Abstract
Antimicrobial use data reported to the National Healthcare Safety Network’s Antimicrobial Use and Resistance Module between January 2019 and July 2022 were analyzed to assess the impact of the COVID-19 pandemic on inpatient antimicrobial use.
Keywords: antibiotic use, hospital epidemiology, antibiotic stewardship, COVID-19, National Healthcare Safety Network
Early in the pandemic of coronavirus disease 2019 (COVID-19), rates of inpatient antimicrobial use (AU) increased due to uncertainty about the optimal treatment of hospitalized patients with COVID-19 [1-7]. However, studies published as early as 2020 showed that bacterial coinfections in hospitalized patients were uncommon at the time of presentation, and guidelines emphasized that antibiotics were not indicated for patients without evidence of bacterial coinfections [4-7].
Over 2600 acute care hospitals across the United States voluntarily submit data on AU electronically to the National Healthcare Safety Network (NHSN) AU Option from inpatient and select outpatient locations [8]. We describe rates of antimicrobials administered to hospitalized patients, as reported to the NHSN AU Option, to characterize changes in AU during the COVID-19 pandemic.
METHODS
We conducted a retrospective analysis of facility-wide inpatient AU reported by acute care hospitals to the NHSN AU Option between January 2019 and July 2022. Only hospitals reporting data for all 43 months of the study period were included in the analysis. Long-term acute care, orthopedic, inpatient rehabilitation, and surgical hospitals were excluded. Facilities reporting zero days present [8], antimicrobial days of therapy greater than days present, or antimicrobial days of therapy as “n/a” (missing) were also excluded.
We used data reported by facilities on the 2021 NHSN Patient Safety Component Annual Hospital Survey to describe hospital characteristics such as facility type, bed size, and teaching status [9]. We analyzed antibiotics administered via oral, parenteral, inhaled, and intramuscular routes. We calculated monthly pooled mean rates as antimicrobial days of therapy [8] per 1000 days present for all antibiotics (combined) reported to the AU Option during the study period. We also reported monthly pooled AU rates for specific antibiotic agents: azithromycin and ceftriaxone because they are commonly used agents for treating community-acquired pneumonia (CAP) and piperacillin with tazobactam because of its broad-spectrum coverage and use in treating hospital-onset infections. We summed the number of new daily cases of COVID-19 in the United States, reported by the Centers for Disease Control and Prevention, to the month-level to highlight changes in COVID-19 case counts during the study period [10]. We used SAS version 9.4 (SAS Institute, Inc, Cary, NC) for all analyses.
RESULTS
There were 2387 facilities that reported data to the NHSN AU Option during the study period. Seventy-one facilities were excluded based on their facility-type. Of those remaining, only 588 reported AU data during all 43 months, and 35 facilities were subsequently excluded due to data quality issues. The final analysis included 553 acute care hospitals (440 general acute care, 52 critical access, 41 Veterans Affairs, 12 children’s, 3 women’s, 2 women’s and children’s, 2 military, 1 oncology). The median hospital bed size was 178 (interquartile range: 80–317) beds, the median number of intensive care unit beds was 20 (interquartile range: 8–49), 78% of facilities were teaching hospitals, and 49% were major teaching hospitals.
The denominator, days present, decreased during the first major wave of COVID-19 in the United States in April of 2020, by approximately 30% when compared with the mean 2019 value. Increases in pooled mean rates for total antibiotics, azithromycin, ceftriaxone, and piperacillin with tazobactam were observed in April 2020 during this first major wave (Figure 1). Compared with April 2019, the total AU rate in April 2020 increased by 7%, azithromycin by 64%, ceftriaxone by 27%, and piperacillin with tazobactam by 5%. Rates of use of azithromycin and ceftriaxone increased during each subsequent rise in COVID-19 cases reported, with larger increases observed in November and December of 2020 and 2021. However, these increases were smaller than those observed in April 2020. Rates of piperacillin with tazobactam and total antibiotics also increased in December 2021, although increases in use were less pronounced than those reported with ceftriaxone and azithromycin (Figure 1).
Figure 1.
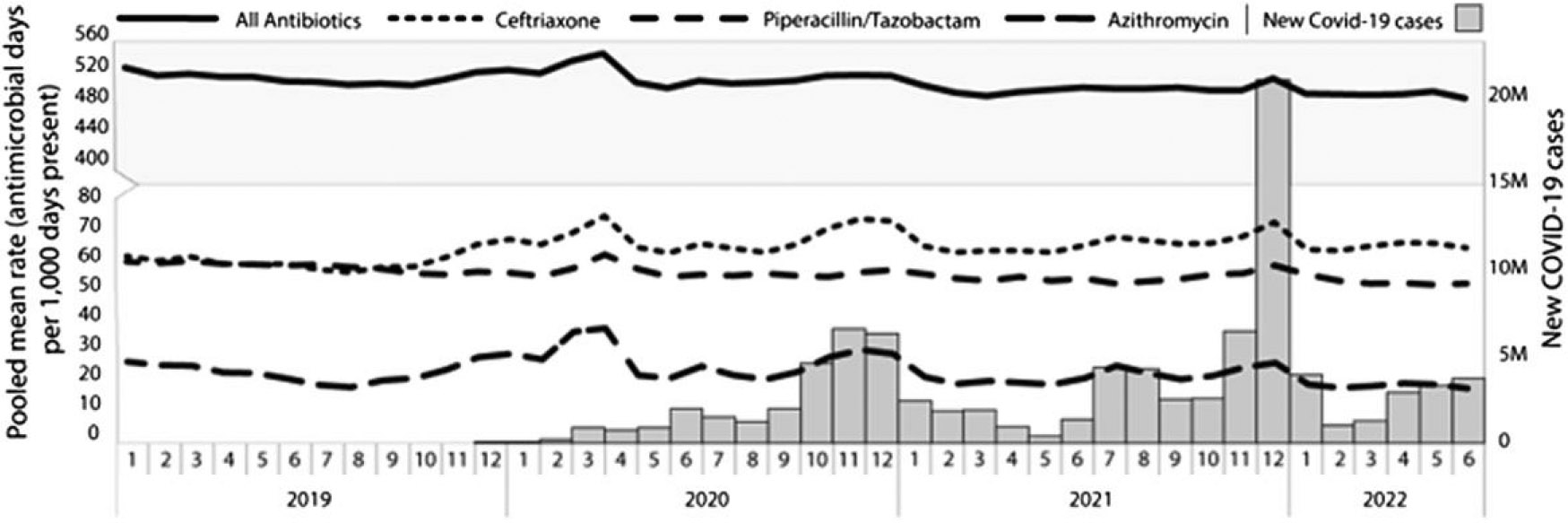
Pooled mean AU rates and new COVID-19 cases, January 2019 through July 2022, by month, among the 553 acute care hospitals reporting to the National Healthcare Safety Network AU Option for all 43 months of the study period. Abbreviations: AU, antimicrobial use; COVID-19, coronavirus disease 2019. Source: https://www.cdc.gov/coronavirus/2019-ncov/science/community-levels.html/.
DISCUSSION
This evaluation of facility-wide inpatient antimicrobial use in US hospitals showed an increase in total AU and in the use of azithromycin, ceftriaxone, and piperacillin with tazobactam in April 2020. This initial increase was likely due to diagnostic uncertainty and limited data about the risk of secondary bacterial infection during the early months of the COVID-19 pandemic and may also have been impacted by a higher acuity of illness in hospitalized patients early in the pandemic. Although ceftriaxone and azithromycin are often used together to treat CAP, a larger rate increase was reported during early months for azithromycin, which may be attributed to publicized information, later disproven, of the potential effectiveness of azithromycin against severe disease early in the pandemic [11]. There was less variation month to month among the total antibiotics group, likely because data are aggregated across many drugs, some of which may have decreased or remained unchanged during the study period, causing other increases to be muted.
Although the use of ceftriaxone and azithromycin increased during each subsequent COVID-19 wave, the magnitude was not as large as the initial increase reported during the spring of 2020, despite subsequent COVID-19 waves being considerably larger. This finding could be due to a combination of factors, including increased availability of diagnostic testing, introduction of vaccines and therapeutics for COVID-19, release of clinical treatment guidelines for managing COVID-19, increased studies demonstrating the rarity of bacterial coinfections on hospital admission, and focus on antibiotic stewardship efforts [7]. The use of piperacillin with tazobactam increased the most during the initial COVID-19 wave and then only increased again during the largest winter 2022 wave. It is likely that the rise in April 2020 was due both to a drop in total days present and a rise in the severity of illness in hospitals, as elective admissions were cancelled, leaving a larger proportion of hospitalized patients at relatively higher risk of developing drug-resistant infections and healthcare-associated infections, which increased during the pandemic [12]. The rise in AU during the winter 2022 surge was mostly likely driven by high rates of hospitalization and secondary infections among patients with COVID-19 as hospital days present were stable during that time [12].
This study has several limitations. The AU Option does not collect patient- or encounter-level data; thus, COVID-19 burden, rates of comorbid conditions and coinfections among hospitalized patients, and appropriateness of treatment cannot be assessed. Additionally, larger facilities that see more patients and have larger denominators (days present) contribute more data to pooled rates; therefore, larger facilities may be overrepresented when reporting the pooled mean metric.
This analysis provides a broad perspective on hospital antibiotic use during the COVID-19 pandemic. Lessons learned from the COVID-19 pandemic suggest that a continued focus on optimizing antibiotic stewardship efforts along with ongoing work on improving the diagnosis and management of respiratory tract infections will help mitigate challenges with antibiotic use in future COVID-19 waves and, potentially, other pandemics. The NHSN AU Option provides a mechanism for healthcare facilities and public health agencies to monitor AU, identify opportunities for quality improvement, and optimize patient safety. Facilities can leverage their AU data to focus antibiotic stewardship efforts and improve the diagnosis and management of patients hospitalized with community-onset respiratory infections.
Footnotes
Potential conflicts of interest. L. A. H. reports an unpaid role as Vice Chair of Clinical Guidelines Committee, American College of Physicians. All remaining authors declare no conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Stevens RW, Jensen K, O’Horo JC, Shah A. Antimicrobial prescribing practices at a tertiary-care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol 2021; 42:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose AN, Baggs J, Wolford H, et al. Trends in antibiotic use in US hospitals during the coronavirus disease 2019 pandemic. Open Forum Infect Dis 2021; 8:ofab236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad PJ, Poles J, Zacharioudakis IM, et al. Coinfections and antimicrobial use in patients hospitalized with coronavirus disease 2019 (COVID-19) across a single healthcare system in New York City: a retrospective cohort study. Antimicrob Steward Healthc Epidemiol 2022; 2:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-10: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26:1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karaba SM, Jones G, Helsel T, et al. Prevalence of co-infection at the time of hospital admission in COVID-19 patients, a multicenter study. Open Forum Infect Dis 2020; 8:ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith L, Karaba SM, Amoah J, et al. Hospital-acquired infections among adult patients admitted for coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol 2022; 43:1054–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 10 December 2022. [PubMed]
- 8.Centers for Disease Control and Prevention. National Healthcare Safety Network antimicrobial use and resistance module protocol. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf. Accessed 18 October 2022.
- 9.Centers for Disease Control and Prevention. National Healthcare Safety Network Patient Safety Component Annual Hospital Survey. Available at: https://www.cdc.gov/nhsn/forms/57.103_pshospsurv_blank.pdf. Accessed 18 October 2022.
- 10.Centers for Disease Control and Prevention. COVID-19 community levels. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/community-levels.html. Accessed 10 December 2022. [PubMed]
- 11.Long B, Gottlieb M. Azithromycin for treatment of COVID-19. Am Fam Physician 2022; 105:Online. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2021. national and state healthcare-associated infections progress report. Available at: https://www.cdc.gov/hai/data/portal/progress-report.html. Accessed 3 January 2022.


