Abstract
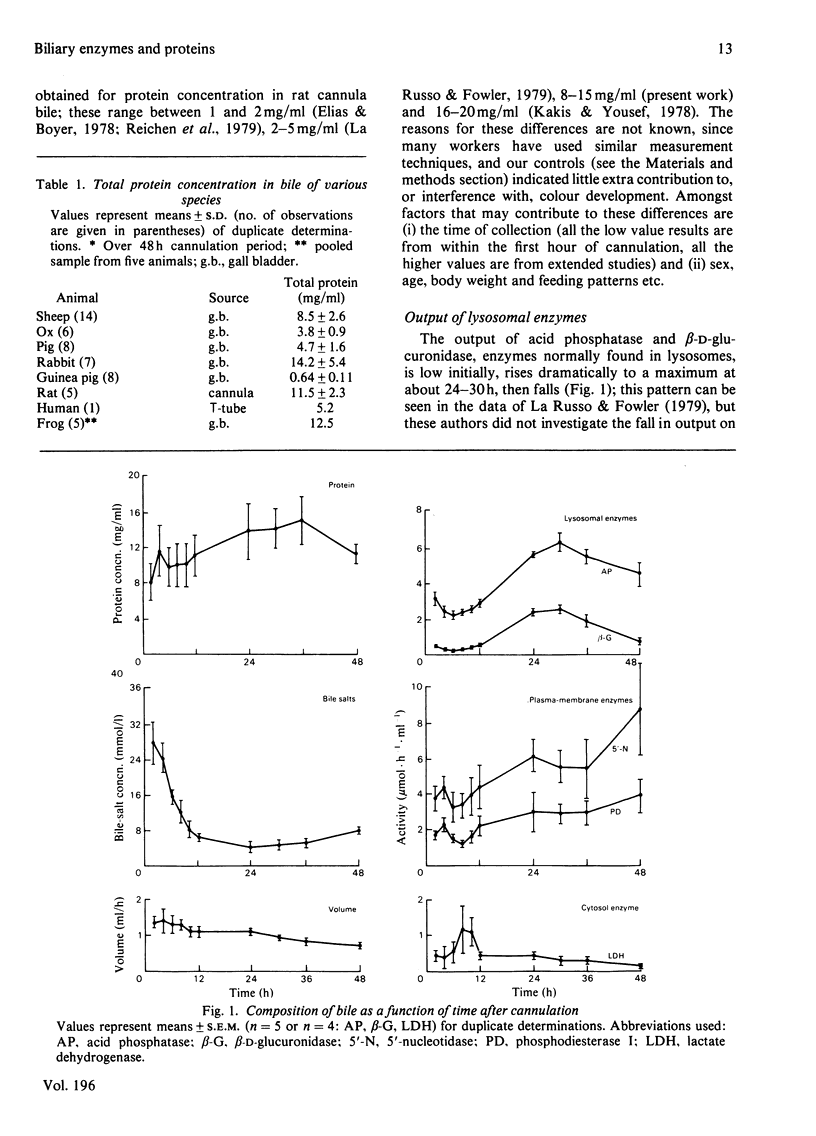
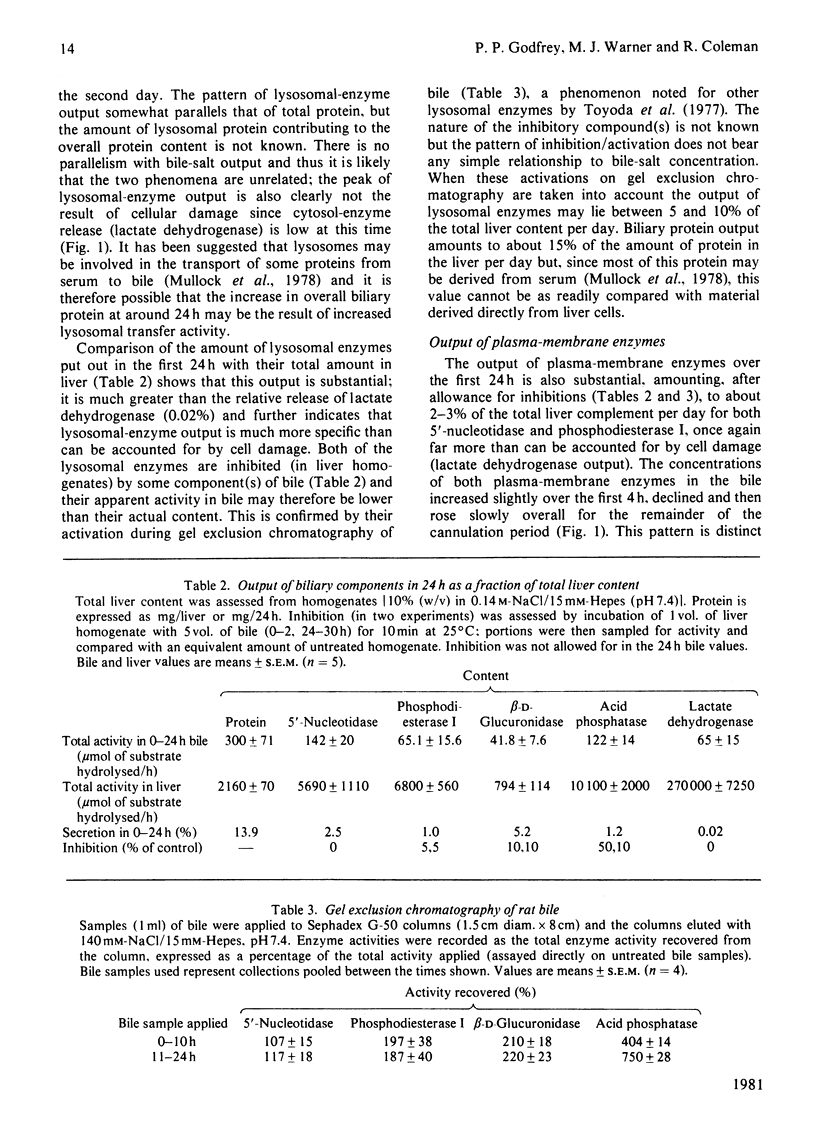
The protein concentration in bile from several species is reported. The changes in output of protein, bile salts and several enzymes have been followed in rat bile over a 48 h cannulation period. Bile-salt concentration dropped rapidly owing to interruption of the enterohepatic circulation but the output of protein, lysosomal enzymes [acid phosphatase (EC 3.1.3.2) and beta-D-glucuronidase (EC 3.2.1.31)] and plasma-membrane enzymes [5'-nucleotidase (EC 3.1.3.5) and phosphodiesterase I (EC 3.1.4.1)] was maintained. Liver cell damage, monitored by output of lactate dehydrogenase, was very low throughout. Protein, lysosomal enzymes and plasma-membrane enzymes showed different patterns of output with time, but all showed a net increase between 12 and 24 h. The output of lysosomal and plasma-membrane enzymes was between 1 and 5% of the total liver complement over the first 24 h; if inhibition by biliary components is taken into account the output of some of these enzymes, particularly acid phosphatase, may be greater. Ultracentrifugation of bile showed that as the concentration of bile salts decreases the proportion of plasma-membrane enzymes in a sedimentable form increases. The results are discussed in relation to other studies of biliary proteins and to studies of the perturbation of membranes and cells with bile salts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginski E. S., Foà P. P., Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem. 1967 Apr;13(4):326–332. [PubMed] [Google Scholar]
- Billington D., Coleman R. Effects of bile salts of human erythrocytes. Plasma membrane vesiculation, phospholipid solubilization and their possible relationships to bile secretion. Biochim Biophys Acta. 1978 May 4;509(1):33–47. doi: 10.1016/0005-2736(78)90005-6. [DOI] [PubMed] [Google Scholar]
- Billington D., Evans C. E., Godfrey P. P., Coleman R. Effects of bile salts on the plasma membranes of isolated rat hepatocytes. Biochem J. 1980 May 15;188(2):321–327. doi: 10.1042/bj1880321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode J. C., Zelder O., Neuberger H. O. Effect of taurocholate, dehydrocholate and secretin on biliary output of alkaline phosphatase and GOT. Helv Med Acta. 1973 Sep;37(2):143–151. [PubMed] [Google Scholar]
- Brightwell R., Tappel A. L. Subcellular distributions and properties of rat liver phosphodiesterases. Arch Biochem Biophys. 1968 Mar 20;124(1):325–332. doi: 10.1016/0003-9861(68)90334-2. [DOI] [PubMed] [Google Scholar]
- Califano J. A., Jr A strategy for future federal support of biomedical research. Clin Res. 1978 Oct;26(5):317–321. [PubMed] [Google Scholar]
- Coleman R., Holdsworth G., Finean J. B. Detergent extraction of erythrocyte ghosts. Comparison of residues after cholate and Triton X-100 treatments. Biochim Biophys Acta. 1976 Jun 4;436(1):38–44. doi: 10.1016/0005-2736(76)90217-0. [DOI] [PubMed] [Google Scholar]
- Coleman R., Holdsworth G. The release of membrane components prior to haemolysis during extraction of intact eryghrocytes with bile salts. Biochim Biophys Acta. 1976 Apr 5;426(4):776–780. doi: 10.1016/0005-2736(76)90146-2. [DOI] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dive C., Heremans J. F. Nature and origin of the proteins of bile. I. A comparative analysis of serum and bile proteins in man. Eur J Clin Invest. 1974 Aug;4(4):235–239. doi: 10.1111/j.1365-2362.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Englert E., Jr, Wales E. E., Jr, Straight R. C. The proteins of human gallbladder bile with and without gallstones. Clin Chim Acta. 1970 Aug;29(2):319–331. doi: 10.1016/0009-8981(70)90053-7. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Kremmmer T., Culvenor J. G. Role of membranes in bile formation. Comparison of the composition of bile and a liver bile-canalicular plasma-membrane subfraction. Biochem J. 1976 Mar 15;154(3):589–595. doi: 10.1042/bj1540589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Hinton R. H., Mullock B. M., Peppard J., Orlans E. Role of endocytic vesicles in the transport of proteins into bile [proceedings]. Biochem Soc Trans. 1980 Feb;8(1):114–114. doi: 10.1042/bst0080114. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Enzyme profiles of mammalian bile. Biochim Biophys Acta. 1975 Apr 21;389(1):47–50. doi: 10.1016/0005-2736(75)90384-3. [DOI] [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Protein composition of rat bile. Can J Biochem. 1978 May;56(5):287–290. doi: 10.1139/o78-044. [DOI] [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. Rat bile as a convenient source of secretory IgA and free secretory component. Eur J Immunol. 1977 Aug;7(8):588–590. doi: 10.1002/eji.1830070818. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Dobrota M., Hinton R. H. Sources of the proteins of rat bile. Biochim Biophys Acta. 1978 Nov 1;543(4):497–507. doi: 10.1016/0304-4165(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Issa F. S., Hinton R. H. Bile 5'-nucleotidase in the serum of jaundiced rats. Clin Chim Acta. 1977 Aug 15;79(1):129–140. doi: 10.1016/0009-8981(77)90470-3. [DOI] [PubMed] [Google Scholar]
- Pope C. E., 2nd, Cooperband S. R. Protein characteristics of serum and bile alkaline phosphatase. Gastroenterology. 1966 May;50(5):631–636. [PubMed] [Google Scholar]
- Price C. P., Hill P. G., Sammons H. G. The nature of the alkaline phosphatases of bile. J Clin Pathol. 1972 Feb;25(2):149–154. doi: 10.1136/jcp.25.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL I. S., BURNETT W. THE PROTEINS OF HUMAN BILE. Gastroenterology. 1963 Dec;45:730–739. [PubMed] [Google Scholar]
- Toyoda S., Eto Y., Aoki K. Bile lysosomal enzymes: characteristics and pathological significance for various hepatobiliary disorders. Clin Chim Acta. 1977 Sep 1;79(2):291–298. doi: 10.1016/0009-8981(77)90421-1. [DOI] [PubMed] [Google Scholar]
- Vyvoda O. S., Coleman R., Holdsworth G. Effects of different bile salts upon the composition and morphology of a liver plasma membrane preparation. Deoxycholate is more membrane damaging than cholate and its conjugates. Biochim Biophys Acta. 1977 Feb 14;465(1):68–76. doi: 10.1016/0005-2736(77)90356-x. [DOI] [PubMed] [Google Scholar]
- Wales E. E., Jr, Englert E., Jr, Winward R. T., Maxwell J. G., Stevens L. E. Disc electrophoresis-immunodiffusion of serum proteins in normal human gallbladder bile. Proc Soc Exp Biol Med. 1969 Oct;132(1):146–149. doi: 10.3181/00379727-132-34168. [DOI] [PubMed] [Google Scholar]
- Yousef I. M., Fisher M. M. In vitro effect of free bile acids on the bile canalicular membrane phospholipids in the rat. Can J Biochem. 1976 Dec;54(12):1040–1046. doi: 10.1139/o76-152. [DOI] [PubMed] [Google Scholar]


