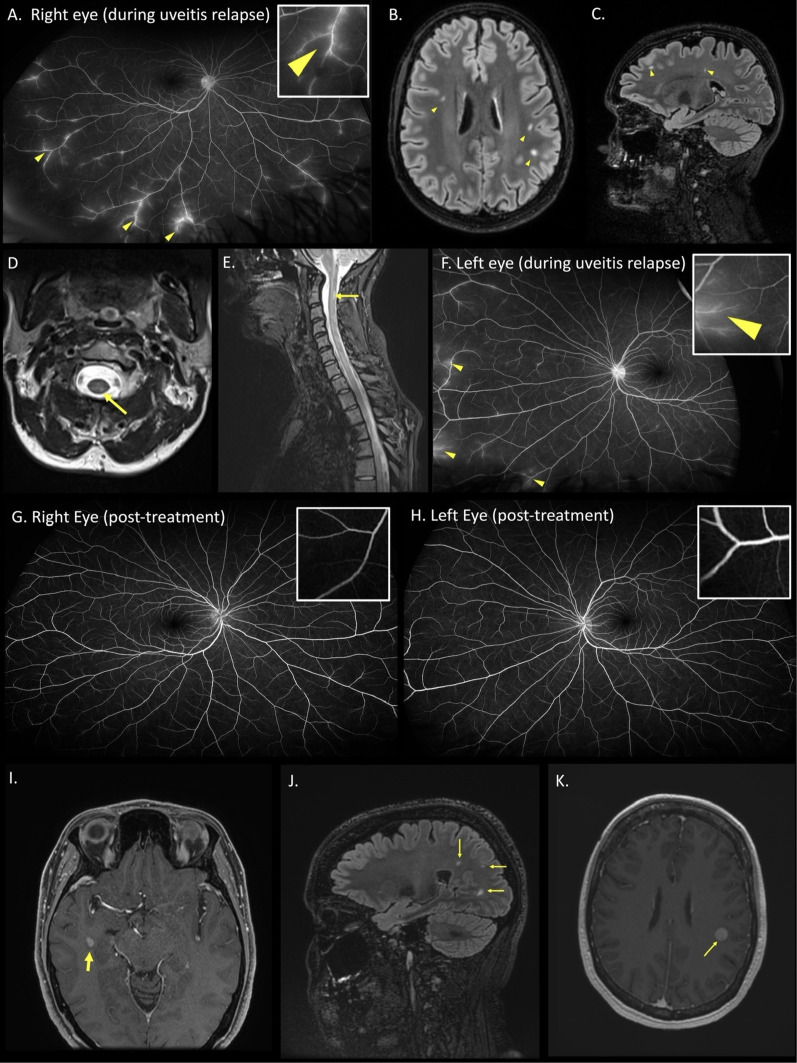
A 28-year-old female presented with painless blurred vision. Visual acuity was 6/12 on the right, and 6/5 on the left. She was managed with corticosteroid eyedrops, but relapsed at 5 months, with peripheral vein leakage on fluorescein angiography (Figure 1A), consistent with IU.
Figure 1.
Case 1 (A) fluorescein angiography of the right eye during a relapse of uveitis. Arteriovenous phase showing leakage of fluorescein dye in peripheral retinal veins (arrowheads and inset) consistent with retinal periphlebitis. (B-C) Axial and Sagittal T2 FLAIR MRI sequences of the brain showing multifocal white matter hyperintense lesions in a distribution typical of multiple sclerosis (arrowheads). (D-E) Axial and sagittal T2 MRI sequences of the spine showing an ill-defined left posterior hyperintense lesion at the level of C2 (arrows). (F) Fluorescein angiography of the left eye upon relapse after treatment interruption of 5 months, showing retinal periphlebitis (arrowheads and inset). (G-H). Progress wide-field fluorescein angiography (early arteriovenous phase) performed 7 months after restarting treatment, showing resolution of retinal periphlebitis (inset). Case 2 (I) Axial T1 post-contrast MRI brain showing a gadolinium-enhancing lesion (arrow) in the right anterior temporal horn white matter. (J) Sagittal T2 FLAIR MRI brain showing non-enhancing juxtacortical white matter lesions (arrows). (K) 4 months later, axial T2 post-contrast MRI brain showed a new enhancing lesion in the left inferior frontoparietal lobe (arrow).
An MRI brain performed to investigate headaches 18 months prior to uveitis onset, revealed multiple non-enhancing white matter periventricular lesions. Progress MRI brain and spine at the onset of uveitis showed interval development of a left inferior-parietal periventricular white matter lesion and short-segment transverse myelitis typical of MS (Figure 1B-E). Cerebrospinal fluid (CSF) analysis revealed intrathecally-restricted oligoclonal bands. She was diagnosed with radiologically isolated syndrome.
She was recommenced on corticosteroid eyedrops, fortnightly intravenous methylprednisolone, and methotrexate 20 mg/week for seven months. Her uveitis responded to treatment, but relapsed twice upon weaning corticosteroids. Natalizumab was commenced with clinical and angiographic resolution of uveitis over two weeks. Other treatments including topical steroids, methylprednisolone and methotrexate were successfully weaned, maintaining remission on natalizumab alone. Complete steroid cessation was achieved for the first time, within two months of natalizumab treatment. After eight months on natalizumab, her John Cunningham virus serology returned positive (index > 4), necessitating transition to another agent given the risk of progressive multifocal leukoencephalopathy. A four-month patient-initiated treatment interruption resulted in another uveitis relapse confirmed on fluorescein angiogram (Figure 1F), coinciding with an enhancing supratentorial lesion. She was commenced on ocrelizumab, with radiological remission of MS and resolution of uveitis (Figure 1G-H).
Case 2
A 47-year-old female presented with a two-month history of subjective visual dysfunction and patchy visual floaters. Visual acuity was 6/6 bilaterally, without relative afferent pupillary defect or colour desaturation. Fundoscopy revealed vitreous debris bilaterally, and she was diagnosed with IU, with corticosteroid eyedrops commenced. An MRI brain showed multiple T2 hyperintense lesions, with an enhancing right anterior temporal lesion (Figure 1I-J) deemed typical for MS, without optic nerve involvement. Visual evoked potentials showed prolonged full-field latencies bilaterally (right 121 ms, left 137 ms) likely reflecting prior subclinical demyelinating optic neuritis.
Despite four months of corticosteroid eyedrops for IU, there was ongoing visual dysfunction and visual floaters which did not abate. Progress MRI brain showed a new enhancing left inferior frontoparietal subcortical lesion (Figure 1K). She was commenced on natalizumab. Six-month progress review showed resolution of symptoms and vitreous opacities on natalizumab alone, with MRI at 12 months demonstrating continued remission.
Discussion
This case series demonstrates the response of IU associated with MS to natalizumab monotherapy. Both patients fulfilled the criteria for radiologically isolated MS following onset of IU, and both their uveitis and MS remained in remission following the use of natalizumab. This suggests a possible linked mechanism for these conditions. It is increasingly recognised that radiologically isolated MS predicts transition to symptomatic MS and represents the same disease process, particularly when additional risk factors such as intrathecally restricted oligoclonal bands, spinal cord lesions, or gadolinium-enhancing or new lesions are present on follow-up imaging, despite absence of clinical demyelination. This was the case in both patients.
A unique challenge in managing MS-related uveitis is that some of the disease-modifying therapies for MS pose a risk of visual complications, such as macular oedema seen in S1P inhibitors. 2 Conversely, TNF-alpha inhibitors frequently used to treat uveitis may trigger demyelination. 3 It would be particularly advantageous for patients to have a unified management approach of MS and its associated uveitis, to reduce the risk of immunosuppression-related adverse effects.
IU is the most common form of uveitis seen in MS, accounting for 61–80% of MS-associated uveitis. 4 It is considered to be T-cell mediated, driven by CD4 + Th1/Th17 cells causing blood-retinal barrier disruption and recruitment of additional effector cells. This may occur because of leukocyte interaction with the retinal extracellular matrix and vascular endothelium through an integrin family of cell adhesion molecules (CAMs), transducing signals downstream to alter cell morphology and facilitate leukocyte recruitment. 5 There is evidence that α4 integrin acts as a mediator of leukocyte adhesion in the pathophysiology of diabetic retinopathy. 6
Targeting integrins is plausible in treating both conditions due to the role of CAMs in their pathophysiology. However, evidence on the use of CAM inhibitors in the treatment of IU is limited. Studies in experimental autoimmune uveitis (EAU) have shown α4-integrin inhibitor peptides reduced the signs of actively induced EAU in mice. 7 Natalizumab, an α4β1 integrin inhibitor and high-efficacy MS disease-modifying therapy, prevents leukocyte migration through the blood-brain barrier. A case report of a patient with tumefactive MS treated with natalizumab reported complete resolution of their uveitis. 8 A recent cohort of uveitis patients with MS showed only one of five patients treated with natalizumab had ongoing uveitis. 9 Further support for the use of CAM inhibitors is noted in a case of a patient with IU in the setting of Crohn's disease, with complete resolution of uveitis in response to vedolizumab, an α4β1 inhibitor. 10
Our case series adds evidence for the potential role of natalizumab in uveitis with co-existent MS, and minimises the need for additional standard immunosuppressive uveitis treatments including corticosteroids. Currently, the role of natalizumab in treating uveitis in the absence of MS is still unknown, however our observations encourage further investigation of integrins as a target in uveitis beyond the MS spectrum.
Acknowledgments
The authors would like to acknowledge Dr Lynette Masters for her expert opinion on the review of the medical imaging.
Footnotes
Author contributions: ADC and SuRa had full access to all of the data in the study and SR is responsible for the overall content as guarantor. ADC, SWR and SuRa contributed to the conception, design of the study and statistical analyses. All authors contributed to acquisition, analysis and interpretation of data. ADC and SuRa contributed to drafting the text and preparing the figure. All authors contributed to the critical review of the manuscript for important intellectual content. SR obtained funding and supervised ADC.
Data availability statement: Deidentified clinical data can be provided to requesting researchers upon reasonable request, with data sharing agreements in place.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SWR has no disclosures relevant to the submitted work. He has received travel support, honoraria, trial payments, research and clinical support to the neurology department or academic projects from NHMRC, MRFF, NBA, Myasthenia Alliance Australia, Lambert Initiative, Beeren foundation, anonymous donors; and from pharmaceutical/biological companies: Alexion, Biogen, CSL, Genzyme, Grifols, Merck, Novartis, Roche, Sandoz, Sanofi, UCB. He is co-founder/shareholder of RxPx health, National IVIG Governance Advisory Council & Specialist Working Group Australia (Neurology) (paid), Australian Medical Services Advisory Committee ad-hoc sub-committee on IVIG (paid), Australian Technical Advisory Group on Immunisation Varicella Zoster working party (unpaid), Medical advisor (unpaid) to various patient and advocacy groups. Funds over the last 5 years including but not limited to travel support, honoraria, trial payments, research and clinical support to the neurology department or academic projects from: NHMRC, MRFF, NBA, Myasthenia Alliance Australia, Lambert Initiative, Beeren foundation, anonymous donors; and from pharmaceutical/biological companies: Alexion, Biogen, CSL, Genzyme, Grifols, Merck, Novartis, Roche, Sandoz, Sanofi, UCB.
SuRa has no disclosures relevant to the submitted work. She has received research funding from the National Health and Medical Research Council (NHMRC, Australia), the Petre Foundation, the Brain Foundation, the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for educational/research sessions coordinated by Biogen, Alexion, Novartis, Excemed and Limbic Neurology. She is on the medical advisory board (non-remunerated positions) of The MOG Project and the Sumaira Foundation.
All other authors have no relevant disclosures.
Ethical considerations: Ethics approval was granted (2019/ETH06041, 2020/STE05763), and patients provided informed written consent.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research funding CIA Ramanathan: National Health and Medical Research GNT1141169, GNT2008339; Royal Australasian College of Physicians Research Establishment Fellowship; University of Sydney SOAR Prize and Rewarding Research Success Grants
Faculty of Medicine and Health, University of Sydney, National Health and Medical Research Council, RACP Foundation, (grant number SOAR and Rewarding Research Success Grants, GNT2008339 CIA Ramanathan, Research Establishment Fellowship CIA Ramanathan).
The funders had no role in study design, data collection, data analysis, data interpretation, manuscript writing, or submission for publication.
ORCID iDs: Stephen W Reddel https://orcid.org/0000-0002-0169-3350
Sudarshini Ramanathan https://orcid.org/0000-0002-0294-9768
References
- 1.Abraham A, Nicholson L, Dick A, et al. Intermediate uveitis associated with MS: diagnosis, clinical features, pathogenic mechanisms, and recommendations for management. Neurol Neuroimmunol Neuroinflamm 2021; 8: e909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarbin MA, Jampol LM, Jager RD, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology 2013 Jul; 120: 1432–1439. [DOI] [PubMed] [Google Scholar]
- 3.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-α Blockers. Curr Neurol Neurosci Rep 2017 Apr; 17: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de-la-Torre A, Silva-Aldana CT, Muñoz-Ortiz J, et al. Uveitis and multiple sclerosis: potential common causal mutations. Mol Neurobiol 2019 Dec; 56: 8008–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YH, Lightman S, Eskandarpour M, et al. Adhesion molecule targeted therapy for non-infectious uveitis. Int J Mol Sci 2022 Jan 3; 23: 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliaki E, Poulaki V, Mitsiades N, et al. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci 2009; 50: 4898–4904. [DOI] [PubMed] [Google Scholar]
- 7.Martín AP, de Moraes LV, Tadokoro CE, et al. Administration of a peptide inhibitor of alpha4-integrin inhibits the development of experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 2005 Jun; 46: 2056–2063. [DOI] [PubMed] [Google Scholar]
- 8.Roemer S, Bissig A, Rocca A, et al. Efficacy of natalizumab in intermediate uveitis related to multiple sclerosis: a case report. Klin Monbl Augenheilkd 2018 Apr; 235: 476–477. [DOI] [PubMed] [Google Scholar]
- 9.Fitoussi R, Gascon P, Denis D, et al. Epidemiological, clinical, and therapeutic profile of uveitis in multiple sclerosis: a multicenter study. Ocul Immunol Inflamm 2024;32: 2185–2189. [DOI] [PubMed] [Google Scholar]
- 10.Fleisher M, Marsal J, Lee SD, et al. Effects of vedolizumab therapy on extraintestinal manifestations in inflammatory bowel disease. Dig Dis Sci 2018 Apr; 63: 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]