Abstract
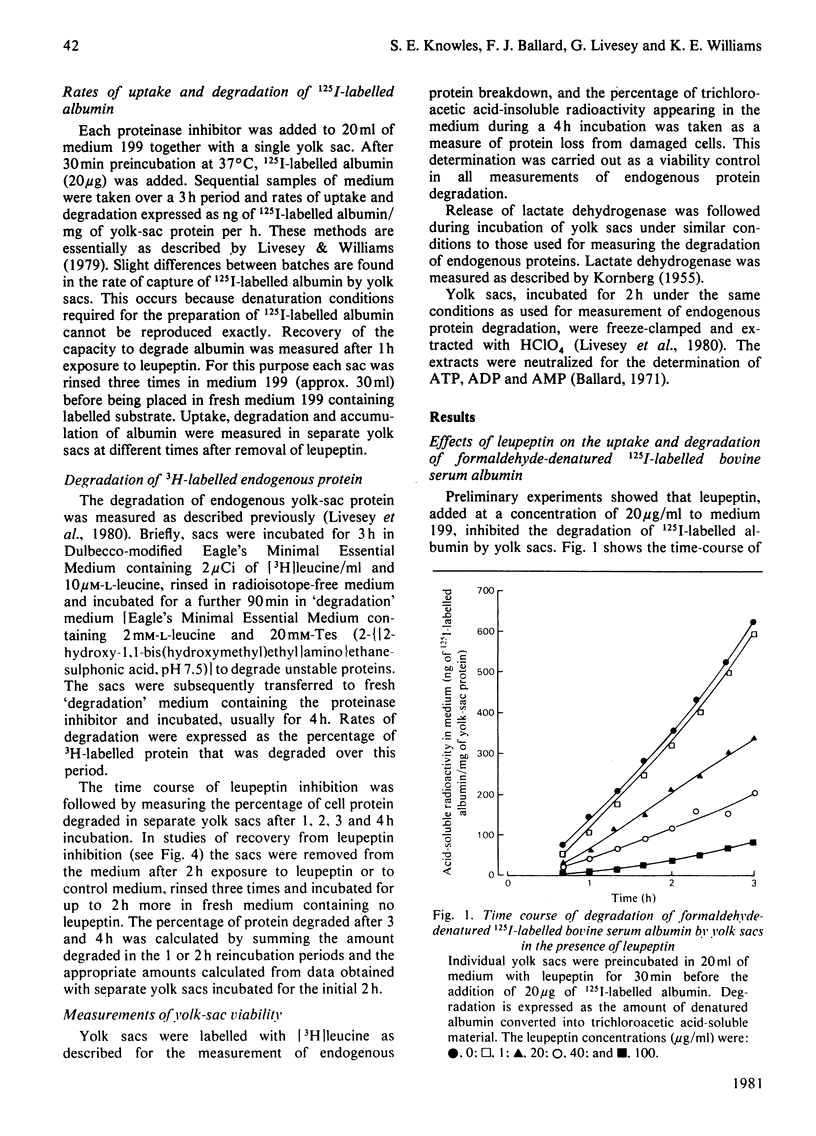
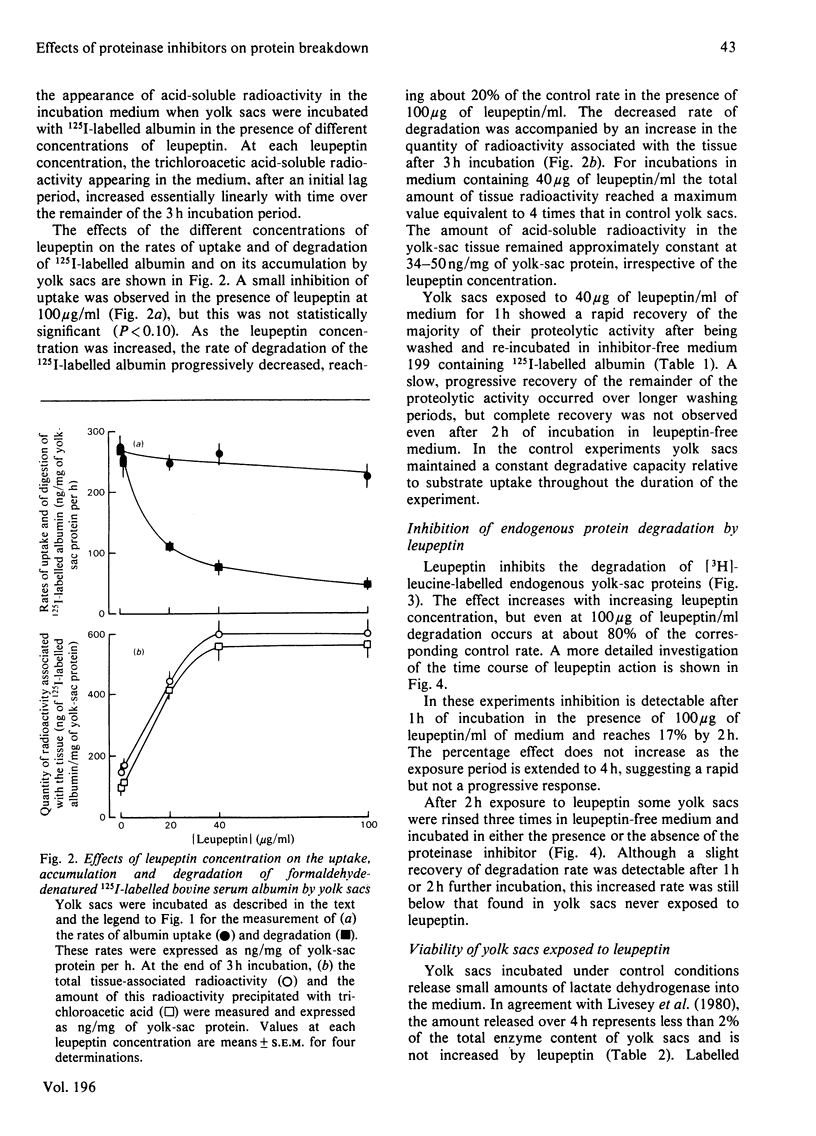
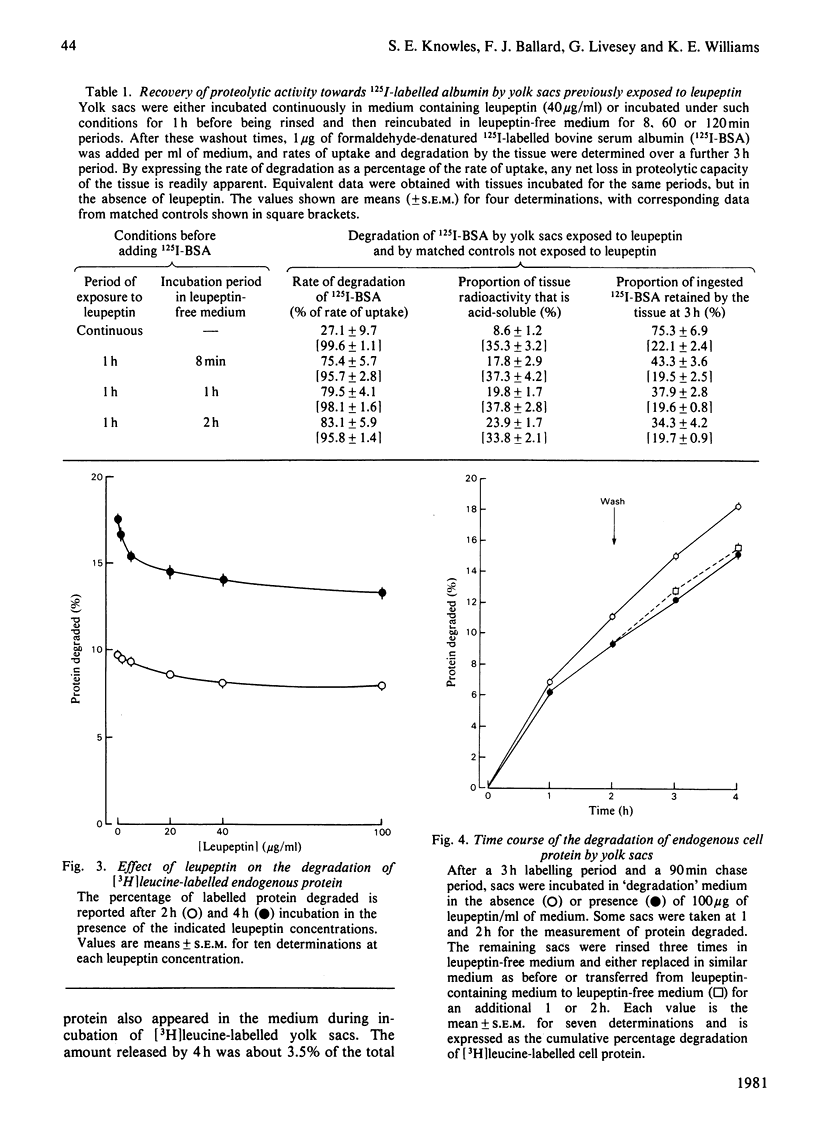
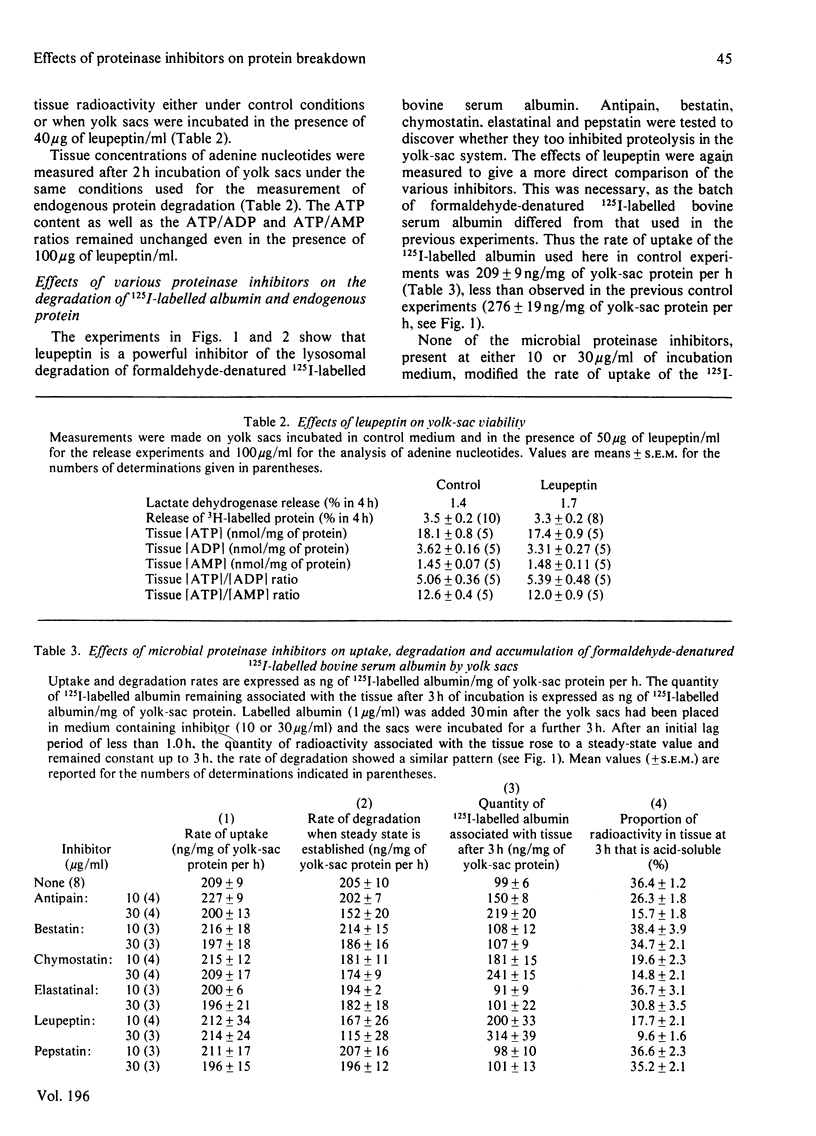
1. The effects of leupeptin and other microbial proteinase inhibitors were measured in rat yolk sacs on the uptake and degradation of formaldehyde-denatured 125I-labelled bovine serum albumin as well as on the degradation of 3H-labelled endogenous protein. 2. Leupeptin, at concentrations between 1 and 100 micrograms/ml, inhibits the degradation of added albumin without affecting pinocytic uptake. Accordingly large amounts of undegraded albumin accumulate within the tissue. 3. Removal of leupeptin produces a rapid recovery of the capacity to degrade albumin. 4. Endogenous protein degradation is rapidly inhibited by leupeptin, but to a far lesser extent than the breakdown of albumin. However, the inhibition is only slightly reversed on removal of leupeptin. 5. Degradation of both albumin and endogenous protein in intact yolk sacs is inhibited by the microbial proteinase inhibitors in the order: leupeptin greater than antipain greater than chymostatin; elastatinal, pepstatin and bestatin are ineffective. 6. Similar results are found when albumin is incubated in yolk-sac homogenates at pH 4 with the inhibitors. 7. The marked inhibitory effects of leupeptin, antipain and chymostatin suggest that cathepsin B and possibly cathepsin L participate in the degradation of 125I-labelled albumin in yolk sacs. By comparison, the smaller inhibitory effects of the proteinase inhibitors on endogenous protein breakdown imply a minor role of lysosomal cathepsins in this process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Dean R. T. Direct evidence of importance of lysosomes in degradation of intracellular proteins. Nature. 1975 Oct 2;257(5525):414–416. doi: 10.1038/257414a0. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Protein degradation in hepatocyte monolayers. Effects of glucagon, adenosine 3':5'-cyclic monophosphate and insulin. Biochem J. 1980 Jan 15;186(1):71–79. doi: 10.1042/bj1860071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman W., Lanting L., Doddema H. J., Bouma J. M., Gruber M. Role of individual cathepsins in lysosomal protein digestion as tested by specific inhibitors. Biochim Biophys Acta. 1974 Nov 25;370(1):297–307. doi: 10.1016/0005-2744(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Libby P., Ingwall J. S., Goldberg A. L. Reduction of protein degradation and atrophy in cultured fetal mouse hearts by leupeptin. Am J Physiol. 1979 Jul;237(1):E35–E39. doi: 10.1152/ajpendo.1979.237.1.E35. [DOI] [PubMed] [Google Scholar]
- Livesey G., Williams K. E. Hydrolysis of an exogenous 125I-labelled protein by rat yolk sacs. Evidence for intracellular degradation within lysosomes. Biochem J. 1979 Dec 15;184(3):519–526. doi: 10.1042/bj1840519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey G., Williams K. E., Knowles S. E., Ballard F. J. Effects of weak bases on the degradation of endogenous and exogenous proteins by rat yolk sacs. Biochem J. 1980 Jun 15;188(3):895–903. doi: 10.1042/bj1880895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Nakamura T., Koyama J. Characterization of macrophage proteases involved in the ingestion of antigen-antibody complexes by the use of protease inhibitors. FEBS Lett. 1978 Aug 15;92(2):299–302. doi: 10.1016/0014-5793(78)80774-1. [DOI] [PubMed] [Google Scholar]
- Roberts A. V., Nicholls S. E., Griffiths P. A., Williams K. E., Lloyd J. B. A quantitative study of pinocytosis and lysosome function in experimentally induced lysosomal storage. Biochem J. 1976 Dec 15;160(3):621–629. doi: 10.1042/bj1600621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Protease inhibitors produced by microorganisms. Acta Biol Med Ger. 1977;36(11-12):1899–1915. [PubMed] [Google Scholar]
- Wang C-C, Touster O. Turnover studies on proteins of rat liver lysosomes. J Biol Chem. 1975 Jul 10;250(13):4896–4902. [PubMed] [Google Scholar]


