Abstract
Sperm capacitation is a complex process that takes place in the female reproductive tract and empowers mammalian sperm with the competence to fertilize an egg. It consists of an intricate cascade of events that can be mimicked in vitro through incubation in a medium containing essential components, such as bicarbonate, albumin, Ca2+, and energy substrates, among others. Genetic and pharmacological studies have underscored the unique significance of the K+ channel SLO3 in membrane potential hyperpolarization, as evidenced by the infertility of mice lacking its expression. Notably, two key molecular events, sperm hyperpolarization and intracellular alkalinization, are central to the capacitation process. SLO3 is activated by alkalinization. However, the molecular mechanisms responsible for intracellular alkalization and activation of SLO3 are not completely understood. In this study, we examined the impact of Na+/H+ exchangers (NHEs) on mouse sperm membrane hyperpolarization during capacitation. Pharmacological inhibition of the NHE1 blocked membrane hyperpolarization. A similar effect was observed in sperm deficient of the Ca2+ channel CatSper because of NHE1 not being activated by Ca2+. In addition, the sperm-specific NHE (sNHE) KO did not show membrane hyperpolarization upon capacitation or induction with cAMP analogs. Our results show that sNHE is dually modulated by cAMP and membrane hyperpolarization probably through its cyclic nucleotide–binding domain and the voltage-sensor motif, respectively. Together, sNHE and NHE1 provide the alkalinization need for SLO3 activation during capacitation.
Keywords: sodium–proton exchange, sperm, potassium channel, adenylate cyclase, cyclic AMP, fertilization, membrane hyperpolarization
The sperm capacitation process in mammalian sperm involves a complex cascade of events that unfolds within the female reproductive tract upon ejaculation (for a review, see Ref. (1)). These events are vital for enabling sperm to successfully fertilize the egg and are replicated in vitro using a specialized capacitating media. These media contain various components, including energy sources, bicarbonate, Ca2+, and albumin. At the molecular level, capacitation is hallmarked by critical processes, such as sperm membrane potential (Em) hyperpolarization and intracellular alkalinization (2, 3, 4). Genetic and pharmacological investigations have unveiled specific transporters that underlie the ionic fluxes involved in such changes. Among them, the atypical sperm-specific Na+/H+ exchanger (NHE; SLC9C1 aka sNHE) and the SLO3 K+ channel have emerged as key players. Their indispensability for capacitation is underscored by the fact that mice deficient in these genes exhibit infertility, albeit without discernible abnormalities in other physiological aspects, as these proteins are uniquely expressed in sperm (2, 5, 6). Given the importance of sperm Em hyperpolarization associated to SLO3 currents, it is intriguing that the molecular events leading to SLO3 opening have not been completely revealed yet, besides knowing that SLO3 responds to alkalinization.
The principal membrane H+ transporters responsible for mediating H+ efflux in response to the rising extracellular pH within the female reproductive tract are NHEs (for a review, see Ref. (7)). Three groups identify NHE isoforms in mammalian sperm: NHE1 and NHE5 (SLC9A subgroup), NHA1 and NHA2 (SLC9B subgroup), and sNHE as a member of the SLC9C subgroup, with the recent identification of SLC9C2 in at least rat and human sperm (8, 9). Their roles and significance in sperm physiology remain an active area of research.
The localization of NHE1 to the sperm midpiece is established (10), but its precise contribution to sperm fertility has been somewhat enigmatic. Notably, the breeding outcomes of NHE1-deficient mice have provided intriguing insights. While mating between Nhe1 KO males and females yielded no successful breeding, a litter could be carried to term when Nhe1+/− (HET) males mated with Nhe1 KO females (11). As for NHE5, its functional importance in sperm physiology is yet to be elucidated, though it has been also localized to the midpiece of mouse sperm (10). Disruptions in the NHA exchangers provoked subfertility in single Nha1 or Nha2 KO males, whereas double KO males were rendered completely infertile, marked by severely compromised sperm motility. These phenotypes were associated with attenuated cAMP synthesis by soluble adenylyl cyclase (sAC) and reduced expression of the full-length sAC isoform (12). Addition of cell-permeable cAMP analogs rescued sperm motility defects, whereas fertility defects seemed to arise from deficient acrosome reaction (13). Regarding sNHE, it is localized to the principal piece of the sperm flagellum. It was found to affect sperm motility, a phenotype amenable to rescue through the addition of cell-permeable cAMP analogs (6). The presence of a cyclic nucleotide–binding domain (CNBD) and a putative voltage-sensor motif on sNHE suggests its potential regulation by cyclic nucleotides and changes in Em. It has been suggested that cAMP modulates intracellular pH (pHi) by regulating sNHE activity (14, 15). While these advances have broadened our understanding of key cellular processes that orchestrate capacitation, many questions remain unanswered regarding the precise physiological roles of these transporters (and even their presence) in sperm function.
In this article, we investigate the role of cAMP and its downstream targets in sperm alkalinization that drives hyperpolarization of Em during capacitation. Unexpectedly, inhibition of PKA catalytic activity did not impair Em hyperpolarization despite sAC activity being necessary for this change in Em. Noncapacitated sperm exposed to cAMP permeable analogs underwent Em hyperpolarization. Our results using a battery of genetic mouse models and pharmacology demonstrate the critical role of NHE1 and sNHE in the pathway leading to Em hyperpolarization, through pH modulation by Ca2+ and cAMP, respectively.
Results
PKA catalytic activity is not required for Em hyperpolarization
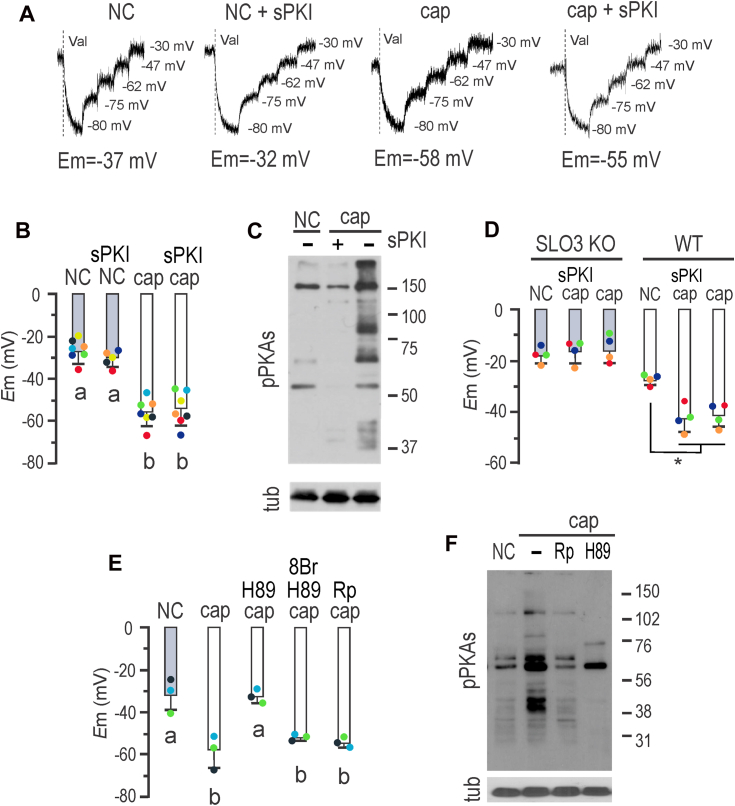
One of the initial events in the capacitation-signaling pathway involves the bicarbonate-induced stimulation of sAC, leading to increased cAMP synthesis, which activates PKA (16). Two pieces of evidence support PKA's role in mouse sperm Em hyperpolarization: (1) the PKA inhibitor H-89 prevented Em hyperpolarization, when present during capacitation, and (2) Em hyperpolarization increased with bicarbonate in a concentration-dependent manner (17). To gain insights into this pathway, we attempted to inhibit Em hyperpolarization using the synthetic and permeable PKA inhibitor peptide sPKI, known to effectively and specifically inhibit its catalytic activity (18). Surprisingly, sperm Em hyperpolarization associated to capacitation was not inhibited by sPKI (Fig. 1, A and B). As a control, we show that sPKI did not affect Em in NC sperm (Fig. 1, A and B). We verified the effective blockade of PKA activity by sPKI through immunoblotting analysis using antibodies against phosphorylated PKA substrates (pPKAs) (19) (Fig. 1C). Furthermore, Em in capacitating medium containing sPKI depended on SLO3 activity, as cells did not hyperpolarize in the presence of BaCl2 (Fig. S1), an effective blocker of SLO3 currents (20). As an additional control, sperm from Slo3 null mice did not hyperpolarize in the presence of sPKI, further disregarding the possibility of nonphysiological hyperpolarization caused by sPKI (Fig. 1D). To resolve this controversy regarding the role of PKA, we assayed the PKA inhibitors H-89 and Rp-cAMPS. Rp-cAMPS is a nonhydrolyzable and cell-permeable analog of cAMP that functions as a selective inhibitor of PKA (21). Figure 1E shows that while 30 μM H-89 inhibited Em hyperpolarization, it was restored by 500 μM 8Br-cAMP. Thus, this indicates that although H-89 impairs the onset of Em hyperpolarization, its mechanism of action is not through PKA inhibition, as a permeable analog of cAMP bypasses this inhibition. In addition, Rp-cAMPS did not inhibit Em hyperpolarization, whereas it inhibited PKA, as shown in the Western blot of Figure 1F.
Figure 1.
Mouse sperm Em hyperpolarization can dispense PKA catalytic activity.A, fluorescence traces showing the values of the sperm Em obtained after sperm incubation in either noncapacitating (NC) or capacitating (cap) conditions containing or not 15 μM sPKI for 60 min. Each experiment displays its calibration curve and the estimated Em value. B, summary of Em measurements of sperm incubated in conditions depicted in A (mean ± SD; n ≥ 5). One-way ANOVA with Tukey’s multiple comparisons test was performed; different letters indicate statistically significant differences (p < 0.001). C, sperms were incubated for 60 min in NC or cap medium containing or not 15 μM sPKI. Each condition was processed for Western blot analysis with a monoclonal anti-pPKAs antibody. Membrane was stripped and analyzed for the presence of tubulin using anti-β-tub (clone E7). D, sperm Em measurements obtained after 60 min incubation of either Slo3 KO (gray boxes) or WT sperm (white boxes) in either NC or cap medium containing or not 15 μM sPKI. One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 4; ∗p < 0.001). Each colored dot represents the value for each independent sample. E, sperm Em measurements obtained after 60 min incubation in NC or cap medium; as indicated, capacitating media were supplemented with either 30 μM H-89, 500 μM 8Br-cAMP (8Br), or 500 μM Rp-cAMPS (Rp). One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 3); different letters indicate statistically significant differences (p < 0.001). Each colored dot represents the value for each independent sample. F, sperms were incubated for 60 min in NC or cap medium; as indicated, cap media were supplemented with either 30 μM H-89 or 500 μM Rp. Each condition was processed for Western blot analysis with a monoclonal anti-pPKAs antibody. Membrane was stripped and analyzed for the presence of tubulin using anti-β-tub (clone E7).
cAMP drives Em hyperpolarization
Figure 1 showed that while H-89 impaired the onset of Em hyperpolarization, this blockade was bypassed by the permeable cAMP analog 8Br-cAMP, known to stimulate phosphorylation of PKAs (22). Thus, we aimed at analyzing the effect of cAMP on Em hyperpolarization. In these experiments, it should be noted that each subset of data has its own controls, for ensuring that all reagents are working as expected. When NC media were supplemented with 8Br-cAMP, Em hyperpolarization was observed, while this effect was not inhibited by sPKI (Fig. 2A). These results, together with the effect of H-89, indicate that while sPKI, Rp-cAMPS, and H-89 inhibit PKA, H-89 exerts an additional nonspecific effect, which is overridden by addition of a permeable cAMP analog. We further studied the effect of cAMP for the onset of Em hyperpolarization, through inhibition of cAMP production during capacitation using the potent and selective sAC inhibitor TDI-10229 (23). As shown in Figure 2B, TDI-10229 prevented Em hyperpolarization when added to capacitating media. The addition of 8Br-cAMP overcame the inhibitory effect of TDI-10229, further supporting the role of cAMP in Em hyperpolarization, independently of PKA catalytic activity.
Figure 2.
Mouse sperm Em hyperpolarization is cAMP regulated and soluble Na+/H+exchanger (sNHE) dependent.A, sperm Em obtained after incubation in either noncapacitating (NC) or capacitating (cap) conditions containing 500 μM 8Br-cAMP alone or in addition to15 μM sPKI for 60 min. Results are expressed as a normalization of percentage of hyperpolarization considering mean NC and cap values as 0% and 100%, respectively (mean ± SD; n = 4). One-way ANOVA with Tukey’s multiple comparisons test was performed; different letters indicate statistically significant differences (p < 0.001). B, sperm Em obtained after incubation in either cap (with either 10 μM TDI10229 alone or in combination with 500 μM 8Br-cAMP) or NC conditions containing or not 500 μM 8Br-cAMP for 60 min. Results are expressed as a normalized percentage of hyperpolarization considering NC mean and cap mean values as 0% and 100%, respectively (mean ± SD; n = 9). One-way ANOVA with Tukey’s multiple comparisons test was performed. Different letters indicate statistically significant differences (p < 0.001). Each colored dot represents the value for each independent sample. C, sperms were incubated for 60 min in NC media containing increasing concentrations of 6Bnz-cAMP, as indicated. Each condition was processed for Western blot analysis with a monoclonal anti-pPKAs antibody. The membrane was stripped and analyzed for the presence of tubulin using anti-β-tub (clone E7). D, summary of densitometry analysis of sperm cells incubated as in A. One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 3). Different letters indicate statistically significant differences (p < 0.05). E, Em measurements of sperm incubated in NC conditions containing either 30 μM 8-pCPT-2′-O-Me-cAMP (8pCPT), 50 nM 6Bnz-cAMP, 500 μM 8Br-cAMP, or 500 μM dibutyryl-cAMP (db-cAMP) for 60 min (mean ± SD; n = 3). One-way ANOVA with Tukey’s multiple comparisons test was performed; different letters indicate statistically significant differences (p < 0.001). F, summary of Em sperm measurements from sNhe KO (gray boxes) or WT mice (black boxes) obtained after incubation in either cap or NC conditions containing 500 μM 8Br-cAMP or 10 mM NH4Cl for 60 min. One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 5; ∗p < 0.05).
cAMP targets during promotion of Em hyperpolarization
Results presented in Figures 1 and 2 indicated that cAMP promotes Em hyperpolarization, independently of PKA catalytic activity. The second messenger cAMP has, besides PKA, two other known effectors: the exchange protein activated by cAMP (EPAC, a guanine-nucleotide-exchange factor) and the CNBD found in cyclic nucleotide–gated ion channels (for a comprehensive review, see Ref. (1)). To shed light on the signaling pathway through which cAMP promotes sperm Em hyperpolarization, we selected specific agonists of cAMP targets, including the widely used 8-pCPT-2′-O-Me-cAMP to activate EPAC and the membrane-permeant PKA selective agonist N6-benzyladenosine-cAMP (6Bnz-cAMP). To select an appropriate concentration of 6Bnz-cAMP, we initially exposed sperm to increasing concentrations of the PKA agonist for 60 min in noncapacitating media to assess PKA activity, as indicated by the in vivo phosphorylation of PKAs. Western blots using anti-pPKAs antibodies revealed that a concentration of 50 nM 6Bnz-cAMP induced a saturating phosphorylation of PKAs (Fig. 2, C and D) and used hereafter to stimulate PKA. Then, the roles of EPAC and PKA in Em were then addressed by direct stimulation. As previously shown, 50 μM 8-pCPT-2′-O-Me-cAMP was used for direct stimulation of EPAC (24). While both general cAMP analogs db-cAMP and 8Br-cAMP promoted Em hyperpolarization, specific activation neither of EPAC with 8-pCPT-2′-O-Me-cAMP nor of PKA with 6Bnz-cAMP induced this Em shift (Fig. 2E), ruling out the direct involvement of EPAC and PKA in this process.
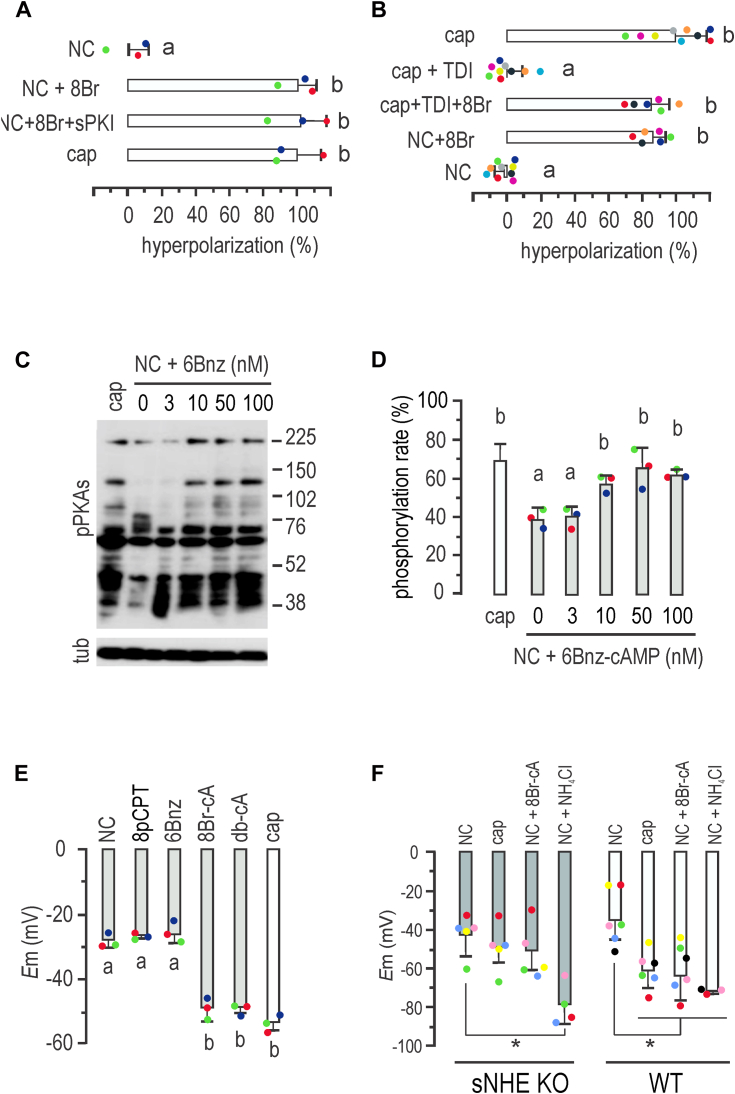
Over 20 years ago, the sNHE/NHE10 (SLC9C1) has been shown to be essential for mice’s male fertility (25), expressed at the principal piece. A unique feature of sNHE/NHE10 (SLC9C1) is the possession of a voltage-sensor domain (found in voltage-gated channels), and a CNBD at cytosolic region. Exchangers of the NHE family regulate pHi, while SLO3 channels are activated by alkalinization. Thus, the presence of a CNBD in Snhe, added to the role of cAMP driving hyperpolarization, led us to hypothesize that sNHE might be the target of cAMP. As pharmacological inhibitors of sNHE are not yet available, we analyzed Em hyperpolarization in sperm from sNHE-null mice. Figure 2F demonstrates that sperm from sNhe KO mice did not undergo hyperpolarization when incubated in capacitating conditions for 60 min. Gain of function was not observed in the presence of the agonist 8Br-cAMP, which could be probably attributed to the absence of the CNBD harbored by sNHE. Alkalinization induced by NH4Cl addition promoted Em hyperpolarization in both WT and sNHE KO sperm, confirming the functional response of SLO3 channels in this KO model. Interestingly, previous research showed that the sAC inhibitor TDI-10229 blocked pHi increase associated with capacitation (23). As mentioned, sNHE is proposed to be also regulated by Em through a voltage-sensor domain, as generally found in voltage-gated channels (26). Thus, Em hyperpolarization could be part of a positive feedback loop, increasing the activity of sNHE. To address this hypothesis, we analyzed the effect of Em hyperpolarization on pHi, through stimulation with the K+ ionophore valinomycin, promoting a pharmacological hyperpolarization (27). Noncapacitated sperm incubated with valinomycin showed an increase of pHi similar to that observed in capacitated cells, as evidenced by fluorescence increase of BCECF-loaded sperm (Fig. 3, A–C), in agreement with previous results (28). Of note, this effect on pHi was absent in sNHE-deficient mice (28).
Figure 3.
Em hyperpolarization induces intracellular alkalinization.A, sperms were incubated in capacitating (cap) or noncapacitating (NC) media either in the absence (NC) or the presence of 1 μM valinomycin (NC + Val). Representative BCECF (pH-sensitive fluorophore) versus propidium iodide (PI) two-dimensional fluorescence dot plot analysis. Squares marked as Q1 (upper left squares) define sperm with high intracellular pH displaying membrane integrity (low PI staining). Numbers in each square indicate percentage of total population detected. B, sperms were incubated in cap or NC media either in the absence (NC) or the presence of 1 μM valinomycin (NC + Val). The two-dimensional dot plots shown in A were used to select sperm with low (live) PI staining. The histogram analysis depicts normalized frequency of sperm and BCECF fluorescence of live sperm populations. C, normalized median fluorescence intensity of BCECF compared to the control capacitating condition (mean ± SD, n = 4, ∗p < 0.05).
Role of NHEs in sperm Em hyperpolarization
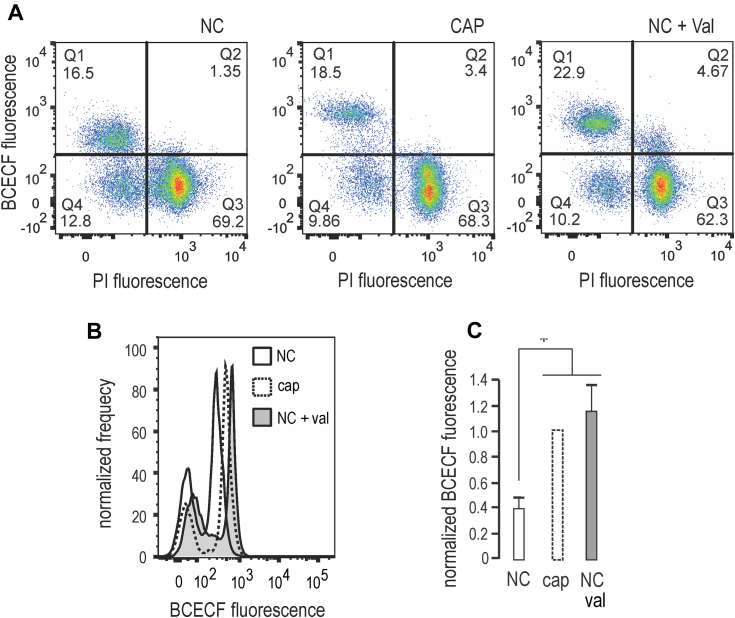
sNHE is not the sole exchanger present in mouse sperm. To explore the role of other NHEs in Em hyperpolarization, we employed the potent inhibitor 5-(N,N-dimethyl)-amiloride (DMA), which targets members of the SLC9A subfamily, including NHE1, NHE2, and NHE3, with Ki values of 0.02, 0.25, and 14 μM, respectively, and negligible effects on NHE4, NHE5, and NHE7 (29). DMA, which does not directly affect SLO3 or CatSper channels as recently shown (30), was added at different concentrations to capacitating media to address its effect on Em hyperpolarization. Figure 4A shows a concentration-dependent inhibition of Em hyperpolarization. A concentration of 1 μM DMA significantly inhibited hyperpolarization. Of note, 10 μM DMA did not affect the phosphorylation of PKAs (Fig. 4B), thus excluding an effect on cAMP synthesis. Considering that NHE1, NHE5, NHA1, NHA2, and sNHE are known to be present in mouse sperm plasma membrane, and that DMA inhibits NHE1, NHE2, and NHE3, then NHE1 might be the primary target of DMA in these cells (29). In line with these findings, recent research demonstrated that DMA reduced K+ currents in mouse sperm by impairing the regulation of pHi (30). Along this line, Figure 4C shows that DMA inhibited alkalinization associated to capacitation in mouse sperm.
Figure 4.
NHE1 activity through Ca2+stimulation is conducive to Em hyperpolarization.A, Em obtained after sperm incubation in either noncapacitating (NC) or capacitating (cap) conditions containing different concentrations of 5-(N,N-dimethyl)-amiloride (DMA) as indicated, for 60 min. One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 4; ∗p < 0.001). B, sperms were incubated for 60 min in NC or in cap medium in the presence or the absence of 10 μM DMA. Each condition was processed for Western blot analysis with a monoclonal anti-pPKAs antibody. C, sperms were incubated for 60 min in NC or cap medium containing or not 10 μM TDI-10229 (TDI) or 1 μM DMA. One-way ANOVA with Dunnett’s multiple comparisons test was performed; different letters indicate statistically significant differences (p < 0.001). D, Em obtained after sperm incubation in either NC or cap conditions containing different concentrations of cariporide, as indicated, for 60 min. One-way ANOVA with Tukey’s multiple comparisons test was performed; different letters indicate statistically significant differences (mean ± SD; n = 4; ∗p < 0.001). E, Em obtained after sperm incubation in NC conditions in the presence or not of 500 μM 8Br-cAMP (8Br) or in cap conditions. As specified, cap conditions were supplemented with either 1 μM DMA or 10 μM cariporide for 60 min. As indicated, these conditions were also supplemented with 500 μM 8Br-cAMP or 10 mM NH4Cl. One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 4; ∗p < 0.01). F, sperm Em from either CatSper1 KO (left panel) or WT mice (right panel) were obtained after sperm incubation in either NC (with boxes) or cap conditions (gray boxes) containing or not either 500 μM 8Br-cAMP or 10 mM NH4Cl for 60 min. One-way ANOVA with Tukey’s multiple comparisons test was performed (mean ± SD; n = 5; ∗p < 0.005).
A second inhibitor, cariporide, which selectively targets NHE1 (31), was also tested. Figure 4D shows that it effectively inhibited Em hyperpolarization when added to capacitating media at 10 μM. The effects of both cariporide and DMA could be bypassed by the addition of either 8Br-cAMP that directly stimulates sNHE (not inhibited by any NHE inhibitor) or NH4Cl that directly stimulates SLO3 (Fig. 4E).
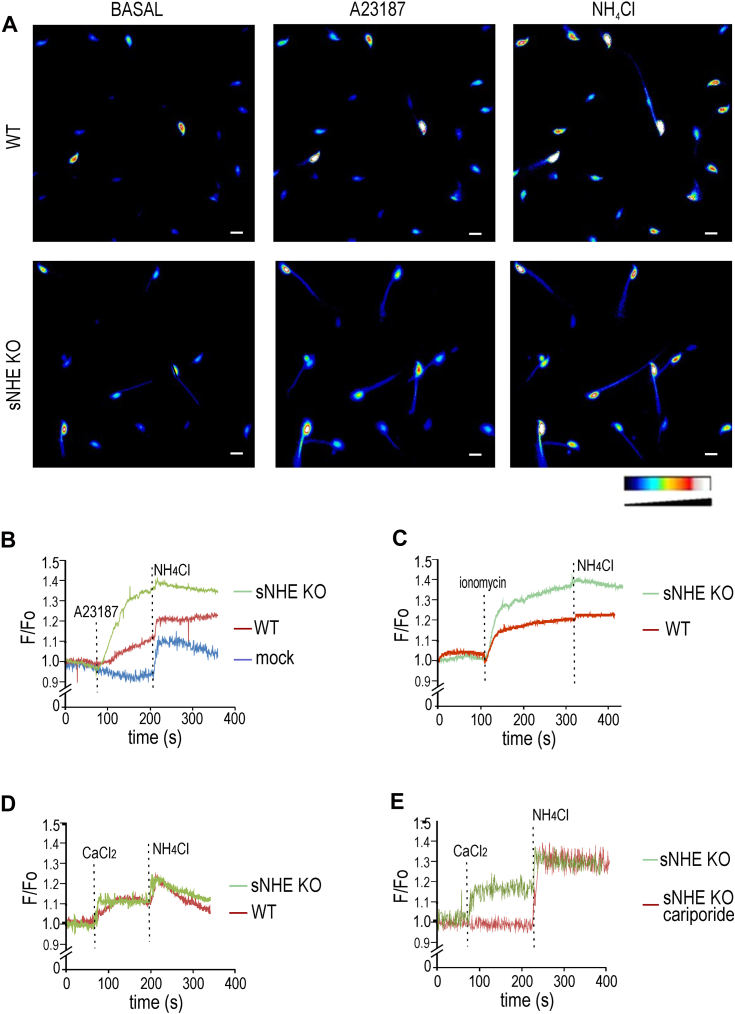
NHE1 possesses a C-terminal domain with extensive disordered regions (32), which binds calmodulin in the presence of Ca2+ (33). In other cell types, this binding induces an alkaline shift in the pHi sensitivity of NHE1, resulting in its activation at less acidic pHi (34). Therefore, the increase in intracellular Ca2+ associated with capacitation could activate NHE1, leading to an increase in pHi. To investigate the role of Ca2+ in Em hyperpolarization, we assessed Em in the CatSper1 KO model. Figure 4,F demonstrates that sperm from CatSper1 KO mice exhibited deficient Em hyperpolarization, which could be restored by the addition of either 8Br-cAMP or NH4Cl, similar to the effects observed when inhibiting NHE1 by either DMA or cariporide (Fig. 4E). Accordingly, Figure 5, A and B shows that the Ca2+ ionophore A23187 promoted pHi increase in sperm loaded with the pH-sensitive dye BCECF. Treatment with Ca2+ ionophore A23187 promoted a pH increase also in sNHE KO but to a greater extent in sNHE KO than in WT sperm. Although it is speculative, this could be attributed to overexpression of NHE1 as a compensatory effect in the sNHE KO model. As a control, NH4Cl was used to evoke pHi increase in both WT and sNHE KO sperm. Same effect was observed when sperms were challenged with ionomycin, as a second Ca2+ ionophore (Fig. 5C). Even though Ca2+ ionophores are used to increase intracellular Ca2+, it can be argued that they also promote pharmacological pHi increase because of H+ extrusion, as ionophores exchange Ca2+ for H+. Thus, as a second approach, cells were incubated in media without added Ca2+ salts (which still contain micromolar concentrations of Ca2+ (35)) and challenged with 1.7 mM Ca2+ (Fig. 5D). As before, pHi increase could be clearly evidenced, both in WT and sNHE KO sperm, further substantiating the role of Ca2+ on pHi. To further address the role of NHE1 as the target of intracellular Ca2+ increase, we incubated sperm from sNHE KO mice with the NHE1 inhibitor cariporide, showed to impair Em hyperpolarization (Fig. 4E). As shown in Figure 5E, cariporide impairs intracellular alkalinization promoted by addition of CaCl2 to a Ca2+ zero medium. Altogether, these results support the role of Ca2+ in the pathway to hyperpolarization and shed light onto the lack of hyperpolarization in CatSper KO sperm.
Figure 5.
Intracellular Ca2+increase promotes cytoplasmic alkalinization in sperm cells. Noncapacitated (NC) sperm cells were loaded with 0.5 μM BCECF-AM for 30 min before smearing onto laminin-precoated coverslips to record fluorescence. A, representative fluorescence images of WT (upper panels) and sNhe KO (lower panels) sperm exposed to 10 μM of the ionophore A23187, followed by 10 mM NH4Cl. Reference bar for fluorescence intensity is depicted. Scale bar represents 10 μm. B, summary average traces of experiments performed in A, including a mock treatment on WT sperm performed with dimethyl sulfoxide instead of A23187. About 122 of 140 cells and 77 of 99 cells analyzed responded in WT and sNHE KO, respectively; n = 5. C, summary average traces of either WT or sNhe KO sperm were exposed to 10 μM ionomycin followed by 20 mM NH4Cl. About 117 of 147 cells and 92 of 110 cells analyzed responded in WT and sNHE KO, respectively; n = 4. D, summary average traces of either WT or sNhe KO sperm incubated in nominal zero Ca2+ (no added Ca2+ salts) challenged with 1.7 mM CaCl2 and followed by 20 mM NH4Cl. About 106 of 139 cells (n = 4) and 69 of 94 cells (n = 3) analyzed responded in WT and sNHE KO, respectively. E, summary average traces of sNhe KO sperm incubated in nominal zero Ca2+ (no added Ca2+ salts) in the presence or not of 10 μM cariporide, and challenged with 1.7 mM CaCl2, followed by 20 mM NH4Cl. About 78 of 101 cells (n = 3) and 20 of 83 cells (n = 3) analyzed responded in the absence or the presence of cariporide, respectively. sNHE, soluble Na+/H+ exchanger.
These findings indicate that cAMP is responsible for Em hyperpolarization, which includes an increase in pHi mediated by NHEs, independent of PKA catalytic activity, and ultimately promoting SLO3 channel opening.
Discussion
The mammalian sperm–specific K+ channel, SLO3, plays a pivotal role in processes leading to sperm capacitation. Slo3 KO mice are unable to undergo a physiologically stimulated acrosome reaction and are consequently infertile (2, 36), underscoring the significance of investigating sperm Em hyperpolarization. However, the regulation of this channel during capacitation remains poorly understood.
Experiments conducted by Escoffier et al. (37) demonstrated that Em hyperpolarization associated to capacitation was inhibited by H-89. It is worth noting that H-89 is now recognized for its nonspecific effects (38). As shown herein, the inhibition exerted by H-89 was overridden by addition of 8Br-cAMP, excluding the role of PKA in this pathway. On the other hand, synthetic short peptides of PKI, such as PKI-(14–22)-amide (sPKI), have gained wide acceptance as pharmaceutical agents for selectively inhibiting PKA activity (39), demonstrating a high level of specificity. Our observations showed that sperm capacitated in the presence of either sPKI or the cell-permeable analog of cAMP Rp-cAMPS that functions as a selective PKA inhibitor, while displaying inhibition of PKAs phosphorylation, exhibited hyperpolarized Em. Furthermore, inhibition of sAC by TDI-10229 (23) blocked Em hyperpolarization, consistent with its impact on impairing pHi alkalinization (23). When the permeable analog 8Br-cAMP was introduced to a noncapacitated sperm, it induced Em hyperpolarization, a finding that aligns with previous reports involving a different cAMP analog (37). This supports the role of cAMP in the road of activating SLO3 channels and excludes PKA as indispensable.
The mouse SLO3 channel was found to be activated by intracellular alkalinization (40), though the precise mechanism of its modulation by pHi remains unresolved (for an in-depth review, see Ref. (41)). An important regulator of SLO3 is the leucine-rich-repeat–containing protein 52 (LRRC52) (42). The significance of LRRC52 for SLO3 activity was demonstrated in Lrrc52 KO mice, where alkalinization failed to hyperpolarize sperm Em to the same extent as in WT sperm, suggesting a crucial role for LRCC52 in SLO3 response to alkalinization (41). In this regard, NHEs have emerged as potential contributors to sperm alkalinization during capacitation. NHEs are responsible for regulating the pH of different cell compartments in a variety of cell types. Of particular importance in sperm physiology, sNHE is located in the principal piece of the sperm flagellum and possesses a CNBD (25). Sperm from sNHE-null mice did not undergo capacitation-associated hyperactivation (6), although this phenotype was restored by the addition of permeable cAMP analogs (43). Similar to slc9c1 KO, disruption of either NHA1 or NHA2 resulted in a reduced sperm motility phenotype, which was also rescued by incubating sperm with cAMP analogs, pointing toward the role of NHEs in proper regulation of sAC activity and/or expression (12). However, our findings herein demonstrate that cAMP analogs did not restore Em hyperpolarization in sNHE-null sperm. Despite the ability of cAMP addition to restore motility in sNHE-null sperm (6, 25), it did not reinstate Em hyperpolarization, indicating the involvement of sNHE in this pathway. Although it awaits further demonstration, the CNBD present in sNHE is probably the target of cAMP, driving sNHE activity (25). Recently, a human case was reported in which a mutation in sNHE resulted in a deletion in the CNBD. These sperm, lacking a functional CNBD, exhibited asthenozoospermia and infertility, indicating its importance in human sperm (44).
Physiological modulation of the NHE family has been a subject of study for many years, mainly through pharmacology. Modified analogs of amiloride were designed to enhance specificity toward NHEs. DMA bears a double substitution of the 5-amino group nitrogen, which increases its potency and selectivity toward NHE1. Our results demonstrated that DMA produced a robust inhibition of Em hyperpolarization when present in capacitating media. Cariporide, a nonrelated amiloride inhibitor of NHE1, with negligible effects on either Na+/Ca2+ exchangers or ENaCs (45), also inhibited Em hyperpolarization. In all instances, Em hyperpolarization could be reinstated by the addition of permeable cAMP analogs. Therefore, these results indicate the participation of both sNHE and NHE1 in triggering Em hyperpolarization of mouse sperm. cAMP could possibly act through the CNBD present in sNHE to increase pHi, triggering the opening of SLO3. This Em hyperpolarization, in turn, could participate in a positive feedback loop onto sNHE, as previously suggested (26, 28). On the other hand, intracellular Ca2+ might activate NHE1 through its extensive disordered regions in the long cytoplasmic C-terminal domain (32, 33). It has been proposed that this binding allows activation of NHE1 at a less acidic pHi (34). Therefore, upon the increase in intracellular Ca2+ associated with capacitation, NHE1 could drive pHi to a more alkaline state. In human sperm, NHE1 has been recently shown to be expressed at low amounts (46). Thus, possible differences in the control of pHi between mouse and human can be expected.
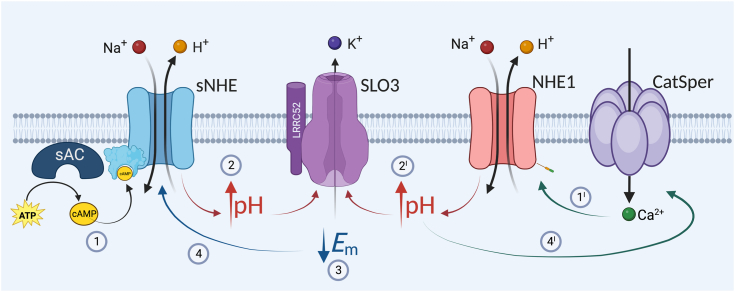
Considering these findings, we propose a mechanism that regulates pHi and Em hyperpolarization in mouse sperm, in which NHE1 and sNHE act synergistically (Fig. 6). Synthesis of cAMP induces alkalinization via sNHE, which, together with the action of NHE1 driven by intracellular Ca2+ increase, promotes the necessary alkalinization to increase the conductance of SLO3. In turn, the positive feedback loop where Em hyperpolarization further activates sNHE is also proposed and awaits further insight.
Figure 6.
Working model proposing a dual action of sNHE and NHE1 on sperm Em hyperpolarization. (1) Synthesis of cAMP induces alkalinization via sNHE, which, together with the action of NHE1 driven by intracellular Ca2+ increase (1′), promote the necessary alkalinization (2 and 2′) to increase the conductance of SLO3 (3). In turn, Em hyperpolarization stimulates a positive feedback loop that further activates sNHE (4). It is worth noting that the sole action of sNHE (inhibition of NHE1) or of NHE1 (in the case of sNhe KO) is not sufficient, under physiological conditions, to promote SLO3 opening. sNHE, soluble Na+/H+ exchanger.
This work paves the way for the study of the role of NHEs in mammalian sperm, considering their pivotal role in capacitation. These insights into the regulatory network of sperm capacitation contribute to our understanding of the fundamental processes that underlie fertilization competence, offering new perspectives for future research in this field.
Experimental procedures
Experimental design
C57BL/6 male mature (10–13 weeks old) male mice (WT, Catsper1 KO (5), Slo3 KO (2) and sNhe KO (25)) were used. In all cases, mice housing and all experimental procedures were conducted in accordance with the corresponding Institutional Animal Care guidelines, reviewed and approved by the Ethical Committees of the Instituto de Biología y Medicina Experimental, Buenos Aires, Argentina #32/2021, Animal Care and Use Committee of the Facultad de Ciencias Bioquímicas y Farmacéuticas de Rosario (UNR), Argentina (#380/2023), and of the Instituto de Biotecnología, UNAM, Mexico. The Guide for Care and Use of Laboratory Animals approved by the National Institutes of Health was strictly met. In all cases, sperms were prepared as detailed later, using high-grade reagents, as follows: bovine serum albumin (BSA, fatty acid free), cariporide (HOE-642), isobutilmetilxantina, and 2′-O-dibutiril adenosín monofosfato-3′,5′ cíclico, carbonyl cyanide 3-chlorophenylhydrazone, dimethyl sulfoxide, Ca2+ ionophore A23187, and ionomycin were purchased from Sigma. PKI 14–22 amide myristoylated (sPKI) was obtained from Tocris. 3-Amino-N-(aminoiminomethyl)-6-chloro-5-(dimethylamino)-2-pyrazinecarboxamide monohydrochloride (DMA), 8-bromo-cyclic 3′,5′-(hydrogen phosphate)-adenosine monosodium salt (8Br-cAMP), N-benzoyl-adenosine cyclic 3′,5′-(hydrogen phosphate) (6Bnz-cAMP), monosodium salt, and 8-[(4-chlorophenyl)thio]-2′-O-methyl-adenosine cyclic 3′,5′-hydrogen phosphate (8pCPT-2-O′-methyl cAMP), monosodium salt, and valinomycin were purchased from Cayman Chemicals. Anti-pPKAs (clone 100G7E) antibodies and horseradish peroxidase–conjugated anti-mouse and anti-rabbit IgG were purchased from Cell Signaling Technology. β-tubulin (clone E7) was purchased by Developmental Studies Hybridoma Bank. The sAC inhibitor TDI-10229 was kindly provided by Drs Levin and Buck, Department of Pharmacology, Weill Cornell Medicine, New York City, USA. 3,3-Dipropylthiadicarbocyanine iodide (DiSC3(5)), BCECF-AM, and pluronic acid from Invitrogen, Thermo Fisher Scientific; while propidium iodide from Santa Cruz Biotechnology. DiSC3(5), BCECF-AM, and pluronic acid were dissolved in dimethyl sulfoxide; propidium iodide was dissolved in hexa-distilled water.
Sperm preparation
Cauda epididymal mouse sperms were collected from adult male mice (10–13 weeks old). Each minced cauda epididymis was placed in 600 μl of Hepes-buffered TYH medium (H-TYH) containing 119.3 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.6 mM glucose, 0.5 mM sodium pyruvate, 1.7 mM Ca2+, and 20 mM Hepes (pH 7.3), accounting for H-TYH medium (“NC medium”). After 15 min of incubation at 37 °C (swim-out), epididymides were removed and the suspension was adjusted with NC medium to a final concentration of 1 to 2 × 107 cells/ml. For capacitation, BSA and NaHCO3 were added to final concentrations of 5 mg/ml and 20 mM, respectively (“cap medium”) and incubated at 37 °C for at least 1 h, or the indicated period.
SDS-PAGE and immunoblotting
After treatment, sperms were collected by centrifugation, washed in 1 ml of PBS, resuspended in Laemmli sample buffer without β-mercaptoethanol, and boiled for 5 min. After centrifugation, 5% β-mercaptoethanol was added to the supernatants and boiled again for 5 min. Protein extracts equivalent to 1 to 2 × 106 sperm per lane were subjected to SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes (Bio-Rad) at 250 mA for 60 min on ice. Membranes were blocked with 3% BSA in Tris-buffered saline (TBS) containing 0.1% Tween-20 (T-TBS). Antibodies were diluted in T-TBS containing 1% BSA as follows: 1/3,000 for anti-pPKAs and 1/10,000 for anti-β-tubulin. Secondary antibodies were diluted 1/10,000 in T-TBS and developed using an enhanced chemiluminescence detection kit (ECL Kallium Biolumina) according to the manufacturer’s instructions. When necessary, polyvinylidene fluoride membranes were stripped at 60 °C for 15 min in 2% SDS, 0.74% β-mercaptoethanol, and 62.5 mM Tris (pH 6.5), and washed six times, 5 min each time, in T-TBS. In all experiments, molecular masses were expressed in kilodaltons (kDas).
Membrane potential assay in cell populations
Sperm Em changes were assessed using DiSC3(5), as previously described (47). After treatment, cells were loaded with 1 μM of the membrane-potential-sensitive dye DiSC3(5) (Molecular Probes) for 2 min. Sperms were transferred to a gently stirred cuvette at 37 °C, and the fluorescence was monitored with a Cary Eclipse fluorescence spectrophotometer at 620/670 nm excitation/emission wavelengths. Carbonyl cyanide 3-chlorophenylhydrazone (0.5 μM) was added as uncoupler of oxidative phosphorylation to avoid mitochondrial contribution to the recorded Em. Recordings were initiated when steady-state fluorescence was reached, and calibration was performed at the end of each measure by adding 1 μM valinomycin and sequential additions of KCl for internal calibration curves, as previously described (48). Sperm Em was obtained from the initial fluorescence (measured as Arbitrary Fluorescence Units) by linearly interpolating it in the theoretical Em values from the calibration curve against arbitrary fluorescence units of each trace. This internal calibration for each determination compensates for variables that influence the absolute fluorescence values.
Determination of pHi by flow cytometry
Sperm pHi changes were assessed using BCECF-AM as previously described (49). After incubation in the appropriate medium, samples were centrifuged at 400g for 4 min at room temperature and resuspended in 200 μl of NC H-TYH medium containing 0.5 μM BCECF-AM for 20 min at 37 °C. Samples were washed again and resuspended in 50 μl of NC H-TYH medium. Before collecting data, 3 μM of propidium iodide was added to monitor viability. Data were recorded as individual cellular events using a MACSQuant Analyzer cytometer (Miltenyi Biotec). Side-scatter area and forward-scatter area (FSC-A) data were collected from 20,000 events per sample to define sperm population as previously described (17). In all cases, doublet exclusion was performed analyzing two-dimensional dot plot FSC-A versus FSC-H. Positive cells for BCECF were collected using fluorescein isothiocyanate filter (FITC; 530/30) together with peridinin chlorophyll protein complex (PerCP; 670LP) filter. Although the two indicators had minimal emission overlap, compensation was done. For calibration curves, samples were split and resuspended with high K+ buffered solutions at pH 6.3, 6.5, 7.0, 7.4, or 8.0 (1.2 mM MgSO4, 1.6 mM CaCl2, 23.8 mM Hepes, 2.78 mM glucose, 3.38 mM sodium pyruvate, and 120 mM KCl; pH previously adjusted with NaOH), and 5 μM nigericin was added to equilibrate intracellular and extracellular pH. Data were analyzed using FlowJo software (version 10.0.7; BD Biosciences).
Analysis of pHi by single cell imaging
Sperms were loaded with the fluorescent pHi indicator as described for flow cytometry. Cells were later adhered to 1 mg/ml laminin-precoated coverslips, allowing their flagella to move continuously. The coverslip was mounted on a chamber (Harvard Apparatus) and placed on the stage of an inverted microscope (Eclipse TE 300; Nikon). Fluorescence illumination was supplied by a Luxeon V Star Lambertian Cyan LED (Lumileds Lighting LLC) attached to a custom-built stroboscopic control box. The LED was mounted into a FlashCube40 assembly with a dichroic mirror (M40-DC400; Rapp Opto Electronic; bandwidths: excitation, 450–490 nm; dichroic mirror, 505 nm; and emission, 520–560 nm). The LED output was synchronized to the Exposure Out signal of an iXon 888 CCD camera via the control box to produce a single flash of 2-ms duration per individual exposure. The camera exposure time was set equivalent to the flash duration (2 ms). Images were collected every 500 ms using iQ software (Andor Technology).
Statistical analysis
Data are expressed as mean ± SD of at least three independent experiments for all determinations. Statistical analyses were performed using the GraphPad Prism 6 software (GraphPad Software, Inc). Student’s t test was used to compare mean values between control and tested groups, whereas differences between mean values of multiple groups were analyzed by one-way ANOVA with multiple comparison tests, as indicated in the figure legends. Significance is indicated in the figure legends.
Data availability
All data are available in the main text or the supporting information.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are thankful to Yoloxochitl Sánchez Guevara for technical support with the sNhe KO mouse strain.
Author contributions
C. M. S., Dario Krapf, and A. D. conceptualization; M. G. B., P. T. R., A. G. N., J. L. D. L. V. B., C. R., G. M. L., Dario Krapf, L. J. S.-E., C. L. T., C. S., and A. D. methodology; I. G. formal analysis; P. T. R., A. G. N., J. L. D. L. V. B., G. M. L., L. J. S.-E., and M. C. investigation; T. N. and C. M. S. resources; Dario Krapf writing–original draft; M. G. B., P. T. R., Diego Krapf, A. G. N., T. N., C. M. S., G. M. L., Dario Krapf, L. J. S.-E., C. L. T., C. S., and A. D. writing–review & editing; P. T. R., A. G. N., J. L. D. L. V. B., and G. M. L. visualization; Diego Krapf, Dario Krapf, C. L. T., and A. D. supervision.
Funding and additional information
Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 2017-3217 (to Dario Krapf); Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 2019-1779 (to Dario Krapf); Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 2021-A-0102 (to Dario Krapf); Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de México Grant PAPIIT IN207122 (to C. L. T.); Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de México Grant PAPIIT IN200919 (to A. D.); Consejo Nacional de Ciencia y Tecnología from Mexico Grant CF-2023-I-291 (to A. D.); National Institutes of Health (NIH) grant RO1HD038082-17A1 (to A. D.); NIH grant R01HD069631 (to C. M. S.); and NIH grant R01HD106968 (to Diego Krapf).
Reviewed by members of the JBC Editorial Board. Edited by Mike Shipston
Supporting information
Supplemental Fig. S1.
Ba2+ blocks Em hyperpolarization even in the presence of sPKI. Sperm Em measurements obtained after incubation for 60 min in capacitating conditions containing either 1 mM BaCl2 or 15 μM sPKI or a combination of both. Results are expressed as a normalization of percentage of hyperpolarization considering mean NC and cap values as 0% and 100%, respectively (mean ± SD; n = 4); one-way ANOVA with Turkey’s multiple comparisons test was performed; different letters indicate statistically significant differences (p < 0.05).
References
- 1.Stival C., Puga Molina Ldel C., Paudel B., Buffone M.G., Visconti P.E., Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv. Anat. Embryol. Cell Biol. 2016;220:93–106. doi: 10.1007/978-3-319-30567-7_5. [DOI] [PubMed] [Google Scholar]
- 2.Santi C.M., Martínez-López P., de la Vega-Beltrán J.L., Butler A., Alisio A., Darszon A., et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matamoros-Volante A., Trevino C.L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J. Cell Sci. 2020;133 doi: 10.1242/jcs.238816. [DOI] [PubMed] [Google Scholar]
- 4.Baro Graf C., Ritagliati C., Torres-Monserrat V., Stival C., Carizza C., Buffone M.G., et al. Membrane potential assessment by fluorimetry as a predictor tool of human sperm fertilizing capacity. Front. Cell Dev. Biol. 2019;7:383. doi: 10.3389/fcell.2019.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren D., Navarro B., Perez G., Jackson A.C., Hsu S., Shi Q., et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu J., Bobulescu I.A., Quill T.A., McLeroy P., Moe O.W., et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc. Natl. Acad. Sci. U. S. A. 2007;104:9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishigaki T., José O., González-Cota A.L., Romero F., Treviño C.L., Darszon A. Intracellular pH in sperm physiology. Biochem. Biophysical Res. Commun. 2014;450:1149–1158. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner C.C., James P.F. Na+/H+ exchangers (NHEs) in mammalian sperm: essential contributors to male fertility. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241914981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner C.C., James P.F. The SLC9C2 gene product (Na+/H+ exchanger isoform 11; NHE11) is a testis-specific protein localized to the head of mature mammalian sperm. Int. J. Mol. Sci. 2023;24:5329. doi: 10.3390/ijms24065329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo A.L., James P.F., Lingrel J.B. Roles of the Na,K-ATPase α4 isoform and the Na+/H+ exchanger in sperm motility. Mol. Reprod. Dev. 2002;62:348–356. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- 11.Bell S.M., Schreiner C.M., Schultheis P.J., Miller M.L., Evans R.L., Vorhees C.V., et al. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am. J. Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 12.Chen S.R., Chen M., Deng S.L., Hao X.X., Wang X.X., Liu Y.X. Sodium–hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balbach M., Hamzeh H., Jikeli J.F., Brenker C., Schiffer C., Hansen J.N., et al. Molecular mechanism underlying the action of zona-pellucida glycoproteins on mouse sperm. Front. Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.572735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windler F., Bönigk W., Körschen H.G., Grahn E., Strünker T., Seifert R., et al. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat. Commun. 2018;9:2809. doi: 10.1038/s41467-018-05253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lishko P.V., Kirichok Y., Ren D., Navarro B., Chung J.J., Clapham D.E. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baro Graf C., Ritagliati C., Stival C., Luque G.M., Gentile I., Buffone M.G., et al. Everything you ever wanted to know about PKA regulation and its involvement in mammalian sperm capacitation. Mol. Cell Endocrinol. 2020;518 doi: 10.1016/j.mce.2020.110992. [DOI] [PubMed] [Google Scholar]
- 17.Escoffier J., Krapf D., Navarrete F., Darszon A., Visconti P.E. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J. Cell Sci. 2012;125:473–485. doi: 10.1242/jcs.093344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weeks K.L., Ke P., Zhang J., Zhang X., Chen X. Protein kinase inhibitor peptide as a tool to specifically inhibit protein kinase A. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.574030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krapf D., Arcelay E., Wertheimer E.V., Sanjay A., Pilder S.H., Salicioni A.M., et al. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J. Biol. Chem. 2010;285:7977–7985. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrighton D.C., Muench S.P., Lippiat J.D. Mechanism of inhibition of mouse Slo3 (KCa5.1) potassium channels by quinine, quinidine and barium. Br. J. Pharmacol. 2015;172:4355–4363. doi: 10.1111/bph.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaap P., van Ments-Cohen M., Soede R.D., Brandt R., Firtel R.A., Dostmann W., et al. Cell-permeable non-hydrolyzable cAMP derivatives as tools for analysis of signaling pathways controlling gene regulation in Dictyostelium. J. Biol. Chem. 1993;268:6323–6331. [PubMed] [Google Scholar]
- 22.Wertheimer E., Krapf D., de la Vega-Beltran J.L., Sánchez-Cárdenas C., Navarrete F., Haddad D., et al. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J. Biol. Chem. 2013;288:35307–35320. doi: 10.1074/jbc.M113.489476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balbach M., Ghanem L., Rossetti T., Kaur N., Ritagliati C., Ferreira J., et al. Soluble adenylyl cyclase inhibition prevents human sperm functions essential for fertilization. Mol. Hum. Reprod. 2021;27:1–13. doi: 10.1093/molehr/gaab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucchesi O., Ruete M.C., Bustos M.A., Quevedo M.F., Tomes C.N. The signaling module cAMP/Epac/Rap1/PLCε/IP3 mobilizes acrosomal calcium during sperm exocytosis. Biochim. Biophys. Acta. 2016;1863:544–561. doi: 10.1016/j.bbamcr.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Wang D., King S.M., Quill T.A., Doolittle L.K., Garbers D.L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 26.Chávez J.C., Ferreira J.J., Butler A., De La Vega Beltrán J.L., Treviño C.L., Darszon A., et al. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem. 2014;289:32266–32275. doi: 10.1074/jbc.M114.607556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graf C.B., Ritagliati C., Stival C., Balestrini P.A., Buffone M.G., Krapf D. Determination of a robust assay for human sperm membrane potential analysis. Front. Cell Dev Biol. 2019;7:101. doi: 10.3389/fcell.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández-Garduño S., Chávez J.C., Matamoros-Volante A., Sánchez-Guevara Y., Torres P., Treviño C.L., et al. Hyperpolarization induces cytosolic alkalization of mouse sperm flagellum probably through sperm Na+/H+ exchanger. Reproduction. 2022;164:125–134. doi: 10.1530/REP-22-0101. [DOI] [PubMed] [Google Scholar]
- 29.Masereel B., Pochet L., Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur. J. Med. Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 30.Kang H., Liu M., Zhang W., Huang R.Z., Zhao N., Chen C., et al. Na+/H+ exchangers involve in regulating the ph-sensitive ion channels in mouse sperm. Int. J. Mol. Sci. 2021;22:1–13. doi: 10.3390/ijms22041612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzzachi S., Guerra L., Martino N.A., Favia M., Punzi G., Silvestre F., et al. Effect of cariporide on ram sperm pH regulation and motility: possible role of NHE1. Reproduction. 2018;155:433–445. doi: 10.1530/REP-17-0456. [DOI] [PubMed] [Google Scholar]
- 32.Nørholm A.-B., Hendus-Altenburger R., Bjerre G., Kjaergaard M., Pedersen S.F., Kragelund B.B. The intracellular distal tail of the Na+/H+ exchanger NHE1 is intrinsically disordered: implications for NHE1 trafficking. Biochemistry. 2011;50:3469–3480. doi: 10.1021/bi1019989. [DOI] [PubMed] [Google Scholar]
- 33.Köster S., Pavkov-Keller T., Kühlbrandt W., Yildiz O. Structure of human Na +/H + exchanger NHE1 regulatory region in complex with calmodulin and Ca 2+ J. Biol. Chem. 2011;286:40954–40961. doi: 10.1074/jbc.M111.286906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakabayashi S., Bertrand B., Ikeda T., Pouysségur J., Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H+-sensitive and Ca2+ regulation-defective. J. Biol. Chem. 1994;269:13710–13715. [PubMed] [Google Scholar]
- 35.Navarrete F.A., García-Vázquez F.A., Alvau A., Escoffier J., Krapf D., Sánchez-Cárdenas C., et al. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell Physiol. 2015;230:1758–1769. doi: 10.1002/jcp.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng X.H., Yang C., Kim S.T., Lingle C.J., Xia X.M. Deletion of the Slo3 gene abolishes alkalizationactivated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5879–5884. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escoffier J., Navarrete F., Haddad D., Santi C.M., Darszon A., Visconti P.E. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod. 2015;92:121. doi: 10.1095/biolreprod.114.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limbutara K., Kelleher A., Yang C.R., Raghuram V., Knepper M.A. Phosphorylation changes in response to kinase inhibitor H89 in PKA-null cells. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-39116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Sabatini B.L. The kinase specificity of protein kinase inhibitor peptide. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.632815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro B., Kirichok Y., Clapham D.E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7688. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon M.D., Ferreira J.J., Li P., Bhagwat S., Butler A., Anderson K., et al. SLO3: a conserved regulator of sperm membrane potential. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241311205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolan J., Walshe K., Alsbury S., Hokamp K., O'Keeffe S., Okafuji T., et al. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 2007;8:320. doi: 10.1186/1471-2164-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quill T.A., Wang D., Garbers D.L. Insights into sperm cell motility signaling through sNHE and the CatSpers. Mol. Cell Endocrinol. 2006;250:84–92. doi: 10.1016/j.mce.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Cavarocchi E., Whitfield M., Chargui A., Stouvenel L., Lorès P., Coutton C., et al. The sodium/proton exchanger SLC9C1 (sNHE) is essential for human sperm motility and fertility. Clin. Genet. 2021;99:684–693. doi: 10.1111/cge.13927. [DOI] [PubMed] [Google Scholar]
- 45.Scholz W., Albus U., Counillon L., Gögelein H., Lang H.J., Linz W., et al. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc. Res. 1995;29:260. [PubMed] [Google Scholar]
- 46.Grahn E., Kaufmann S.V., Askarova M., Ninov M., Welp L.M., Berger T.K., et al. Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm. Nat. Commun. 2023;14:5395. doi: 10.1038/s41467-023-40855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritagliati C., Baro Graf C., Stival C., Krapf D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech. Dev. 2018;154:33–43. doi: 10.1016/j.mod.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Ritagliati C., Luque G.M., Stival C., Baro Graf C., Buffone M.G., Krapf D. Lysine acetylation modulates mouse sperm capacitation. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-31557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luque G.M., Xu X., Romarowski A., Gervasi M.G., Orta G., De la Vega-Beltrán J.L., et al. Cdc42 localized in the CatSper signaling complex regulates cAMP-dependent pathways in mouse sperm. FASEB J. 2021;35 doi: 10.1096/fj.202002773RR. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text or the supporting information.