Abstract
Obesity presents a significant challenge in managing patients with craniopharyngioma (CP). Cyst fluid (CF), rich in inflammatory mediators, is implicated in CP‐related obesity, though the precise mechanism remains unclear. This study investigated the impact of CF or C‐X‐C motif chemokine ligand‐1 (CXCL1) injections on body weight, Lee index, plasma lipid profiles, hepatic lipid accumulation, leptin levels, NF‐κB pathway, the suppressor of cytokine signaling 3 (SOCS3) expression, and leptin sensitivity in rats. Bioinformatics was employed to identify differentially expressed genes (DEGs) between CF/CXCL1‐treated and control SY5Y cells, as well as to confirm enriched pathways. Western blotting was used for experimental validation, including the effects of sodium salicylate (SS) on leptin sensitivity in SY5Y cells. Injecting CF or CXCL1 into the brain, without hypothalamic damage, led to increased body weight, Lee index, and hepatic lipid accumulation in rats, alongside elevated fasting blood glucose, triglycerides, and total cholesterol, while high‐density lipoprotein cholesterol levels decreased. Additionally, CF and CXCL1 could induce elevated leptin levels, a higher leptin‐to‐body weight ratio, and resistance to exogenous leptin by activating the NF‐κB pathway and upregulating the expression of SOCS3 in rats. Further validation confirmed that CF and CXCL1 suppress leptin signaling by activating the NF‐κB pathway and upregulating SOCS3. Moreover, SS mitigated the inhibitory effects of CF or CXCL1 on leptin signaling, preserving leptin sensitivity in SY5Y cells. These results highlight the obesogenic role of CF and CXCL1, offering insights into the development of morbid obesity through inflammatory factor‐mediated leptin resistance, independent of hypothalamic damage. SS may serve as a promising therapeutic approach for CP‐associated obesity, though additional clinical studies are necessary to confirm its efficacy.
Keywords: craniopharyngioma, inflammatory mediator, leptin resistance, NF‐κB pathway, obesity, SOCS3
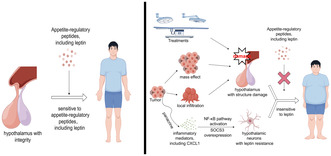
The difference in sensitivity to appetite‐regulatory peptides between normal controls and patients with CP. Left: In normal individuals, the hypothalamus accurately senses levels of various appetite‐regulatory peptides to maintain energy balance. Right: In patients with CP, the hypothalamus can be affected by the tumor's mass effect, invasion, or treatment‐induced structural damage. Additionally, inflammatory mediators secreted by tumor cells activate the NF‐κB pathway and upregulate SOCS3 expression in hypothalamic neurons. These disruptions contribute to leptin resistance, impair the hypothalamus's ability to regulate energy balance, and ultimately lead to morbid obesity in patients with CP.
Abbreviations
- BP
biological processes
- CC
cellular components
- CF
cyst fluid
- CP
craniopharyngioma
- CXCL1
C‐X‐C motif chemokine ligand‐1
- DEGs
expressed genes
- ELISA
enzyme‐linked immunosorbent assay
- FBG
fasting blood glucose
- FC
fold change
- GO
gene ontology
- HDL
high‐density lipoprotein cholesterol
- HI
hypothalamic involvement
- KEGG
Kyoto encyclopedia of genes and genomes
- MF
molecular function
- NF‐κB
nuclear factor kappa B
- SD
standard deviation
- SOCS3
suppressor of cytokine signaling 3
- SS
sodium salicylate
- STAT3
signal transducer and activator of transcription 3
- TC
total cholesterol
- TG
triglycerides
1. INTRODUCTION
Craniopharyngioma (CP) is an uncommon intracranial neoplasm characterized by benign histopathology (WHO I), yet it exhibits aggressive behavior. 1 Its proximity to and invasion of critical adjacent structures often results in significant functional impairment, leading to a high prevalence of long‐term morbidities such as endocrine dysfunction, 2 psychiatric disorders, 3 and morbid obesity. 4
Morbid obesity in patients with CP substantially diminishes quality of life and exacerbates the risk of metabolic disorders, including cardiovascular disease, type II diabetes mellitus, and nonalcoholic fatty liver disease. 5 , 6 , 7 Additionally, obesity in this population is notably resistant to conventional treatments, 8 complicating the management of its prevalence. The hypothalamic nuclei play a pivotal role in regulating energy balance, as evidenced by extensive research demonstrating that hypothalamic damage induces obesity in rodent models and patients with sellar tumors. 9 , 10 , 11 However, hyperphagia and obesity occur in a significant proportion (over 50%) of patients with CP without hypothalamic involvement (HI) or third ventricle floor abnormalities, 12 suggesting that mechanisms beyond hypothalamic damage may contribute to obesity in CP.
The hypothalamus orchestrates energy expenditure and feeding behavior by detecting peripheral metabolic signals. 13 Leptin, a key metabolic regulator, acts on hypothalamic nuclei to suppress food intake and promote energy expenditure. 14 Disruption of leptin signaling, commonly seen as leptin resistance, destabilizes energy homeostasis and body composition. 15 , 16 In line with previous findings, 10 , 17 , 18 , 19 , 20 , 21 our study also identified hyperleptinemia in patients with CP, indicative of leptin resistance. 22 Various factors, including inflammatory mediators, are implicated in leptin resistance, 23 with evidence showing that these mediators contribute to its pathogenesis. 24 Notably, two independent studies revealed that CP cyst fluid (CF), rich in inflammatory mediators, plays a role in obesity development. 25 , 26 Furthermore, in one of our studies, a positive correlation was observed between C‐X‐C motif chemokine ligand‐1 (CXCL1) mRNA expression and leptin levels, and CXCL1 was associated with significant weight gain and new‐onset obesity in patients with CP. 27 These results suggest that inflammatory mediators, particularly CXCL1, in CF may promote morbid obesity in patients with CP by inducing leptin resistance in hypothalamic neurons.
Building on this, this study aims to investigate the obesogenic effects of CF and CXCL1 and to elucidate the role of leptin resistance in morbid obesity. Sodium salicylate (SS), a known COX inhibitor, has demonstrated anti‐inflammatory effects on adipose tissue and improved insulin sensitivity in both animal models and obese individuals. 28 , 29 However, whether SS ameliorates leptin resistance remains unclear. Therefore, this study explores the potential of SS to mitigate CF‐ or CXCL1‐induced leptin resistance.
2. MATERIALS AND METHODS
2.1. Cyst fluid collection
The study design was approved by the Ethics Committee of Beijing Tiantan Hospital, and informed consent was obtained from all patients or their guardians for the use of CF in research. CF was collected from four patients diagnosed with adamantinomatous craniopharyngioma during tumor resection using syringes, ensuring no blood contamination. The pooled CF was subsequently stored at −80°C until used in animal experiments. We confirmed a pronounced pro‐inflammatory cytokine profile within CF compared to cerebrospinal fluid by the ELISA test (date was not shown).
2.2. Animal groups and interventions
The animal experimental protocol received approval from the Ethical Committee of Experimental Animals at the Beijing Neurosurgery Institute. Young male Sprague–Dawley rats (6 weeks of age, body weight ranges from 160 to 180 g), sourced from Charles River Laboratories, were housed in a controlled environment with regulated temperature, humidity, and a 12‐h light–dark cycle. Throughout the study, the rats were given unrestricted access to water and a standard Chow diet. Experimental interventions began 1 week after their acclimatization to the animal care facility.
The rats were randomly divided into four groups: control (n = 6), sham (n = 6), CF (n = 6), and CXCL1 (n = 6). The control group did not undergo stereotactic surgery, while the sham, CF, and CXCL1 groups underwent stereotactic surgery for implantation of an osmotic minipump (Alzet, model 2006, Durect Cupertino, CA, USA) containing sterile saline, CF, or CXCL1 (10 μg/mL, Peprotech, Inc.), respectively. Tena‐Suck et al. 25 reported that syringe insertion and CF are capable of inducing brain tissue damage. Therefore, we implant the osmotic minipump allowing the substances to enter the lateral ventricle slowly, to reduce the hypothalamic damage caused by syring insertion or acute inflammation caused by a high dose of CF. Avoiding hypothalamic damage is critical for confirming the independent obesogenic role of CF and CXCL1 in CP. Thus, we implanted an osmotic minipump allowing CF into the lateral ventricle slowly and chose the lateral ventricle rather than the hypothalamus as the insertion site. The implantation procedure followed the established protocol. 30 In brief, anesthesia was induced via intraperitoneal injection of sodium pentobarbital (25 mg/kg), and the anesthetized rats were positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The pump was placed in a subcutaneous dorsal pocket and connected to a brain infusion cannula via a vinyl catheter (Alzet brain infusion kit 2, Durect Cupertino, CA, USA). Each cannula was implanted into the left lateral ventricle at coordinates relative to bregma (−0.8 mm anteroposterior; −1.3 mm lateral; −4.5 mm vertical from dura) and secured with dental cement. 30 We take steps (details were shown in Supplementary Material 1) to reduce severe side effects, such as fever, epilepsy attack, acute hydrocephalus, and death, in experimental rats following stereotactic surgery and improve the success rate of the animal model, including choosing healthy rats, precise and gentle surgical techniques, a sterile environment, proper anesthesia, accurate targeting, and providing 1‐week postoperative care. In the present animal experiment, a single rat exhibited a mild fever persisting for 2 days postsurgery, and the rat's body temperature normalized following the administration of antibiotics.
After 4 weeks postsurgery, body weight and nose‐to‐anus length were recorded to calculate the Lee index, a reliable measure of obesity in rodents analogous to the human body mass index. 31 Blood samples were collected from the medial canthus using a heparinized capillary tube under anesthesia; Subsequently, euthanasia was performed to facilitate the collection of liver and brain tissues. The Lee index is calculated by dividing the cube root of body weight (in grams) by the length (in centimeters). 31 An overview of the experimental design is provided in Figure 1A.
FIGURE 1.
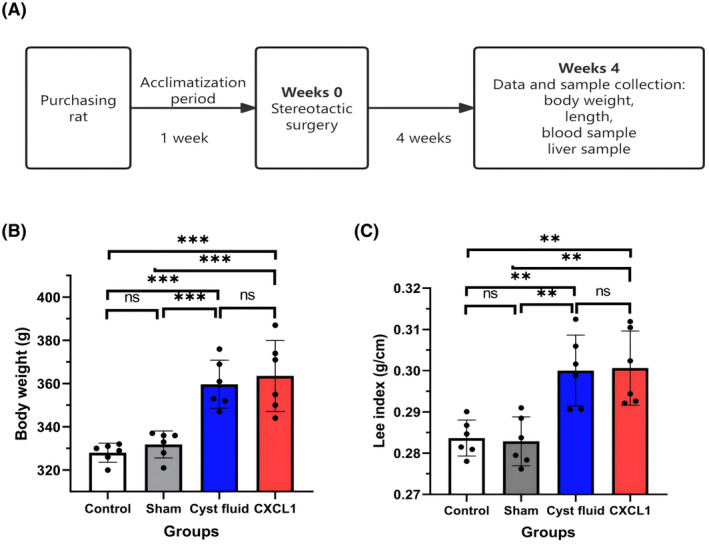
(A) Overview of the animal experiments; (B) Body weight of rats after 4 weeks of stereotactic surgery; (C) Lee index of rats after 4 weeks of stereotactic surgery. Data are expressed as mean ± standard deviation. ns, no significance; **p < .01; ***p < .001.
2.3. Levels of leptin, blood glucose, and blood lipids measurement
Each blood sample was collected in a heparinized Eppendorf tube and maintained at 4°C for 1 h before centrifugation at 5000 rpm for 10 min. The resulting plasma was then transferred to a freezer and stored at −80°C for subsequent analyses. Leptin and blood lipid levels, including triglycerides (TG), total cholesterol (TC), and high‐density lipoprotein cholesterol (HDL), were measured using enzyme‐linked immunosorbent assay (ELISA) kits from Gelatins Biological Reagent Co., LTD (GLC8776, GLC1435, GLC‐A10116, GLC‐A10126). Fasting blood glucose (FBG) was determined using a glucose assay kit with O‐toluidine from Beyotime Biotechnology (Catalog No. S0201S, Shanghai, China).
2.4. Lipid accumulation in the liver
Liver weights were recorded for each rat, and liver samples were stored at −80°C for further analysis. Lipid droplets in the liver were quantified through oil red O staining, following established protocols. 32 Fresh liver tissues were embedded in an optimal cutting temperature compound (Fisher Healthcare, Houston, TX), sectioned at 10 μm thickness on a cryostat, and fixed in 4% paraformaldehyde (PFA)/PBS for 10 min. After rinsing with water and 60% isopropanol, the sections were stained with oil red O (Millipore Sigma, St. Louis, MO) for 5 min, followed by rinsing with water and isopropanol. The slides were then mounted with glycerin jelly containing 7% (w/v) gelatin in a water‐glycerol mixture (50/50).
2.5. Histological change and impact on NF‐κB pathway in hypothalamic tissue
After the mice were sacrificed, the brain tissue was collected. The hematoxylin–eosin (H&E) staining was conducted to reveal histological changes and examine hypothalamic damage. 33 First, the brain tissue was prepared into 4 μm continuous frozen sections. Then, the section was stained with hematoxylin for 3 min, rinsed with tap water for 2 min, treated with 1% hydrochloric acid alcohol for 2 s, rinsed with tap water for 2 min, treated with 1% ammonia water for 20 s, stained with 0.5% eosin alcohol for 10 s, dehydrated with alcohol gradient, transparentized with xylene, and sealed with neutral resin. Finally, pathological changes in the brain were observed under a light microscope. Western blotting (WB) was conducted to detect the NF‐κB signal pathway and downstream expression of SOCS3 in hypothalamic tissue samples. The specific methodology employed for this procedure is comprehensively detailed in the section dedicated to WB. Additionally, the reverse‐transcription quantitative polymerase chain reaction (RT‐qPCR) was adopted to examine the expression of SOCS3 from the sample of hypothalamic tissue of each group. The detail was described in our previous studies. 22 , 27 Briefly, total RNA was extracted from the samples using a Steadypure universal RNA extraction kit (Accurate Biotechnology, Hunan, China, Cat No. AG21017). The sequences of the SOCS3 primer used were 5′‐GCCTCAAGACCTTCAGCTCCAAG‐3′ (forward) and 5′‐CGGTTACGGCACTCCAGTAGAATC‐3′ (reverse). 34 SYBR Green qPCR Mix (Biosharp Biotechnology, BL698A) was used to perform qPCR on QuantStudio 5 RealTime PCR System following the manufacturer's protocol. The housekeeping gene GAPDH was used for normalization. The relative mRNA expression was determined using the 2−ΔΔCT method and was expressed as log2 (X + 1).
2.6. Leptin sensitivity test
To assess whether CF or CXCL1 induces leptin resistance, a leptin sensitivity test was conducted, as outlined in Figure 4A. Four weeks poststereotactic surgery, baseline measurements of food intake, body weight, and the Lee index were recorded. Starting in the fifth week, rats received daily intraperitoneal injections of rat leptin (150 μg/kg) at 17:00 for three consecutive days. 35 Changes in daily food intake, body weight, and the Lee index were monitored throughout the fifth week.
FIGURE 4.
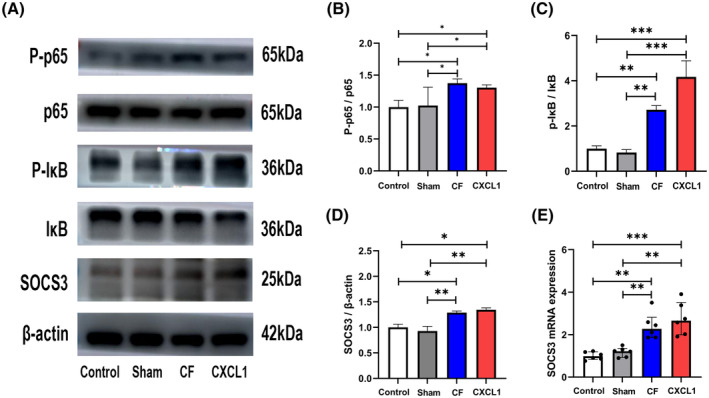
Cyst fluid and the inflammatory mediator CXCL1 activate the NF‐κB pathway and upregulate the expression of SOCS3 in the hypothalamus. (A) Representative images of NF‐κB pathway‐related protein and SOCS3 expression bands. (B, C) Western blots showing that cyst fluid or CXCL1 activates the NF‐κB pathway in the hypothalamus. (D, E) CF and CXCL1 could upregulate the expression of SOCS3 at the protein level and mRNA level. *p < .05; **p < .01; ***p < .001; CF, cyst fluid; CXCL1, C‐X‐C motif chemokine ligand‐1; IκB, inhibitor of nuclear factor‐kappa B Alpha; NF‐κB, nuclear factor kappa B; p65, p65 protein; P‐IκB, phosphorylated inhibitor of nuclear factor‐kappa B Alpha; P‐p65, phosphorylated p65 protein; SOCS3, suppressor of cytokine signaling 3; SS, sodium salicylate.
2.7. Cell culture and leptin receptor overexpression
Human neuroblastoma SY5Y cells, which closely resemble neurons, have been widely used to study the mechanisms of leptin resistance. 36 , 37 In this study, SY5Y cells were chosen as a substitute for hypothalamic neurons, which lack passage characteristics, to reduce variability in obtaining primary neurons from different rats. SY5Y cells (CL‐0208), generously provided by Procell Life Science & Technology Co., Ltd., were cultured in Dulbecco's modified Eagle's medium with 10% (v/v) heat‐inactivated fetal calf serum at 37°C in a humidified incubator containing 5% CO2 and 95% air. Following the manufacturer's protocol, Lip2000 Transfection Reagent (Biosharp Biotechnology) was used to transfect the human leptin receptor construct into SY5Y cells, and stable transfectants were selected using G418 antibiotic. 36 As a result, the SY5Y cells used in subsequent experiments stably overexpressed the leptin receptor.
2.8. Bioinformatic analysis
A bioinformatic analysis was conducted to examine the effects of CF and chemokine CXCL1 on biological processes and pathway activation. Initially, SY5Y cells were pretreated with sterile saline (5 μL/mL), CF (5 μL/mL), or CXCL1 (100 ng/mL) for 4 h. Nine samples were then collected, consisting of three control group samples treated with saline, three CF group samples treated with CF, and three CXCL1 group samples treated with CXCL1. The cells were washed with ice‐cold PBS before RNA extraction, followed by the alignment of sequencing data to the human reference genome (hg38). Transcript quantification was normalized using transcript per million values and log(x + 1) transformed. Further details of RNA extraction and sequencing have been described in previous work. 38 For statistical analysis, the CF and CXCL1 groups were combined into a single CF/CXCL1 group. Differentially expressed genes (DEGs) between the CF/CXCL1 group and the control group were identified using the “limma” R package, with significance defined as log2 fold change (FC) >1 and an adjusted p‐value <.05. Finally, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses for the DEGs were performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID; version 6.8; https://david.ncifcrf.gov/).
2.9. Experiment validation
The experiment was divided into four groups: the control group (SY5Y cells cultured under normal conditions), the leptin group (SY5Y cells treated with human leptin at 200 ng/mL for 2 h), the CF group (SY5Y cells pretreated with cystic fluid at 5 μL/mL for 4 h, followed by treatment with human leptin at 200 ng/mL for 2 h), and the CXCL1 group (SY5Y cells pretreated with CXCL1 at 100 ng/mL for 4 h, followed by treatment with human leptin at 200 ng/mL for 2 h). After these treatments, cells were harvested for WB analysis.
2.10. Drug intervention
To assess the potential of SS, a nonsteroidal anti‐inflammatory agent, in mitigating the effects of CF and CXCL1 on leptin resistance, another set of experiments was performed with four groups: the CF group (cells pretreated with CF at 5 μL/mL for 4 h, then exposed to leptin at 200 ng/mL for 2 h), the CF + SS group (cells pretreated with CF at 5 μL/mL and SS at 1 μg/mL for 4 h, followed by leptin treatment at 200 ng/mL for 2 h), the CXCL1 group (cells pretreated with CXCL1 at 100 ng/mL for 4 h, then treated with leptin at 200 ng/mL for 2 h), and the CXCL1 + SS group (cells pretreated with CXCL1 at 100 ng/mL and SS at 1 μg/mL for 4 h, followed by leptin treatment at 200 ng/mL for 2 h). After these treatments, the cells were collected for subsequent WB analysis.
2.11. Western blotting
For WB, cells were washed twice with ice‐cold PBS and lysed in RIPA buffer (Beyotime Biotechnology, P0013B, Shanghai, China) on ice for 30 min. Following lysis, supernatants were collected after centrifugation at 12 000 g for 10 min at 4°C. Protein concentrations were determined using a BCA protein assay kit. Proteins were separated by 10% SDS‐PAGE and transferred onto polyvinylidene difluoride membranes (IPVH00010, Millipore, Merck, Billerica, MA, USA). The membranes were subsequently probed with the indicated primary antibodies and horseradish peroxidase (HRP)‐conjugated secondary antibodies, followed by detection using an Enhanced Chemiluminescence Reagent (WBKLS0100, Millipore). The density of protein bands was quantified using Multi Gauge V 3.1 software, with results expressed as fold changes relative to the control group (pretreated with sterile saline), normalized to β‐actin. Phosphorylated STAT3 (Tyr705) and STAT3 antibodies were obtained from Assay Biotechnology, while phosphorylated IκB Alpha (Ser32/36), IκB Alpha, phosphorylated p65, and p65 antibodies were sourced from Proteintech Group, Inc. SOCS3 antibody was purchased from Immunoway Biotechnology Company.
2.12. Statistical methods
For statistical analysis, parametric variables were reported as means with standard deviations (SD) and evaluated using the Student's t‐test. Data analysis was conducted using IBM Statistical Package for the Social Sciences (SPSS, version 19.0) and GraphPad Prism (version 9.0). A paired t‐test was employed to assess differences in body weight before and after injections. One‐way ANOVA followed by Tukey's or Sidak's post hoc tests was applied to compare baseline data across the four experimental groups. Statistical significance was set at p < .05.
3. RESULTS
3.1. Body weight and Lee index in rats
The results of the study concerning body weight and Lee index across the four experimental groups are shown in Figure 1B,C. Both the CF and CXCL1 groups demonstrated significantly higher body weight and Lee index compared to the control and sham groups. No statistically significant differences were observed between the sham group and the control group for these measures. Similarly, no significant differences in body weight or Lee index were detected between the CF and CXCL1 groups.
3.2. Leptin and metabolic profiles in rats
Following the 4‐week intervention, leptin concentrations in both the CF and CXCL1 groups were significantly elevated compared to the control and sham groups (Figure 2A, all p < .001). The leptin concentration‐to‐body weight ratios also showed marked increases in the CF and CXCL1 groups relative to the control and sham groups (Figure 2B, all p < .01). Additionally, the CF and CXCL1 groups exhibited significantly higher levels of FBG (Figure 2C, all p < .01), TC (Figure 2D, all p < .05), and TG (Figure 2E, all p < .05), while HDL concentrations were significantly reduced in these groups compared to the control and sham groups (Figure 2F, all p < .05).
FIGURE 2.
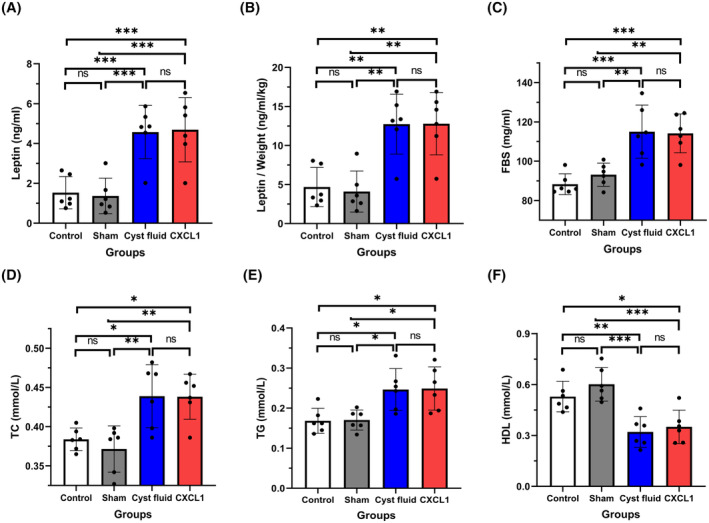
(A) Plasma leptin levels in the four groups; (B) Ratio of plasma leptin to body weight in the four groups; (C) Plasma fasting blood glucose (FBG) levels in the four groups; (D) Plasma total cholesterol (TC) levels in the four groups; (E) Plasma triglyceride (TG) levels in the four groups; (F) Plasma high‐density lipoprotein (HDL) levels in the four groups. Data are expressed as mean ± standard deviation. ns, no significance; *p < .05; **p < .01; ***p < .001.
3.3. Parameters of the liver of rats
The mean liver weights were 10.01 ± 0.87 g in the control group, 10.13 ± 0.77 g in the sham group, 20.40 ± 1.08 g in the CF group, and 20.53 ± 1.08 g in the CXCL1 group (Figure 3A). Corresponding liver‐to‐body weight ratios were 2.98%, 3.00%, 5.04%, and 5.06%, respectively (Figure 3B). These results indicate significantly higher liver weights and liver‐to‐body weight ratios in the CF and CXCL1 groups compared to the control and sham groups (Figure 3A,B, all p < .05). Consistently, oil red O staining revealed substantial hepatic lipid accumulation in the CF and CXCL1 groups, characterized by larger and more numerous lipid droplets (Figure 3C–E).
FIGURE 3.
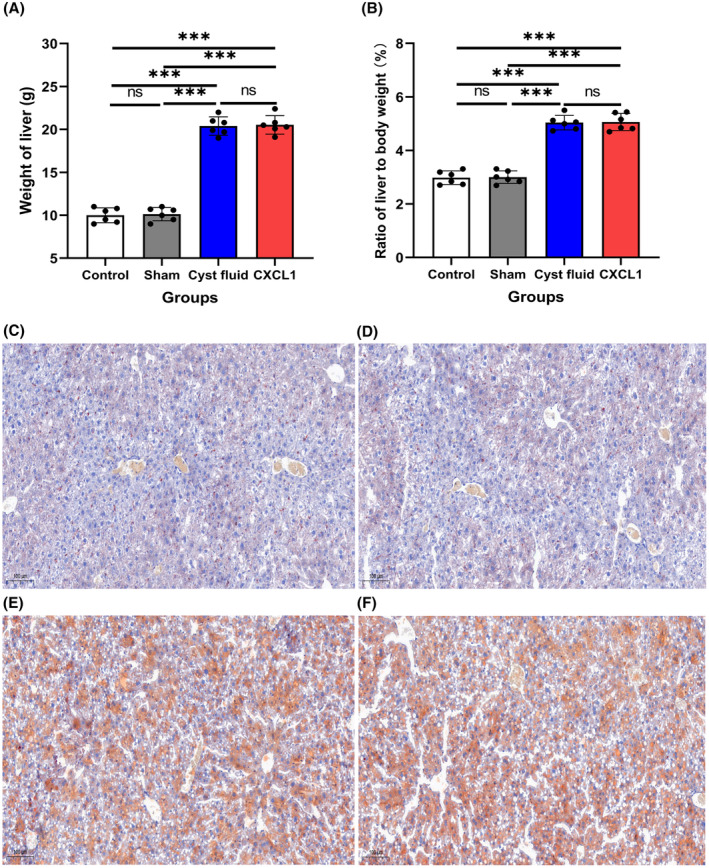
(A) Mean liver weight across the four groups; (B) Mean liver‐to‐body weight ratio across the four groups; (C–F) Representative images of oil red O‐stained liver sections: (C) control group, (D) sham group, (E) cyst fluid group, and (F) CXCL1 group. Data are expressed as mean ± standard deviation. ns, no significance; ***p < .001.
3.4. Impact of CF and CXCL1 on the NF‐κB pathway and expression of SOCS3 in the hypothalamus
Figure S1 presents images of cortical sections stained using the H&E technique for the four experimental groups. No significant morphological alterations, such as pyknosis, neuropil degradation, necrosis, cell shrinkage, inflammation, vacuolation, and edema, were observed across these groups. Furthermore, the WB analysis indicated activation of the NF‐κB pathway (Figure 4A–C) and elevated expression of SOCS3 (Figure 4A,D) in the CF and CXCL1 groups. Additionally, an increased level of SOCS3 mRNA expression was detected in the CF and CXCL1 groups (Figure 4E).
3.5. Effect of leptin on food intake, body weight, and Lee index of four groups
Given leptin's critical role in regulating energy intake, the study investigated whether CF and the inflammatory mediator CXCL1 contribute to obesity by altering the leptin response. A leptin sensitivity test was performed at the beginning of the fifth week. As expected, leptin administration produced an anorexigenic effect in the control and sham groups, evidenced by reduced food intake (Figure 5B,E), body weight (Figure 5C,F), and Lee index (Figure 5D,G). In contrast, the CF and CXCL1 groups displayed diminished sensitivity to leptin, as indicated by stable food intake (Figure 5B,E), weight gain (Figure 5C,F), and elevated Lee index values (Figure 5D,G), suggesting the presence of leptin resistance.
FIGURE 5.
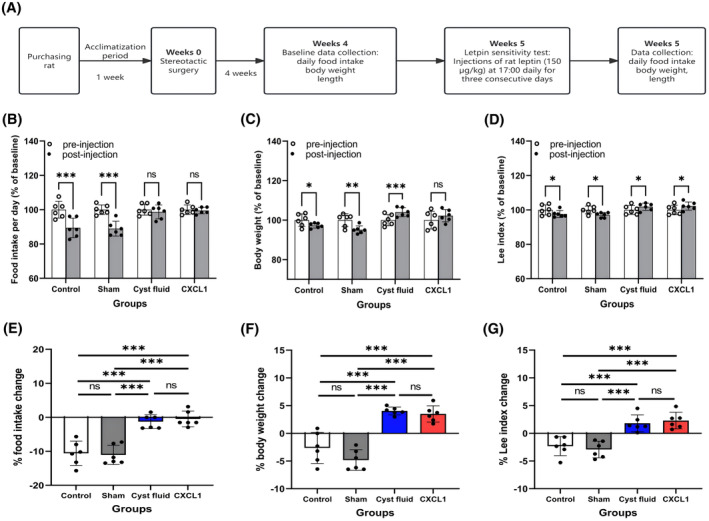
(A) Flowchart of the leptin sensitivity test; (B) Food intake, (C) body weight, and (D) Lee index as a percentage of baseline after leptin injection in the four groups; (E) Effect of leptin on food intake; (F) Effect of leptin on body weight; (G) Effect of leptin on Lee index. Data are expressed as mean ± standard deviation. ns, no significance; *p < .05; **p < .01; ***p < .001.
3.6. Identification of differentially expressed genes, biological processes, and pathways associated with the contribution of CF and CXCL1 to obesity
As illustrated in Figure S2A, SY5Y cells treated with CF or CXCL1 exhibited 811 DEGs (661 upregulated, 150 downregulated) compared to the control group. GO and KEGG pathway analyses identified the most significantly enriched terms. KEGG analysis revealed that these DEGs in the CF/CXCL1 group were strongly associated with the NF‐κB, TNF, and IL‐17 signaling pathways (Figure S2B). In the biological process (BP) category, these DEGs were notably enriched in processes such as cytokine response, positive regulation of NIK/NF‐κB signaling, and I‐κB kinase/NF‐κB signaling (Figure S2C). Additionally, GO analysis indicated that these DEGs were linked to terms such as extracellular matrix, membrane raft, CXCR cytokine receptor binding, and structural components of chromatin (Figure S2D,E).
3.7. CF and chemokine CXCL1 inhabit leptin‐induced JAK‐STAT3 pathway activation
Based on the KEGG analysis results, it was hypothesized that CF and CXCL1 induce leptin resistance by activating the NF‐κB pathway, upregulating SOCS3 expression, and consequently inhibiting JAK‐STAT3 pathway activation. As shown in Figure 5, leptin treatment normally activates the JAK‐STAT3 pathway, as evidenced by increased phosphorylated STAT3 levels (Figure 6A,B). However, pretreatment with CXCL1 and CF significantly reduced leptin signaling activation (Figure 6A,B) by elevating phosphorylated p65 (Figure 6A,C), phosphorylated IκB (Figure 6A,D), and SOCS3 expression (Figure 6A,E).
FIGURE 6.
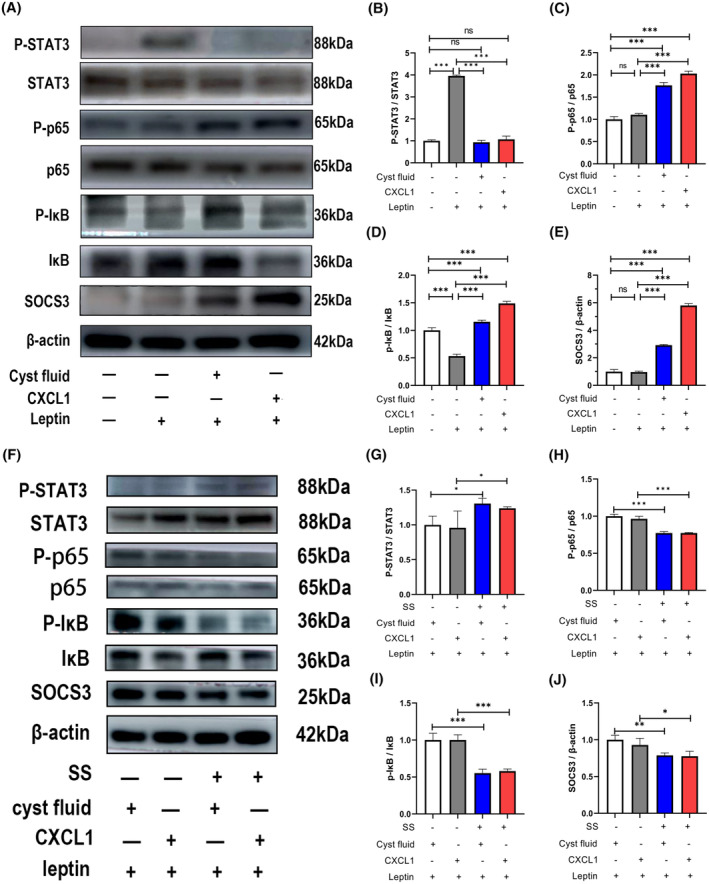
Cyst fluid and the inflammatory mediator CXCL1 induce leptin resistance in SY5Y cells, while SS can rescue SY5Y cells from the inhibitory effects of cyst fluid and CXCL1 on leptin signaling. (A) Representative Western blots showing that cyst fluid or CXCL1 induces leptin resistance in SY5Y cells; (B) Leptin activates the JAK‐STAT3 pathway in SY5Y cells pretreated without cyst fluid or CXCL1. In contrast, leptin fails to activate the JAK‐STAT3 pathway in SY5Y cells pretreated with cyst fluid or CXCL1, indicating leptin resistance; (C–E) SY5Y cells pretreated with cyst fluid or CXCL1 show activation of the NF‐κB pathway and overexpression of SOCS3; (F) Representative Western blots showing that SS preserves leptin sensitivity in SY5Y cells; (G–J) SY5Y cells pretreated with cyst fluid plus SS or CXCL1 plus SS exhibit higher levels of phosphorylated STAT3 and lower levels of phosphorylated p65, phosphorylated IκB, and SOCS3 compared to cells pretreated with only cyst fluid or CXCL1, indicating that SS maintains leptin sensitivity. ns, no significance; *p < .05; **p < .01; ***p < .001. CXCL1, C‐X‐C motif chemokine ligand‐1; IκB, inhibitor of nuclear factor‐kappa B Alpha; NF‐κB, nuclear factor kappa B; p65, p65 protein; P‐IκB, phosphorylated inhibitor of nuclear factor‐kappa B Alpha; P‐p65, phosphorylated p65 protein; P‐STAT3, phosphorylated signal transducer and activator of transcription 3; SOCS3, suppressor of cytokine signaling 3; SS, sodium salicylate; STAT3, signal transducer and activator of transcription 3.
3.8. The impact of SS on the activation of NF‐κB pathway and expression of SOCS3
As shown in Figure 5A–E, CF and CXCL1 inhibited the JAK‐STAT3 pathway and induced leptin resistance in SY5Y cells by activating the NF‐κB pathway and upregulating SOCS3 expression (Figure 6). To assess whether SS could counteract the detrimental effects of CF and CXCL1 on leptin‐induced JAK‐STAT3 pathway activation, SY5Y cells were pretreated with SS. As expected, SS pretreatment resulted in decreased levels of phosphorylated p65 (Figure 6F,H), phosphorylated IκB (Figure 6F,I), and SOCS3 expression (Figure 6F,J), thereby reactivating the JAK‐STAT3 pathway (Figure 6F,G). These results suggest that SS effectively mitigates the inhibitory effects of CF and CXCL1 on the JAK‐STAT3 pathway and restores leptin sensitivity in SY5Y cells (Figure 6F,G).
4. DISCUSSION
CP is classified as a benign tumor with a 5‐year survival rate exceeding 90%. However, survivors, particularly pediatric patients, often suffer from significantly diminished quality of life due to persistent adverse effects. 39 Among these, weight issues are the most frequently reported by caregivers, with 57.3% of survivors classified as obese, 29.3% as overweight, and 1.2% as underweight. 40 These weight issues not only contribute to an increased risk of cardiovascular mortality and lower self‐esteem among survivors but also place additional burdens on caregivers, reducing their quality of life. 6 , 40 Therefore, understanding the specific mechanisms driving obesity in patients with CP is of paramount clinical importance. Although tumor wall cells possess the ability to secrete inflammatory mediators, 41 , 42 we chose to utilize CF instead of tumor wall cells in our animal model. This decision was made to prevent mechanical damage and the mass effect associated with orthotopic transplantation on the hypothalamus, as well as to address the inadequate concentration of inflammatory mediators within the central nervous system that results from ectopic transplantation. Utilizing CF allows for a targeted examination of the independent effects of inflammatory mediators on obesity. In this study, the obesogenic effects of CF and the inflammatory mediator CXCL1 were confirmed, supporting the hypothesis that both contribute to leptin resistance and obesity by activating the NF‐κB pathway and overexpressing SOCS3. Elucidating the cross‐talk between tumors and the hypothalamus via inflammatory mediator secretion will deepen our understanding of obesity pathogenesis in CP.
Over the past few decades, numerous studies have highlighted the clinical consequences of hypothalamic damage caused by tumors or their treatments, including psychosocial disorders, hyperphagia, sleep disturbances, decreased energy expenditure, and hypopituitarism, all of which contribute to morbid obesity in patients with CP. 43 Consequently, obesity in patients with CP is often viewed as a natural result of hypothalamic damage. Consistent with previous findings, this study demonstrated that CF injection in rats led to abnormal physiological outcomes, including increased body weight (Figure 1B), elevated Lee index (Figure 1B), altered plasma metabolic profiles (Figure 2C–F), and hepatic lipid accumulation (Figure 3). Considering that reduced levels of inflammatory mediators (e.g., CXCL1, CXCL9, FGF2, GrB, IL‐1, IL‐6, IL‐8, MMP8, MMP9, and CTSS) are linked to anorexia nervosa, which is characterized by decreased food intake and significant weight loss, 44 and that CF, enriched with inflammatory mediators, promotes weight gain as observed in this study, it is reasonable to conclude that inflammatory mediators play a critical role in energy balance regulation. Furthermore, our previous research identified elevated mRNA expression levels of three inflammatory mediators (CXCL1, CXCL8, and TNF) as independent risk factors for weight gain or new‐onset obesity in patients with CP. 27 Extending these findings, we investigated the specific role of CXCL1 in obesity and found that CXCL1 injection into the brain elicited similar effects on body weight as CF injection (Figures 1, 2, 3), reinforcing the causal relationship between CXCL1 and obesity in rats. These results provide compelling evidence that CP tumor tissue contributes to weight gain not only through hypothalamic structural damage but also by secreting inflammatory mediators.
Appetite‐regulating peptides bind to the hypothalamus and either stimulate or inhibit appetite. These peptides are categorized into orexigenic peptides (which stimulate appetite, such as ghrelin) 45 and anorexigenic peptides (which inhibit appetite, such as leptin, insulin, and glucagon‐like peptide‐1). 46 Despite the presence of hyperleptinemia 47 and hyperinsulinemia 48 in obese patients with CP, these biofactors seem ineffective in suppressing appetite, as many CP survivors still experience hyperphagia, 40 indicative of leptin and insulin resistance. A high preoperative leptin level that fails to prevent weight gain, while serving as an independent predictor of significant weight gain and new‐onset obesity, further supports the existence of leptin resistance in patients with CP. 22 Hypothalamic damage contributes to this resistance by reducing sensitivity to endogenous leptin and ghrelin. 19 Intracerebroventricular leptin injections also fail to reduce food intake once the arcuate nucleus is damaged. 49 , 50 Following CF or CXCL1 brain injections, without hypothalamic damage caused by syringe insertion and a high dose of CF (Figure S1), rats exhibited hyperleptinemia (Figure 2A), an elevated leptin‐to‐body weight ratio (Figure 2B), activation of NF‐κB pathway in the hypothalamus (Figure 4), higher level of SOCS3 expression in the hypothalamus (Figure 4), and resistance to exogenous leptin (Figure 5). To date, this study is the first to link CF or inflammatory mediator CXCL1 to leptin resistance and obesity at a cellular functional level rather than through hypothalamic structural damage. These findings offer novel insights into the mechanisms driving morbid obesity in CP survivors, particularly those without HI or with mild HI.
Lifestyle modifications, such as diet and exercise, are fundamental for managing alimentary obesity, but these interventions may be challenging for many CP survivors due to hyperphagia and visual impairments. 51 Additionally, obesity in patients with CP is often unresponsive to lifestyle interventions. 8 While bariatric surgery has been performed in CP individuals with morbid obesity, the total weight reduction after 24 months was less than that of normal controls, 52 , 53 suggesting that bariatric surgery may be less effective for CP‐related obesity. Therefore, pharmacotherapy may offer a more viable approach to preventing and treating obesity in patients with CP. Given the complexity of obesity's pathophysiological mechanisms, individualized pharmacotherapeutic interventions based on specific underlying mechanisms are crucial for improving the management of morbid obesity in patients with CP.
Leptin, the product of the ob gene, was identified and cloned from rodent adipose tissue in 1994, establishing its vital role in body weight regulation. 54 Most obese individuals, however, are not leptin‐deficient but instead exhibit elevated circulating leptin levels, indicating leptin resistance. As a result, research has increasingly focused on overcoming leptin resistance. 24 Growing evidence suggests that human obesity is linked to hypothalamic inflammation, which disrupts insulin and leptin signaling, thereby impairing energy balance regulation and promoting weight gain. 55 The vitro experiments confirmed that CF enriched with inflammatory mediators, as well as the pro‐inflammatory factor CXCL1, induces leptin resistance by activating the NF‐κB pathway and upregulating SOCS3 (Figure 6A–E). Additionally, the study found that SS, a nonsteroidal anti‐inflammatory drug, inhibits SOCS3 overexpression and preserves leptin sensitivity (Figure 6F–J). Thus, this study demonstrated that SS has the potential to attenuate leptin resistance and may represent a novel therapeutic option for preventing and treating morbid obesity in patients with CP.
5. CONCLUSION
Our findings offer significant insights into the complex pathogenesis of obesity in patients with CP. Beyond hypothalamic damage, leptin resistance driven by inflammatory mediators present in CP cyst fluid merits greater attention. Additionally, preliminary evidence suggests that SS may have the potential to mitigate leptin resistance. However, further multicenter prospective studies are necessary to confirm its clinical efficacy in managing obesity in patients with CP.
AUTHOR CONTRIBUTIONS
From designing the study to its execution, encompassing data collection and analysis, all authors have contributed substantially to this manuscript and have been actively engaged in drafting, revising, and reviewing these provisions. Moreover, all authors have given their final approval for the forthcoming version, collectively agreeing to assume responsibility for all facets of the work.
FUNDING INFORMATION
This study was supported by the Beijing Municipal Science and Technology Commission (Grant No. Z191100006619087), the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202108), Ningbo Top Medical and Health Research Program (2022020304), Zhejiang Province Medical and Health Science and Technology Project (2023KY1071).
DISCLOSURES
The authors declare no conflicts of interest.
ETHICS STATEMENT
The Ethics Committee of Beijing Tiantan Hospital approved the study design (KY‐2021‐041‐02), and informed consent was obtained from all patients or their guardians for the use of cystic fluid in scientific research. The animal protocol was approved by the Ethical Committee of Experimental Animals at the Beijing Neurosurgery Institute.
Supporting information
Figure S1.
Figure S2.
Data S1.
ACKNOWLEDGMENTS
The authors extend their gratitude to the members of the Department of Neurosurgery, Beijing Tiantan Hospital, for their invaluable advice and support throughout this study. Furthermore, we extend our sincere gratitude to the reviewers of this manuscript. Their insights have been instrumental in enhancing the scientific merit and rigor of this study, thereby increasing its visibility and impact within the research community. Consequently, all authors acknowledge and thank the reviewers for their invaluable feedback and constructive comments.
Xiao Y, Wu W, Liu F, et al. Inflammatory mediator contributes to leptin resistance and obesity in craniopharyngioma. The FASEB Journal. 2024;38:e70242. doi: 10.1096/fj.202402216RR
Youchao Xiao, Wentao Wu, and Fangzheng Liu contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data used in this study are available upon reasonable request from Youchao Xiao and Wentao Wu.
REFERENCES
- 1. Müller HL, Merchant TE, Warmuth‐Metz M, Martinez‐Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5:1‐19. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Xiao Y, Wu W, et al. Effects of craniotomy and endoscopic endonasal transsphenoidal surgery on bodyweight in adult‐onset craniopharyngioma: a single‐center retrospective study. J Clin Med. 2023;12:1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao R, Lu P, Fan Y, et al. Clinical analysis of risk factors of postoperative psychiatric disorders in patients with adult craniopharyngioma. Front Neurol. 2021;12:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Müller HL, Emser A, Faldum A, et al. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J Clin Endocrinol Metab. 2004;89:3298‐3305. [DOI] [PubMed] [Google Scholar]
- 5. Müller HL, Bueb K, Bartels U, et al. Obesity after childhood craniopharyngioma—German multicenter study on pre‐operative risk factors and quality of life. Klin Padiatr. 2001;213:244‐249. [DOI] [PubMed] [Google Scholar]
- 6. Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. Lancet. 2001;357:425‐431. [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann A, Bootsveld K, Gebhardt U, Daubenbüchel AM, Sterkenburg AS, Müller HL. Nonalcoholic fatty liver disease and fatigue in long‐term survivors of childhood‐onset craniopharyngioma. Neuro‐Oncology. 2016;18:iii19. [DOI] [PubMed] [Google Scholar]
- 8. Müller HL. Craniopharyngioma and hypothalamic injury: latest insights into consequent eating disorders and obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lustig RH. Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment. Front Endocrinol (Lausanne). 2011;2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roth CL. Hypothalamic obesity in craniopharyngioma patients: disturbed energy homeostasis related to extent of hypothalamic damage and its implication for obesity intervention. J Clin Med. 2015;4:1774‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann A, Postma FP, Sterkenburg AS, Gebhardt U, Müller HL. Eating behavior, weight problems and eating disorders in 101 long‐term survivors of childhood‐onset craniopharyngioma. J Pediatr Endocrinol Metab. 2015;28:35‐43. [DOI] [PubMed] [Google Scholar]
- 12. Duan D, Wehbeh L, Mukherjee D, et al. Preoperative BMI predicts postoperative weight gain in adult‐onset craniopharyngioma. J Clin Endocrinol Metab. 2021;106:e1603‐e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elfers CT, Roth CL. Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Front Endocrinol (Lausanne). 2011;2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caron A, Lemko HMD, Castorena CM, et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. elife. 2018;7:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ioffe E, Moon B, Connolly E, Friedman JM. Abnormal regulation of the leptin gene in the pathogenesis of obesity. Proc Natl Acad Sci U S A. 1998;95:11852‐11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakao K. Translational science: newly emerging science in biology and medicine‐lessons from translational research on the natriuretic peptide family and leptin. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:538‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel L, Cooper CD, Quinton ND, et al. Serum leptin and leptin binding activity in children and adolescents with hypothalamic dysfunction. J Pediatr Endocrinol Metab. 2002;15:963‐971. [DOI] [PubMed] [Google Scholar]
- 18. Lustig RH, Hinds PS, Ringwald‐Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double‐blind, placebo‐controlled trial. J Clin Endocrinol Metab. 2003;88:2586‐2592. [DOI] [PubMed] [Google Scholar]
- 19. Roth C, Wilken B, Hanefeld F, Schröter W, Leonhardt U. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. Eur J Endocrinol. 1998;138:89‐91. [DOI] [PubMed] [Google Scholar]
- 20. Shaikh MG, Grundy RG, Kirk JMW. Hyperleptinaemia rather than fasting hyperinsulinaemia is associated with obesity following hypothalamic damage in children. Eur J Endocrinol. 2008;159:791‐797. [DOI] [PubMed] [Google Scholar]
- 21. Guran T, Turan S, Bereket A, et al. The role of leptin, soluble leptin receptor, resistin, and insulin secretory dynamics in the pathogenesis of hypothalamic obesity in children. Eur J Pediatr. 2009;168:1043‐1048. [DOI] [PubMed] [Google Scholar]
- 22. Xiao Y, Wu W, Cai K, Jin L, Jia Y, Qiao N. Clinical significance of plasma leptin and its receptors mRNA expression in craniopharyngiomas: a prospective study. Biomol Ther. 2023;13:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon O, Kim KW, Kim MS. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci. 2016;73:1457‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sáinz N, Barrenetxe J, Moreno‐Aliaga MJ, Martínez JA. Leptin resistance and diet‐induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35‐46. [DOI] [PubMed] [Google Scholar]
- 25. Tena‐Suck ML, Hernández‐Campos ME, Ortiz‐Plata A, Salinas‐Lara C, Colín‐González AL, Santamaría A. Intracerebral injection of oil cyst content of human craniopharyngioma (oil machinery fluid) as a toxic model in the rat brain. Acta Histochem. 2014;116:448‐456. [DOI] [PubMed] [Google Scholar]
- 26. Ainiwan Y, Chen Y, Mao C, et al. Adamantinomatous craniopharyngioma cyst fluid can trigger inflammatory activation of microglia to damage the hypothalamic neurons by inducing the production of β‐amyloid. J Neuroinflammation. 2022;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao Y, Wu W, Liu F, et al. The clinical significance of inflammatory mediators in predicting obesity and progression‐free survival in patients with adult‐onset craniopharyngioma. BMC Cancer. 2024;24:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhong B, Ma S, Wang DH. Ablation of TRPV1 abolishes salicylate‐induced sympathetic activity suppression and exacerbates salicylate‐induced renal dysfunction in diet‐induced obesity. Cells. 2021;10:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan M, Konstantopoulos N, Lee J. Reversal of obesity‐ and diet‐reversal of obesity‐ and diet‐induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673‐1677. [DOI] [PubMed] [Google Scholar]
- 30. Morales‐Medina JC, Dominguez‐Lopez S, Gobbi G, Beck‐Sickinger AG, Quirion R. The selective neuropeptide Y Y5 agonist [cPP1‐7,NPY19‐23,Ala31,Aib32,Gln34]hPP differently modulates emotional processes and body weight in the rat. Behav Brain Res. 2012;233:298‐304. [DOI] [PubMed] [Google Scholar]
- 31. Naomi R, Teoh SH, Rusli RNM, Embong H, Bahari H, Kumar J. Elateriospermum tapos yoghurt as a therapeutic intervention for obesity‐associated cognitive impairments and anxiety‐like behaviour in a high fat diet maternal obese rat model. Nutrients. 2023;15:2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang KP, Goodson ML, Vang W, Li H, Page AJ, Raybould HE. Leptin signaling in vagal afferent neurons supports the absorption and storage of nutrients from high‐fat diet. Int J Obes. 2021;45:348‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yin H, Shen J, Qian X, et al. Dimethyl phthalate exposure induces cognitive impairment via COX2‐mediated neuroinflammation. Ecotoxicol Environ Saf. 2024;284:117039. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Zhu X, Wu Y. Mer activation ameliorates nerve injury‐induced neuropathic pain by regulating microglial polarization and neuroinflammation via SOCS3 in male rats. Naunyn Schmiedeberg's Arch Pharmacol. 2024;397:7037‐7050. [DOI] [PubMed] [Google Scholar]
- 35. Isoda M, Ebihara K, Sawayama N, et al. Leptin sensitizing effect of 1,3‐butanediol and its potential mechanism. Sci Rep. 2021;11:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hosoi T, Kuwamura A, Thon M, Tsuchio K, El‐Hafeez AAA, Ozawa K. Possible involvement of 4‐hydroxy‐2‐nonenal in the pathogenesis of leptin resistance in obesity. Am J Physiol Cell Physiol. 2019;316:C641‐C648. [DOI] [PubMed] [Google Scholar]
- 37. Preninka AI, Kuriya K, Yazawa K, et al. Homocysteine causes neuronal leptin resistance and endoplasmic reticulum stress. PLoS One. 2022;17:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia Y, Ma L, Cai K, et al. Immune infiltration in aggressive papillary craniopharyngioma: high infiltration but low action. Front Immunol. 2022;13:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foreman NK, Faestel PM, Pearson J, et al. Health status in 52 long‐term survivors of pediatric brain tumors. J Neuro‐Oncol. 1999;41:47‐53. [DOI] [PubMed] [Google Scholar]
- 40. Kayadjanian N, Hsu EA, Wood AM, Carson DS. Caregiver burden and its relationship to health‐related quality of life in craniopharyngioma survivors. J Clin Endocrinol Metab. 2023;109:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Apps JR, Carreno G, Gonzalez‐Meljem JM, et al. Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol. 2018;135:757‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Donson AM, Apps J, Griesinger AM, et al. Molecular analyses reveal inflammatory mediators in the solid component and cyst fluid of human adamantinomatous craniopharyngioma. J Neuropathol Exp Neurol. 2017;76:779‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Iersel L, Brokke KE, Adan RAH, Bulthuis LCM, Van Den Akker ELT, Van Santen HM. Pathophysiology and individualized treatment of hypothalamic obesity following craniopharyngioma and other suprasellar tumors: a systematic review. Endocr Rev. 2018;40:193‐235. [DOI] [PubMed] [Google Scholar]
- 44. Tyszkiewicz‐Nwafor M, Jowik K, Paszynska E, Dutkiewicz A, Słopien A, Dmitrzak‐Weglarz M. Expression of immune‐related proteins and their association with neuropeptides in adolescent patients with anorexia nervosa. Neuropeptides. 2022;91:1‐8. [DOI] [PubMed] [Google Scholar]
- 45. Patterson M, Bloom SR, Gardiner JV. Peptides ghrelin and appetite control in humans—potential application in the treatment of obesity. Peptides. 2011;32:2290‐2294. [DOI] [PubMed] [Google Scholar]
- 46. Martelli D, Brooks VL. Leptin increases: physiological roles in the control of sympathetic nerve activity, energy balance, and the hypothalamic–pituitary–thyroid axis. Int J Mol Sci. 2023;24:2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holmer H, Pozarek G, Wirfält E, et al. Reduced energy expenditure and impaired feeding‐related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab. 2010;95:5395‐5402. [DOI] [PubMed] [Google Scholar]
- 48. Erfurth EM. Craniopharyngioma—an update on metabolic and cognitive complications and new therapy. J Intern Med. 2023;294:269‐280. [DOI] [PubMed] [Google Scholar]
- 49. Dawson R, Pelleymounter MA, Millard WJ, Liu S, Eppler B. Attenuation of leptin‐mediated effects by monosodium glutamate‐induced arcuate nucleus damage. Am J Phys. 1997;273:E202‐E206. [DOI] [PubMed] [Google Scholar]
- 50. Tang‐Christensen M, Holst JJ, Hartmann B, Vrang N. The arcuate nucleus is pivotal in mediating the anorectic effects of centrally administered leptin. Neuroreport. 1999;10:1183‐1187. [DOI] [PubMed] [Google Scholar]
- 51. Van Schaik J, Meeteren AYNS, Vos‐kerkhof E, et al. Treatment and outcome of the Dutch Childhood Craniopharyngioma Cohort study: first results after centralization of care. Neuro‐Oncology. 2023;25:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bretault M, Boillot A, Muzard L, et al. Bariatric surgery following treatment for craniopharyngioma: a systematic review and individual‐level data meta‐analysis. J Clin Endocrinol Metab. 2013;98:2239‐2246. [DOI] [PubMed] [Google Scholar]
- 53. Wijnen M, Olsson DS, Van Den Heuvel‐Eibrink MM, et al. Efficacy and safety of bariatric surgery for craniopharyngioma‐related hypothalamic obesity: a matched case‐control study with 2 years of follow‐up. Int J Obes. 2017;41:210‐216. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425‐432. [DOI] [PubMed] [Google Scholar]
- 55. Sonnefeld L, Rohmann N, Geisler C, Laudes M. Is human obesity an inflammatory disease of the hypothalamus? Eur J Endocrinol. 2023;188:37‐45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Data S1.
Data Availability Statement
The data used in this study are available upon reasonable request from Youchao Xiao and Wentao Wu.


