Abstract
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a debilitating multifactorial illness characterized by profound fatigue persisting for more than six months, post-exertional malaise, cognitive impairments, and a range of systemic symptoms. Until now, no accepted causal treatment regimens have been available; therapeutic options include different approaches, such as alleviation of symptoms and promotion of energy conservation. In this study, we report the case of a 49-year-old female presented to our center suffering from ME/CFS for more than 15 years, characterised by a strong energy loss and neurological and systemic symptoms; previous therapies remained unsuccessful. Therefore, we decided to perform double-filtration apheresis. After comprehensive laboratory evaluation, including investigation of persistent viral infections, the patient was treated eight times with double-filtration apheresis within a period of 2 years, which resulted in a remarkable sustained clinical remission and significant improvement in her quality of life. Therefore, we conclude that double-filtration apheresis could be an effective therapeutic tool for the treatment of ME/CFS.
Keywords: ME/CFS, double-filtration apheresis, autoantibodies
Introduction
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex multifactorial illness that affects millions of people of all ages worldwide. This disease is usually lifelong in adults. The prevalence of ME/CFS is difficult to measure and there are no specific physical diagnostic signs or biomarkers. The disease is often unrecognized and patients suffering from it remain undiagnosed.1 It is estimated that prevalence ranges from 0.1 and 0.7% in the general population with 2/3 of the cases being women.2,3 Many affected people are unable to work, and their quality of life is strongly decreased.4 The etiology of ME/CFS remains unclear. Mounting evidence suggests a complex interplay between genetic predisposition, immune dysregulation, viral infections, and environmental factors.5–7 Infectious Mononucleosis caused by the Epstein-Barr Virus (EBV) has been consistently associated with ME/CFS.8,9 To date, there is no causal treatment for this disease. Previous studies have reported elevated autoantibodies against β2 adrenergic receptors (ADRB2) and muscarinic 3 and 4 acetylcholine receptors in a subset of patients with ME/CFS.10–12 Additionally, patients with high antibody titers against ADRB2 more frequently had antinuclear antibodies (ANA).10 Another study with 468 CFS patients reported that 25% had decreased serum levels of immunoglobulin mostly in IgG3 subclass, while elevated immunoglobulin levels with an excess of IgM and IgG2 in particular were found in another 25%.10
Double-filtration apheresis (Toxopherese®) is a well-tolerated extracorporeal filtration-based medical application that efficiently removes pathogenic and excess components such as immune complexes and specific toxins from patient plasma. Therapeutic apheresis has been proposed as a treatment option for ME/CFS.12–14 We present a case of severe ME/CFS that was successfully treated with double-filtration apheresis and achieved full clinical remission.
Case Presentation
Patient Description and Case History
The female 49-year-old patient presented in our practice suffering from severe fatigue syndrome for more than 15 years combined with a strongly impaired energy metabolism, a deep depression and post-exertional malaise significantly impacting the quality of her life. She also suffered from insomnia and needed several hours to get up in the morning; she still worked but was mentally and physically exhausted afterwards. She had unexplainable weight increase and swelling of her hands, feet, and neck, and additionally showed neurological disturbances of unclear etiology, such as trembling of the hands and feet; everyday tasks such as opening a bottle or lifting/moving objects were only possible to a very limited degree. She experienced recurrent vertigo and headache almost daily, combined with stabbing pain in the skin of her head and diffuse hair loss. The diagnosis for ME/CFS has also been based on the Canadian consensus criteria which were sufficiently fulfilled.
Upon admission, the patient had rashes on the dorsal parts of both lower legs. She was diagnosed with hypothyroidism combined with cold nodules 8 years previously and was treated with L-thyroxine since then. In 2006, she developed infectious mononucleosis, from which she never completely recovered. Previous therapies remained unsuccessful in this regard. Medications administered before the beginning of treatment in our practice included L-thyroxine, melatonin, alprazolam, alpha-lipoic acid, torasemide, and progesterone. Because of her profession, she had been exposed to potentially toxic substances for more than 25 years.
Physical and Laboratory Examination Results
The first physical examination did not reveal any particular findings except for a strong meteoristic abdomen. A comprehensive laboratory analysis was conducted before therapeutic treatment and revealed chronic inflammation with slightly elevated white blood cell count (7.17/nl), thrombocytosis (423/nl), increased malondialdehyde-modified (MDA)-LDL (42.2 U/l), neurotoxic impairment with strongly increased autoimmune antibodies against β2 adrenergic receptors (ADRB2) (16.6 U/mL), borderline antinuclear antibodies (ANA) of 1:80, dental foci with increased RANTES (90 ng/mL), and leaky-gut syndrome (zonulin 47.5 ng/mL). Eosinophilic cationic protein (ECP) was also increased (23.5 µg/l). Excess immunoglobulin was detected for IgG (> 202 mg/l), IgA (> 187 mg/l), and IgM (362 mg/l). The Lymphocyte Transformation Test (LTT) showed increased T-lymphocyte activity specific for herpes simplex I and II, Cytomegalovirus, Varicella Zoster, Staphylococcus and Chlamydia trachomatis.
The presence of other autoimmune diseases has been investigated but could be ruled out. Laboratory findings including respective reference ranges are summarised in the table below (Table 1).
Table 1.
Laboratory Findings of Blood Analysis Before Treatment
| Laboratory Marker | Reference Range | Measured Value Before Treatment |
|---|---|---|
| White blood cell count | <6.8/nla | 7.17/nl |
| Thrombocytes | 150–370/nl | 423/nl |
| MDA-LDL | <35 U/l | 42.2 U/l |
| ADRB2 autoantibodies | <8 U/mL | 16.6 U/mL |
| ANA | <1:80 | 1:80 |
| RANTES | <30 ng/mL | 90 ng/mL |
| Zonulin | <26 ng/mL | 47.5 ng/mL |
| ECP | <16 µg/l | 23.5 µg/l |
| IgG | <110 mg/l | >202 mg/l |
| IgA | <25 mg/l | >187 mg/l |
| IgM | <84 mg/l | 362 mg/l |
Notes: aExplanatory note: Values higher than 10.4/nl indicate an acute inflammation. Values >6,8/nl and <10.4/nl indicate a chronic inflammation.
Application of Double-Filtration Apheresis and Outcome
Double-filtration apheresis (Toxopherese®) was used as a treatment procedure. During each apheresis, plasma was separated from the cell-rich fraction using a separation filter and then filtered. The amount of plasma to be filtered was calculated taking into consideration the sex, height, body weight and hematocrit of the patient. Heparin was given as anticoagulant. The filtered plasma was combined with the remaining blood components, reinfused, and the eluate was collected. No plasma/replacement fluid had to be given afterwards.
The patient’s vital signs were monitored during the procedure. Each treatment lasted for an average of 1 hour and 30 minutes. No side effects occurred during the treatments. After extracorporeal apheresis, the appearance of the eluate was visually evaluated and photographed.
The patient received the first apheresis, followed by the second one two days later. The third and fourth treatments followed three months later at the same frequency as treatments 1 and 2. The procedure was well tolerated, and the patient reported that swelling of the hands, feet, neck, and eyes improved one day after the first apheresis. Before the third treatment, the patient said that the shiver attacks did not occur anymore, and that her depressive mood and dizziness had significantly improved. The difficulties in getting up also significantly improved, as did the hair loss. Family members confirmed improvements in clinical symptoms.
Laboratory analysis of the eluate after the first double-filtration apheresis showed increased C3d (complement cleavage products) circulating immune complexes (55.7 mg/l; reference range: < 29 mg/l). The third apheresis revealed no changes in the amount of C3d immune complexes. However, laboratory blood analysis showed that white blood cell count decreased to 6.88/nl but were still slightly increased, demonstrating chronic inflammation (reference range: < 6.8/nl). The thrombocyte levels were also elevated. Zonulin, a marker of a leaky gut decreased from 47.5 ng/mL to 37 ng/mL (reference range: < 25 ng/mL). Eosinophilic cationic protein as a marker for inflammation and allergies also decreased from 23.5 µg/l to 16.3 µg/l almost into the normal range (< 16 µg/l). Excess IgM immunoglobulins were reduced to the normal range (< 1 mg/l; reference range: < 84 mg/l). Increased IgA levels were strongly reduced (from > 187 mg/l to 108 mg/l; reference range: < 25 mg/l). However, IgG levels remained unchanged (> 202 mg/l).
Following the 4th double-filtration apheresis, the patient presented again in our practice and confirmed that the shiver attacks completely disappeared, as well as brain fog and vertigo. She felt more active and concentrated at work. Sleep and headaches improved. The episodes of depression disappeared. Autoantibodies against ADRB2 decreased from 16.6 U/mL to 10.2 U/mL (reference range: < 8 U/mL) while MDA-LDL decreased into the normal range (< 35 U/l). The fifth and sixth treatments were conducted 6 months later at the same frequency as before. Laboratory investigations of the eluate revealed that C3d circulating immune complexes were within the normal range (< 29 mg/l) for the first time. After a break of one year the patient underwent the seventh and eighth extracorporeal apheresis. When she presented to our center before the seventh treatment, she still had no clinical symptoms except for mild hair loss. Her quality of life significantly improved and she was able to live a normal life. Analysis of the eluate showed that C3d circulating immune complexes were still within the normal range (< 29 mg/l). Laboratory blood analysis showed that autoantibodies against ADRB2 decreased further to 9.9 U/mL (reference range: < 8 U/mL).
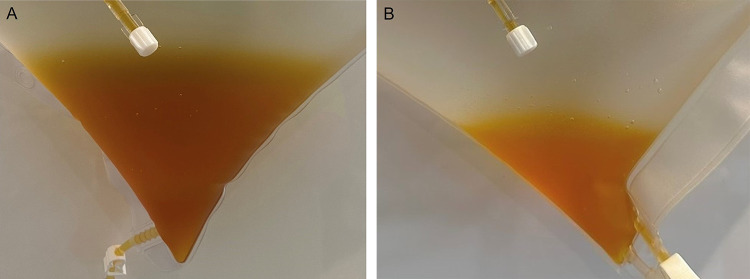
A visual evaluation of the eluate has been conducted after each treatment. A comparison of the eluates after the first and eighth treatments revealed that the liquid was brighter and had fewer streaks (Figure 1). After the conduction of many apheresis treatments in our center we have seen a strong correlation of the eluate appearance and the patients’ clinical condition/laboratory findings. The eluate of chronically ill patients generally contains visible precipitates (streaks) that can result from the presence of excess complement factors/immune complexes (eg C1q or C3d). Additionally, the colour can be dark yellow to brown. These visual abnormalities decrease over the course of several apheresis treatments. Also, for the case presented improvement in clinical symptoms and laboratory findings could also be reflected in the visual determination of the eluate received after each double-filtration apheresis.
Figure 1.
Eluate after first double-filtration apheresis (A); Eluate after eighth double-filtration apheresis (B).
Discussion
We report the case of a female adult suffering from ME/CFS for more than 15 years, who was successfully treated with double-filtration apheresis. Laboratory markers normalized after treatment. Initially, increased levels of autoantibodies against ADRB2, immunoglobulins, and antinuclear antibodies were observed in the patient. The occurrence of increased ß2-adrenerg autoantibodies in a subset of patients with ME/CFS correlating with increased IgG levels has previously been described,10 and it has been demonstrated that the severity of fatigue correlates with autoantibodies against ADRB2.15 On the other hand, a reduction in these antibodies has been associated with clinical improvement; an earlier study conducted with 10 patients suffering from ME/CFS already showed effectiveness in removing autoantibodies against ß2-adrenoreceptor by applying five cycles of immunoadsorption (IA), which is a different type of therapeutic apheresis based on adsorption.12 In this study, 70% of the patients who were treated experienced a rapid improvement in symptoms, with 3 of these patients experiencing long-lasting improvement for 6–12+ months. In a subsequent conformational trial five patients who responded to the first IA treatment were retreated with an adapted treatment protocol.13 As a result, four of the five patients had clinical improvement lasting six–12 months, providing evidence that IA seems to have clinical efficacy in the treatment of ME/CFS.
In another study, a cohort of 27 patients with Long-Covid including chronic fatigue syndrome, was treated twice with therapeutic apheresis.14 This study showed that after treatment, autoantibodies against β1 and β2 adrenergic receptors and muscarinic M3 and M4 acetylcholine receptors were reduced. A reduction in autoantibodies against ADRB2 was also observed in the present case.
Furthermore, our patient presented with increased C3d (complement cleavage product) circulating immune complexes in the eluate. A recent study investigated potential biomarkers in patients with Long-Covid demonstrating increased complement activation.16 It has been proposed that tissue injury may also be complement-mediated in Long-Covid patients and that complement activation may be driven by antigen–antibody complexes. Altered complement activation has furthermore been proposed to contribute to the development of neurodegenerative diseases.17 This might also play a role in ME/CFS. In our patient, the laboratory marker C3d circulating immune complexes was increased at the beginning of treatment, leading to the conclusion that the complement system was activated such that the resulting immune complexes could not be eliminated appropriately. Under physiological conditions, immune complexes are transiently formed to eliminate foreign antigens such as viruses or other infectious agents. However, if their formation exceeds the absorptive capacity of phagocytes, these complexes increase and may lead to tissue damage and further activation of the complement system. Various triggers can activate the complement system. As our patient showed increased T lymphocyte activity specific for herpes simplex I and II, Cytomegalovirus, Varicella Zoster in LTT before treatment initiation, it can be assumed that these herpes viruses may have contributed to complement activation. Treatment with extracorporeal double-filtration apheresis decreased C3d immune complexes, ß2-adrenerg autoimmune antibodies, and excess immunoglobulin formation. In addition, the patient’s clinical symptoms completely resolved.
Conclusion
In conclusion, the above-referenced studies support that therapeutic apheresis could be an effective treatment option for patients with ME/CFS. Here, we report a case of severe ME/CFS. The patient was treated with eight cycles of double-filtration apheresis until sustained clinical remission. Concomitant laboratory analyses revealed a reduction of increased laboratory parameters. Therefore, we consider double-filtration apheresis to be an effective and well-tolerated therapeutic option for treating ME/CFS.
Funding Statement
There is no funding to report.
Ethical Approval and Consent
Ethical approval is not required to publish the case details in accordance with local or national guidelines. The patient provided written informed consent for the publication of this case report and accompanying images.
Disclosure
Dr Harald Burgard reports to be a shareholder of MedSelect GmbH, outside the submitted work. The author reports no other conflicts of interest in this work.
References
- 1.Nacul L, Authier FJ, Scheibenbogen C, et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina. 2021;57(5):510. doi: 10.3390/medicina57050510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nacul LC, Lacerda EM, Pheby D, et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med. 2011;9:91. doi: 10.1186/1741-7015-9-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estévez-López F, Castro-Marrero J, Wang X, et al. Prevalence and incidence of myalgic encephalomyelitis/chronic fatigue syndrome in Europe-the Euro-epiME study from the European network EUROMENE: a protocol for a systematic review. BMJ Open. 2018;8(9):e020817. doi: 10.1136/bmjopen-2017-020817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tschopp R, König RS, Rejmer P, Paris DH. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a preliminary survey among patients in Switzerland. Heliyon. 2023;9(5):e15595. doi: 10.1016/j.heliyon.2023.e15595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. doi: 10.1136/bmj.38933.585764.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanElzakker MB. Chronic fatigue syndrome from vagus nerve infection: a psychoneuroimmunological hypothesis. Med Hypotheses. 2013;81(3):414–423. doi: 10.1016/j.mehy.2013.05.034 [DOI] [PubMed] [Google Scholar]
- 7.Glassford JAG. The neuroinflammatory etiopathology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front Physiol. 2017;8:88. doi: 10.3389/fphys.2017.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015;313(11):1101–1102. doi: 10.1001/jama.2015.1346 [DOI] [PubMed] [Google Scholar]
- 9.Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124(1):189–193. doi: 10.1542/peds.2008-1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loebel M, Grabowski P, Heidecke H, et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav Immun. 2016;52:32–39. doi: 10.1016/j.bbi.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Kuratsune H, Hidaka Y, et al. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. Int J Mol Med. 2003;12(2):225–230. [PubMed] [Google Scholar]
- 12.Scheibenbogen C, Loebel M, Freitag H, et al. Immunoadsorption to remove ß2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS One. 2018;13(3):e0193672. doi: 10.1371/journal.pone.0193672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tölle M, Freitag H, Antelmann M, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: efficacy of repeat immunoadsorption. J Clin Med. 2020;9(8):2443. doi: 10.3390/jcm9082443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achleitner M, Steenblock C, Dänhardt J, et al. Clinical improvement of Long-COVID is associated with reduction in autoantibodies, lipids, and inflammation following therapeutic apheresis. Mol Psychiatry. 2023;28:1–6. doi: 10.1038/s41380-023-02084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitag H, Szklarski M, Lorenz S, et al. Autoantibodies to vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med. 2021;10(16):3675. doi: 10.3390/jcm10163675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervia-Hasler C, Brüningk SC, Hoch T, et al. Persistent complement dysregulation with signs of thromboinflammation in active long covid. Science. 2024;383(6680):eadg7942. doi: 10.1126/science.adg7942 [DOI] [PubMed] [Google Scholar]
- 17.Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601–617. doi: 10.1038/s41582-020-0400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]