Abstract
Objectives:
To compare the demographic, clinical, and biochemical characteristics of newly diagnosed versus known patients with type 1 diabetes mellitus (T1DM) presenting with diabetic ketoacidosis (DKA) in a tertiary care center in the Western region of Saudi Arabia.
Methods:
We retrospectively reviewed 147 children and adolescents diagnosed with T1DM who presented with DKAs between January 2019 and December 2023. Data on age, gender, nationality, economic status, episode severity, presenting symptoms, and biochemical markers were collected and analyzed.
Results:
The mean patient age was 7.24 years, with known patients being older (mean age: 8.24 years) than newly diagnosed patients (mean age: 6.34 years). Most patients (55.8%) belonged to the middle-childhood age group (6 to 11 years). Among known patients, the most prevalent symptoms included vomiting, reported by 62 (88.6%) individuals, and abdominal pain, which affected 55 (78.6%). In contrast, new patients exhibited a strikingly high incidence of polyuria, with 68 (88.3%) cases, and polydipsia, affecting 65 (84.4%) individuals.
Conclusion:
The DKA incidence was higher in newly diagnosed patients, particularly in the middle-childhood age group. Economic factors may contribute to disease manifestations, and newly diagnosed patients had longer DKA symptom durations. The higher DKA incidence and severity in newly diagnosed patients, particularly in certain age groups, underscores the importance of increased disease awareness and early diagnosis.
Keywords: type 1 diabetes mellitus, ketoacidosis, demographic, clinical characteristics, biochemical markers, pediatrics
Type 1 diabetes mellitus (T1DM) is a prevalent and chronic condition among children. It is marked by an absolute deficiency of insulin owing to the autoimmune destruction of pancreatic insulin-producing beta cells over months or years.1 The prevalence of T1DM is 95 cases per 100,000 individuals, with an incidence of 15 cases per 100,000, based on the documented global occurrence.2 Recently, the Kingdom of Saudi Arabia (KSA) has experienced an increase in the incidence and prevalence of T1DM. Based on the latest available information, the incidence rate in KSA is 29 cases per 100,000 individuals, whereas the prevalence rate is 109.5 per 100,000.3 According to the Diabetes Atlas, the annual incidence of T1DM among Saudi Arabian children aged between 0 and 19 years is reported to be 3.8 per 1000.4
According to the World Health Organization, diabetic ketoacidosis (DKA) is the leading cause of death in the twenty-first century, with an annual mortality rate of 10% in pediatric patients.5 The overall pediatric mortality rate of DKA varies from 3.4% to 13.4% in developing nations and from 0.2% to 0.4% in developed nations. This mortality risk is higher in children with severe DKAs.6
Diabetic ketoacidosis is an acute complication of T1DM characterized by hyperglycemia, ketoacidosis, and ketonuria. It occurs when an absolute or relative insulin insufficiency prevents glucose from entering cells for energy use, causing the liver to rapidly break down fat into ketones for use as an energy source. Overproduction of ketones causes them to accumulate in the blood and urine and acidify the blood.7 This condition may manifest as an initial clinical presentation in new cases or known cases owing to different etiologies, such as physiological stress, infections, and inadequate adherence to insulin therapy being the most commonly reported.8,9
Studies have indicated a negative correlation between the prevalence of DKA and age among individuals diagnosed with T1DM, with its occurrence diminishing with age. Additionally, males exhibit a slightly higher prevalence of DKA than females.10 The clinical spectrum of DKA includes prominent symptoms like polyuria, polydipsia, weight loss, fatigue, altered mental status, and respiratory distress.11
In patients with DKAs, key biochemical markers include a decrease in plasma pH and bicarbonate anions (HCO3-), and elevated plasma glucose and glycated hemoglobin (HbA1C) levels. However, a comprehensive understanding of these markers is essential for understanding the severity and metabolic derangements associated with DKA.12
This study aimed to analyze and compare the demographic, clinical, and biochemical parameters between new versus known patients with T1DM presenting with DKA at a tertiary care center in the Western region of Saudi Arabia.
Methods
This retrospective review included 147 children and adolescents diagnosed with T1DM who presented with DKAs at (blinded for review), between January 2018 and December 2023. This study was approved by the Research Ethical Committee of the hospital.
The Cochrane sample size formula was used to determine the recommended sample size, considering the total number of patients admitted with DKA between 2019 and 2023. A sample size of 147 cases was carefully determined, taking into account a 95% confidence interval (CI), a 20% prevalence of T1DM-related DKA cases, and a 6% margin of error. Patient records were thoroughly assessed using an electronic documentation system, focusing on key variables such as age, gender, nationality, economic status, and episode severity. The inclusion of these variables is critical as they offer insight into potential correlations, allowing for a comprehensive examination of their influence on the severity and manifestations of the condition.
Age categorization followed the guidelines set forth by the National Institute of Child Health and Human Development and encompassed distinct stages: infants (6 to 12 months), toddlers (13 months to 2 years), early childhood (2 to 5 years), middle childhood (6 to 11 years), and early adolescents (12 to 18 years).13 This broad age range was chosen to capture the developmental stages that could significantly affect the presentation and management of T1DM-related DKA. By encompassing these diverse age groups, this study aimed to discern potential variations in the condition across critical developmental milestones, offering a perspective on how age influences disease manifestations and severity.
Gender was categorized into male and female, and nationality was categorized into Saudi and non-Saudi. The self-reported economic status of the patient was categorized based on a report by the Saudi Ministry of Human Resources and Social Development, where an income of less than 10,238 riyals, the average monthly wage, was considered low, and an income more than this was considered good (2018).
Normal pH ranges typically fall between 7.35 and 7.45, whereas the normal bicarbonate concentration ranges from 22 to 28 mmol/L based on the local center’s laboratory values. Hence, DKA severity was categorized based on The European Society for Pediatric Endocrinology guidelines, where mild DKA was defined as a venous pH of less than 7.3 and a bicarbonate concentration of less than 15 mmol/L, moderate DKA as a pH of less than 7.2 and a bicarbonate concentration of less than 10 mmol/L, and severe DKA as a pH of less than 7.1 and a bicarbonate concentration of less than 5 mmol/L.14 The duration of DKA was documented in hours (less than 24 h, between 24 and 48 h, and more than 48 h).15 To ensure consistency in the classification of DKA severity, the individuals responsible for this categorization underwent specific training and periodic assessments for inter-rater reliability.
Presenting symptoms reported by the patient or caregiver, such as polyuria, polydipsia, polyphagia, nausea, vomiting, abdominal pain, diarrhea, blurry vision, headache, and decreased activity were documented. Signs observed by healthcare providers, including tachycardia, tachypnea, fever, Glasgow Coma Scale (GCS), drowsiness, Kussmaul breathing, and shortness of breath were also recorded. These observations and reported symptoms were gathered and recorded while maintaining consistency in data collection procedures.
All efforts were made to maintain patient anonymity throughout data collection by de-identifying records via pseudonymization of patient data for an assigned number, ensuring that all individuals remained anonymous throughout the study to adhere to ethical standards.
This comprehensive methodology addresses the pertinent aspects of data collection and analysis, encompassing demographic details, clinical severity, and manifestations of DKA in pediatric patients with T1DM while acknowledging and mitigating the potential biases inherent in retrospective studies.
Statistical analysis
Categorical data are expressed as frequencies and percentages (%), whereas parametric data are expressed as means±standard deviations (minimum-maximum). Data were analyzed using Statistical Package for Social Sciences, version 22 (IBM Corp., Armonk, New York, USA). The Shapiro-Wilk test was used to determine whether the data sample followed a normal distribution. Pearson’s Chi-squared test was used to compare categorical data, the Mann-Whitney U test was used for independent non-normally distributed parametric data (such as age, complaint duration, bicarbonate concentration, HbA1C level), and the unpaired Student’s t-test was used for normally distributed parametric data (such as GCS). Binary logistic regression was used to determine the predictors of newly diagnosed DKA (expected β ± standard error) and 95% confidence intervals (95% CIs). Statistical significance was defined at p-values of <0.05.
Results
The mean age of the patients was 7.24 years (range: 1-18 years) with a standard deviation of 3.77. Known patients were typically older (mean age: 8.24 years) compared with newly diagnosed patients (mean age: 6.34 years) (p<0.001). Notably, most fell in the middle-childhood age group, which ranged from 6 to 11 years and accounted for (55.8%). The distribution across age groups varied, with more toddlers that ranged from 13 months to 2 years among the newly diagnosed patients (15.6%) and early adolescents that ranged from 12 to 18 years among the known patients (20%) (p=0.003). Among the participants, 54.4% were male, and 52.4% were Saudi nationals (Table 1).
Table 1.
- Demographic characteristics of known versus newly diagnosed patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis.
| Characteristics | Known patients (n=70, 47.6%) | New patients (n=77, 52.4%) |
|---|---|---|
| Age (years) | 8.24±3.52 | 6.34±3.77 |
| Age groups | ||
| Toddler | 1 (1.4%) | 12 (15.6%) |
| Early childhood | 14 (20.0%) | 19 (24.7%) |
| Middle childhood | 41 (58.6%) | 41 (53.2%) |
| Early adolescence | 14 (20.0%) | 5 (6.5%) |
| Gender | ||
| Male | 39 (55.7%) | 41 (53.2%) |
| Female | 31 (44.3%) | 36 (46.8%) |
| Nationality | ||
| Saudi | 33 (47.1%) | 44 (57.1%) |
| Non-Saudi | 37 (52.9%) | 33 (42.9%) |
| Economic status | ||
| Good | 31 (44.3%) | 49 (63.6%) |
| Poor | 39 (55.7%) | 28 (36.4%) |
Values are presented as numbers and percentages (%).
There was a clear distinction regarding economic status between the newly diagnosed and known patients with DKA. A higher percentage of new patients (63.6%) had a good economic status compared with known patients (44.3%) (p=0.021).
Among the admitted children, 47.6% had a known history of T1DM, and 52.4% were new patients. The known patients tended to be older (p<0.0001) and were predominantly in middle childhood (58.6%). Conversely, new patients were more prevalent in middle (53.2%) and early childhood (24.7%) (p=0.003) (Appendix 1).
The distribution of DKA severity levels (mild, moderate, and severe) exhibited no statistically significant difference between the known and new patients. Mild DKA was slightly more prevalent in known (34.3%) than in new patients (28.6%) (p=0.060), moderate DKA was more prevalent in known (45.7%) than in new patients (33.8%), and severe DKA was more prevalent in new (37.7%) than in known patients (20%). Notably, the GCS scores, indicative of neurological status, demonstrated consistency across all patients (average GCS: 14.56 ± 1.33), with minimal variation observed between known (14.61 ± 1.41) and new patients (14.52 ± 1.25) (p pss both groups (Table 2).
Table 2.
- Clinical characteristics of known versus newly diagnosed patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis (DKA).
| DKA severity | Known patients (n=70, 47.6%) | New patients (n= 77, 52.4%) |
|---|---|---|
| Mild | 24 (34.3%) | 22 (28.6%) |
| Moderate | 32 (45.7%) | 26 (33.8%) |
| Severe | 14 (20.0%) | 29 (37.7%) |
Values are presented as number and percentages (%).
There was a distinct clinical manifestations of diabetic ketoacidosis (DKA) in known and new patients. Among known patients, the most prevalent symptoms included vomiting, reported by 62 (88.6%) individuals, and abdominal pain, which affected 55 (78.6%). In contrast, new patients exhibited a strikingly high incidence of polyuria, with 68 (88.3%) cases, and polydipsia, affecting 65 (84.4%) individuals. Furthermore, 45 (58.4%) of new patients reported vomiting, while only 11 (15.7%) of known patients experienced polydipsia. Other manifestations, such as nausea and fever, were less common overall, with 21.4% of known patients and 11.7% of new patients experiencing nausea, and fever reported in 14.3% of known patients and 6.5% of new patients (Table 3).
Table 3.
- Clinical symptoms in known versus new patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis.
| Clinical manifestations | Known patients (n=70, 47.6%) | New patients (n=77, 52.4%) |
|---|---|---|
| Vomiting | 62 (88.6%) | 45 (58.4%) |
| Abdominal pain | 55 (78.6%) | 43 (55.8%) |
| Polyuria | 14 (20.0%) | 68 (88.3%) |
| Polydipsia | 11 (15.7%) | 65 (84.4%) |
| Nausea | 15 (21.4%) | 9 (11.7%) |
| Fever | 10 (14.3%) | 5 (6.5%) |
| Headache | 8 (11.4%) | 5 (6.5%) |
| Diarrhea | 4 (5.7%) | 8 (10.4%) |
| Polyphagia | 3 (4.3%) | 6 (7.8%) |
| Blurry vision | 4 (5.7%) | 3 (3.9%) |
Values are presented as number and percentages (%).
Tachycardia was prevalent among the 2 groups with 45 (64.3%) of known patients and 47 (61%) of new patients exhibiting this symptom. Decreased activity was also quite common among new patients, affecting 53 (68.8%) compared to 37 (52.9%) of known patients, indicating a potential decline in overall health status. Drowsiness was reported in 22 (31.4%) of known patients and 32 (41.6%) of new patients, suggesting a greater level of fatigue or altered consciousness in newly diagnosed cases. Additionally, Kussmaul breathing was observed in a similar proportion of both groups, with 24 (34.3%) of known patients and 27 (35.1%) of new patients, highlighting its significance as a compensatory mechanism for metabolic acidosis (Table 4).
Table 4.
- Clinical signs in known versus new patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis.
| Clinical manifestations | Known patients (n=70, 47.6%) | New patients (n=77, 52.4%) |
|---|---|---|
| Tachycardia | 45 (64.3%) | 47 (61.0%) |
| Decreased activity | 37 (52.9%) | 53 (68.8%) |
| Tachypnea | 32 (45.7%) | 37 (48.1%) |
| Drowsiness | 22 (31.4%) | 32 (41.6%) |
| Kussmaul breathing | 24 (34.3%) | 27 (35.1%) |
| Infection | 23 (32.9%) | 18 (32.9%) |
| Shortness of breath | 16 (22.9%) | 18 (23.4%) |
Values are presented as number and percentages (%).
Discussion
Ketoacidosis is a severe, acute complication of T1DM that poses a significant clinical challenge, particularly in pediatric patients. Notably, the onset of T1DM predominantly occurs during the pivotal age ranges of 4-6 and 10-14 years.16,17 This study comprehensively investigated various factors influencing DKA in children with T1DM. Specifically, the differences between new versus known patients may provide insights into their demographic, clinical, and biochemical profiles. T1DM development involves multifactorial influences, including age, race, genetic predisposition, dietary habits, and viral infections.12
This study emphasized a higher incidence of DKA among newly diagnosed patients (53.2%) compared with local studies (29.8%) in the central region of Kingdom of Saudi Arabia (KSA) by Babiker et al17 and (41.7%) in the eastern region of KSA by Albuali et al.12 An international study conducted in Italy by Passanisi et al18 reported slightly lower yet comparable results (51.5%). The discrepancies between our findings and the local studies could be attributed to regional variations in healthcare access, public awareness of diabetes symptoms, and differences in early diagnosis practices.
Figure 1.
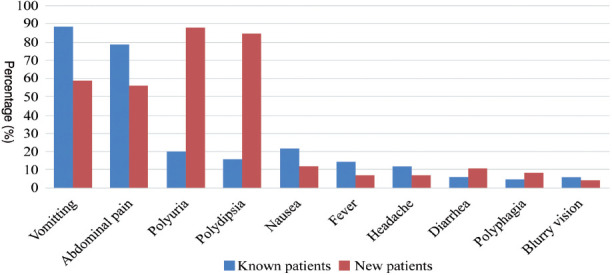
- Clinical symptoms in known versus new patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis.
Figure 2.
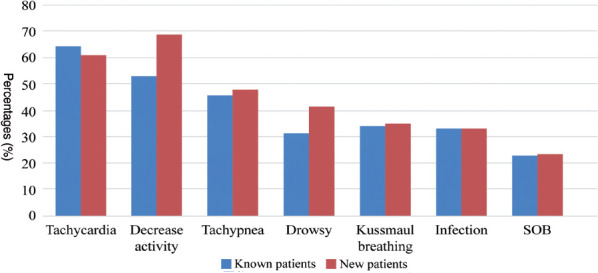
- Clinical signs in known versus new patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis
The majority of known patients with DKA were in the middle-childhood age group (58.6%), with (20%) in adolescence, in early childhood (20%), and in the toddler (1.4%) age group. This corresponds to the findings of Babiker et al,17 where 50% of the known patients were older than 10 years, 44.2% were between 5 and 10 years, and 5.71% were younger than 5 years. In this study, new patients comprised (15.6%) of the study population, of which the majority (53.2%) were in middle childhood and the minority (6.5%) were adolescents. Babiker et al17 reported that 47.6% of new patients were in middle childhood, and (28%) were older than 10 years.12 The higher proportion of middle-childhood patients may be linked to delayed diagnosis and possible stressors such as changes in school or lifestyle, that may exacerbate diabetes symptoms.
Regarding gender distribution, males were slightly predominant in known (55.7%) and new (53.2%) patients with DKA, which is consistent with other studies conducted in KSA. A study by Albuali et al12 showed that 120 out of 211 (56.9%) children with new onset T1DM were male.12 In contrast, a study among 373 children by Naeem et al19 showed that the proportion of females (55.5%) was slightly higher than that of males (44.5%). The variation in gender distribution across studies might be attributed to population-specific factors.
Economic status exhibited marked differences between new and known patients. Known patients were more likely to have a low economic status (55.7%), whereas new patients exhibited a good economic status (63.6%), indicating potential disparities in healthcare access for known patients with lower incomes or increased awareness of symptoms, leading to earlier diagnosis among higher-income families.
Most patients presented with moderate DKA (39.5%), followed by mild (31.3%) and severe DKA (29.3%), consistent with the findings of Albuali et al12 Known patients commonly exhibit moderate DKA severity, followed by mild and severe DKA. Conversely, new patients often present with severe DKA, followed by moderate and mild DKA, indicating the potential for delayed recognition of DKA symptoms in this group. This is probably because known patients are more likely to have received prior education on DKA management and warning signs, enabling earlier detection and intervention.
Among the presenting complaints, vomiting and abdominal pain were reported by a substantial number of patients. The prevalence was higher among known (88.6% and 78.6%) than new patients (58.4% and 55.8%), which is consistent with the findings of Naeem et al,19 who reported similar discrepancies in vomiting and abdominal pain between known and new patients with DKA. Importantly, new patients exhibited a longer duration of acute complaints (1-14 days) than known patients (1-4 days). Similarly, Naeem et al19 found that the duration of polyuria was longer in new patients (11.4-15.2 days) than in known patients (1.3-2.9 days), providing further evidence that new patients are prone to present in healthcare facilities later compared with known patients owing to their decreased familiarity with the symptoms of DKA and its potentially life-threatening consequences.17
In this study, the biochemical profiles of patients with DKA revealed intriguing trends, particularly when newly diagnosed and known patients with T1DM were compared. In the patient cohort, the pH level predominantly fell between 7.2 and 7.3 (43.5%), whereas the bicarbonate level ranged from 8 to less than 12 mmol/L (42.1%). Notably, this study highlighted significantly higher pH and bicarbonate levels in known patients than in new patients (p=0.030 and p<0.0001, respectively), as this may relate to disease severity. These findings provide insights into the biochemical aspects of DKA and reveal the distinct differences between new and known patients with T1DM. These variations highlight the complexity and variability of the biochemical characteristics of DKA. Lower pH and bicarbonate levels in newly diagnosed patients could indicate more severe acidosis upon presentation, highlighting the need for close monitoring and immediate intervention to correct metabolic abnormalities. Compared with Albuali et al’s12 research, the results of this study align in some respects and diverge in others. Their study analyzed 211 pediatric patients admitted to the intensive care unit for DKA and reported that (41.7%) of them were new cases. These findings emphasize the correlation between DKA outcomes and metabolic abnormalities upon presentation.12
Study limitations
This study had a few limitations. First, the retrospective design may have resulted in recall bias and potentially led to missing or incomplete data given its reliance on historical medical records. Second, the study’s confinement to a single center may limit its broader applicability across KSA and the world.
In conclusion, this study highlights the substantial differences between patients with newly diagnosed versus known T1DM presenting with DKA and emphasizes the critical need for healthcare providers to address these differences. The higher incidence of DKA in new patients, particularly in certain age groups, underscores the importance of increased awareness and early diagnosis of this condition. Economic factors also contribute to disease manifestations, emphasizing the need to address gaps in healthcare access. Public health campaigns, educational initiatives, and collaborations with primary care physicians are crucial for patient education, early detection, and timely interventions. Although the study’s single-center design limits its generalizability, future research should consider a multicenter approach to validate and further investigate T1DM in the pediatric population. Early recognition, improved access to care, and educational initiatives are essential to reduce the impact of DKA in pediatric patients with T1DM.
Acknowledgment
We would like to thank Editage (www.editage.com) for their writing support on the manuscript.
Appendix 1.
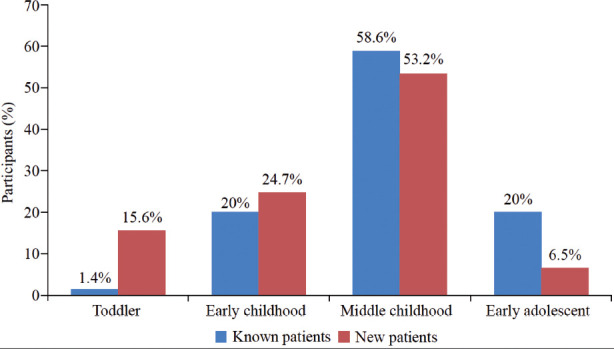
- Age categorization of known versus newly diagnosed patients with type 1 diabetes mellitus presenting with diabetic ketoacidosis.
Footnotes
References
- 1.Lucier J, Weinstock RS.. Diabetes mellitus Type 1. 2023. Treasure Island (FL): StatPearls Publishing; 2024. Jan-. [Google Scholar]
- 2.Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M.. Prevalence and Incidence of type 1 diabetes in the world: A Systematic Review and meta-analysis. Health Promot Perspect 2020; 10: 98-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Herbish AS, El-Mouzan MI, Al-Salloum AA, Al-Qurachi MM, Al-Omar AA.. Prevalence of type 1 diabetes mellitus in Saudi Arabian children and adolescents. Saudi Med J 2008; 29: 1285-1288. [PubMed] [Google Scholar]
- 4.International Diabetes Federation; 2021. In: J Lucier, Mathias PM. IDF diabetes atlas, 10th ed. [Google Scholar]
- 5.Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain Vet al. ISPAD clinical practice consensus guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2014; 15: 154-179. [DOI] [PubMed] [Google Scholar]
- 6.Baloch SH, Ibrahim PMN, Lohano PD, Gowa MA, Mahar S, Memon R.. Pediatric risk of mortality III score in predicting mortality among diabetic ketoacidosis patients in a pediatric Intensive Care Unit. Cureus 2021; 13: e19734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medscape References. Diabetic Ketoacidosis (DKA): practice essentials, background, pathophysiology. [updated 2025. Aug 13; cited 2024]. Available from: https://emedicine.medscape.com/article/118361-overview?form=fpf
- 8.Buschard K. The etiology and pathogenesis of type 1 diabetes – A personal, non-systematic review of possible causes, and interventions. Front Endocrinol 2022; 13: 876470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostopoulou E, Sinopidis X, Fouzas S, Gkentzi D, Dassios T, Roupakias Set al. Diabetic ketoacidosis in children and adolescents; diagnostic and therapeutic pitfalls. Diagnostics (Basel) 2023; 13: 2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen ET, Stafford JM, Saydah S, D’Agostino RB, Dolan LM, Lawrence JMet al. Increase in prevalence of diabetic ketoacidosis at diagnosis among youth with type 1 diabetes: the SEARCH for diabetes in youth study. Diabetes Care 2021; 44: 1573-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher E, Siu HYH.. Diabetic ketoacidosis as first presentation of type 1 diabetes mellitus in a young child: Important differential diagnosis for respiratory distress. Can Fam Physician 2020; 66: 425-426. [PMC free article] [PubMed] [Google Scholar]
- 12.Albuali WH, Yousef AA, Al-Qahtani MH, AlQurashi FO, Albuali HW, Yousef HAet al. A clinical and biochemical comparative study of diabetic ketoacidosis (DKA) in newly diagnosed vs. known cases of Type 1 diabetic children. Rev Diabet Stud 2023; 19: 28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasundaram P, Avulakunta I; 2023. Human growth and development [online]. Darshini. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] [Google Scholar]
- 14.Rodríguez-Merchán B, Casteràs A, Domingo E, Nóvoa FJ, López Y, Cabezas-Agricola JMet al. Capillary beta-hydroxybutyrate determination for monitoring diabetic ketoacidosis. Endocrinol Nutr 2011; 58: 347-352. [DOI] [PubMed] [Google Scholar]
- 15.Al Shaikh A, Farahat F, Saeedi M, Bakar A, Al Gahtani A, Al-Zahrani Net al. Incidence of diabetic ketoacidosis in newly diagnosed type 1 diabetes children in western Saudi Arabia: 11-year experience. J Pediatr Endocrinol Metab 2019; 32: 857-862. [DOI] [PubMed] [Google Scholar]
- 16.Babar B, Aamir AH.. Seasonal variation and severity of diabetic ketoacidosis in patients at a tertiary care hospital in Pakistan. Pak J Med Sci 2022; 38: 1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babiker A, Aljahdali GL, Alsaeed MK, Almunif AF, Mohamud MS, Al Mutair Aet al. Frequency and risk factors of diabetic ketoacidosis in a specialized Children’s Hospital, Riyadh: A cross-sectional study. Oman Med J 2022; 37: e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passanisi S, Salzano G, Basile P, Bombaci B, Caime F, Rulli I, et al. Prevalence and clinical features of severe diabetic ketoacidosis treated in pediatric intensive care unit: A 5-year monocentric experience. Ital J Pediatr. ProQuest 2023; 49: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naeem MA, Al-Alem HA, Al-Dubayee MS, Al-Juraibah FN, Omair A, Al-Ruwaili ASet al. Characteristics of pediatric diabetic ketoacidosis patients in Saudi Arabia. Saudi Med J 2015; 36: 20-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


