Abstract
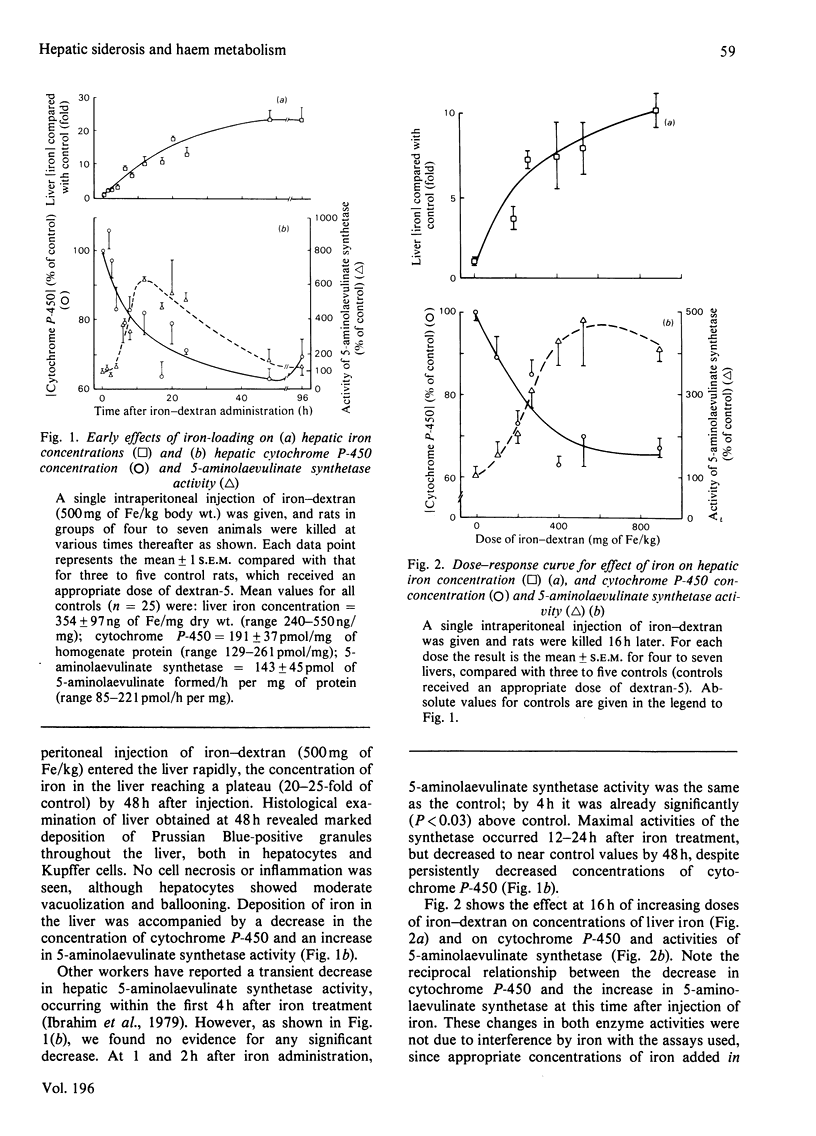
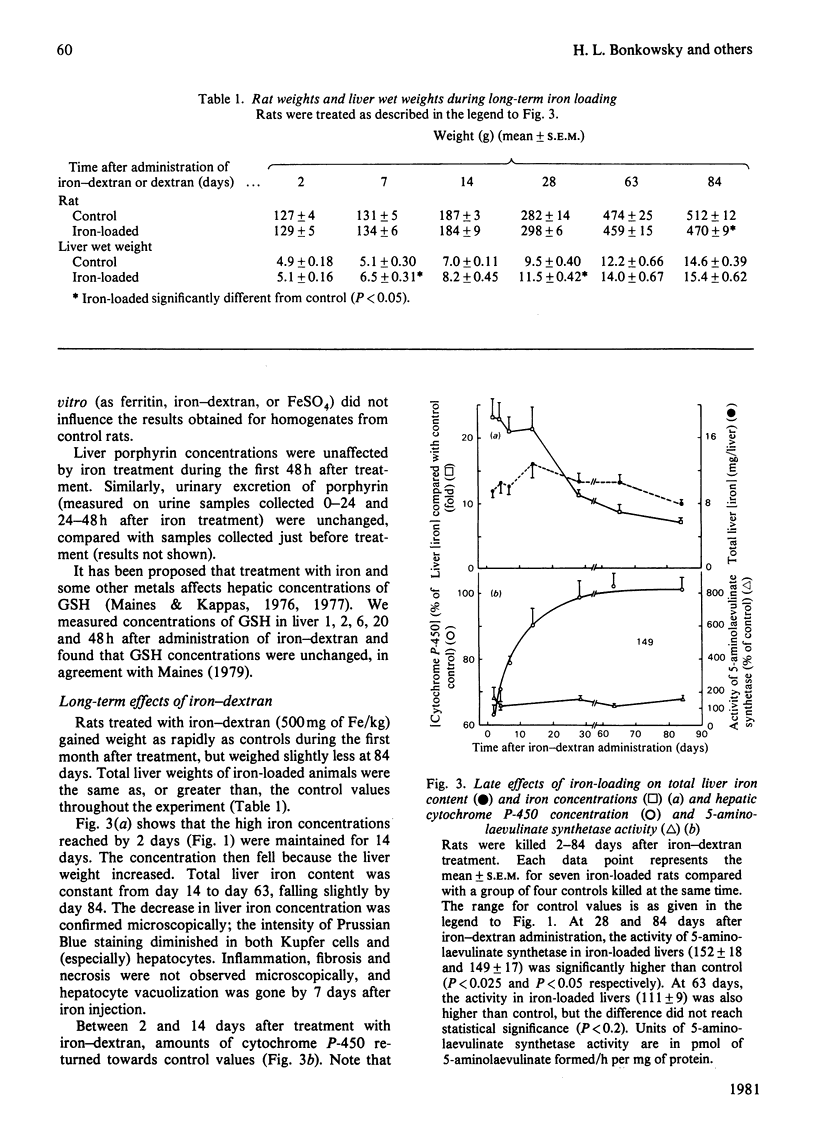
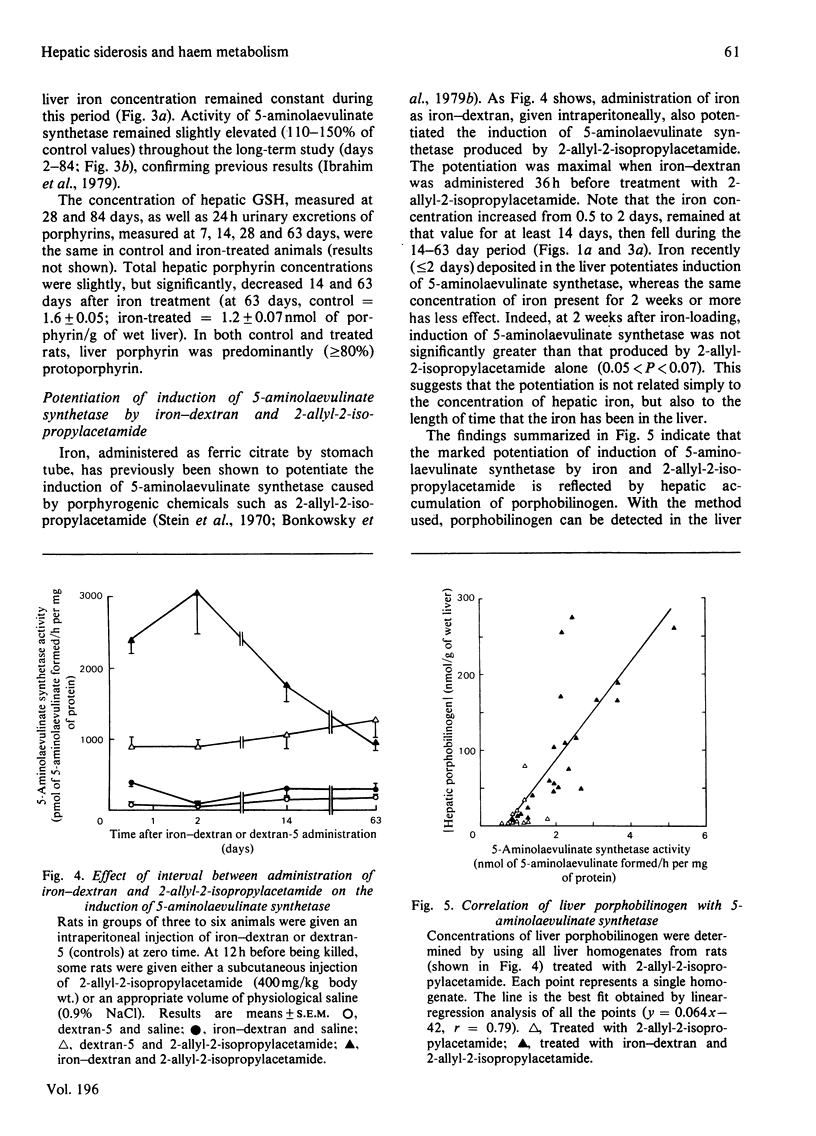
We have determined the dose-response curves (100-900 mg of Fe/kg body wt.) and the time course over 84 days for the effects of a single injection of iron-dextran on rat hepatic 5-aminolaevulinate synthetase, cytochrome P-450, iron content, and GSH (reduced glutathione). Porphyrins in liver and urine have also been measured. (1) At 2 days after treatment, a dose of 500 mg of Fe/kg produced a 20-fold increase in iron concentration, which was maintained for 14 days. Total hepatic iron remained constant over 63 days, falling slightly by 84 days. (2) The activity of 5-aminolaevulinate synthetase was maximally increased (6-fold) 12-24 h after iron treatment. By 48 h the activity fell to less than twice the control value and thereafter remained slightly above the control value (1.1-1.5-fold) until 84 days after iron treatment. Liver GSH concentrations were unaffected by iron. Porphyrins in liver and urine were either unchanged or decreased. (3) Hepatic cytochrome P-450 decreased after iron treatment to a minimum (63% of control) at 48 h after iron administration and gradually returned to the control value by 28 days. (4) Iron-dextran potentiated 2 allyl-2-isopropyl-acetamide-induced synthesis of hepatic 5-aminolaevulinate. Potentiation occurred if the drug was given at the same time or 36 h after iron administration, but did not occur if the drug was given 14 or 64 days after iron administration. (5) The results are discussed in relation to proposed mechanisms for the effects of iron on hepatic haem metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbritti G., De Matteis F. Decreased levels of cytochrome P-450 and catalase in hepatic porphyria caused by substituted acetamides and barbiturates. Importance of the allyl group in the molecule of the active drugs. Chem Biol Interact. 1972 Mar;4(4):281–286. doi: 10.1016/0009-2797(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Barry M., Sherlock S. Measurement of liver-iron concentration in needle-biopsy specimens. Lancet. 1971 Jan 16;1(7690):100–103. doi: 10.1016/s0140-6736(71)90838-5. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Guzelian P. S. Degradation of endogenous hepatic heme by pathways not yielding carbon monoxide. Studies in normal rat liver and in primary hepatocyte culture. J Clin Invest. 1980 May;65(5):1135–1140. doi: 10.1172/JCI109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Carpenter S. J., Healey J. F. Iron and the liver: subcellular distribution of iron and decreased microsomal cytochrome P-450 in livers of iron-loaded rats. Arch Pathol Lab Med. 1979 Jan;103(1):21–29. [PubMed] [Google Scholar]
- Bonkowsky H. L., Healey J. F., Sinclair P. R., Mayer Y. P., Erny R. Metabolism of hepatic haem and 'green pigments' in rats given 2-allyl-2-isopropylacetamide and ferric citrate. A new model for hepatic haem turnover. Biochem J. 1980 May 15;188(2):289–295. doi: 10.1042/bj1880289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Pomeroy J. S. Human hepatic delta-aminolaevulinate synthase: requirement of an exogenous system for succinyl-coenzyme A generation to demonstrate increased activity in cirrhotic and anticonvulsant-treated subjects. Clin Sci Mol Med. 1977 May;52(5):509–521. doi: 10.1042/cs0520509. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Sinclair P. R., Sinclair J. F. Hepatic heme metabolism and its control. Yale J Biol Med. 1979 Jan-Feb;52(1):13–37. [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Sparks R. G. Iron-dependent loss of liver cytochrome P-450 haem in vivo and in vitro. FEBS Lett. 1973 Jan 15;29(2):141–144. doi: 10.1016/0014-5793(73)80545-9. [DOI] [PubMed] [Google Scholar]
- Delta-Aminolevulinic acid synthase from chick embryo liver mitochondria. II. Immunochemical correlation between synthesis and activity in induction and repression. J Biol Chem. 1976 Mar 10;251(5):1347–1353. [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Hanstein W. G., Sacks P. V., Muller-Eberhard U. Properties of liver mitochondria from iron-loaded rats. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1175–1184. doi: 10.1016/0006-291x(75)90797-4. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Cytochrome P-450 as a microsomal peroxidase utilizing a lipid peroxide substrate. Arch Biochem Biophys. 1971 Nov;147(1):14–27. doi: 10.1016/0003-9861(71)90304-3. [DOI] [PubMed] [Google Scholar]
- Högberg J., Orrenius S., O'Brien P. J. Further studies on lipid-peroxide formation in isolated hepatocytes. Eur J Biochem. 1975 Nov 15;59(2):449–455. doi: 10.1111/j.1432-1033.1975.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim N. G., Hoffstein S. T., Freedman M. L. Induction of liver cell haem oxygenase in iron-overloaded rats. Biochem J. 1979 May 15;180(2):257–263. doi: 10.1042/bj1800257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner J. P., Lee G. R., Nacht S. The role of iron in the pathogenesis of porphyria cutanea tarda. An in vitro model. J Clin Invest. 1972 Dec;51(12):3044–3051. doi: 10.1172/JCI107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner J. P., Steinmuller D. P., Lee G. R. The role of iron in the pathogenesis of porphyria cutanea tarda. II. Inhibition of uroporphyrinogen decarboxylase. J Clin Invest. 1975 Sep;56(3):661–667. doi: 10.1172/JCI108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw M., Neethling A. C., Percy V. A., Carstens M., Shanley B. C. Effects of hexachlorobenzene feeding and iron overload on enzymes of haem biosynthesis and cytochrome P 450 in rat liver. Clin Sci Mol Med. 1977 Aug;53(2):111–115. doi: 10.1042/cs0530111. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D. Role of trace metals in regulation of cellular heme and hemoprotein metabolism: sensitizing effects of chronic iron treatment on acute gold toxicity. Drug Metab Rev. 1979;9(2):237–255. doi: 10.3109/03602537908993893. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Paine A. J., Legg R. F. Apparent lack of correlation between the loss of cytochrome P-450 in hepatic parenchymal cell culture and the stimulation of haem oxygenase activity. Biochem Biophys Res Commun. 1978 Mar 30;81(2):672–679. doi: 10.1016/0006-291x(78)91589-9. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Edmondson P., Stephenson B., Sarkar D., Freyholtz H. Direct spectrofluorophotometric determination of porphyrin in diluted urine. Ann Clin Res. 1976;8 (Suppl 17):156–161. [PubMed] [Google Scholar]
- Selden C., Seymour C. A., Peters T. J. Activities of some free-radical scavenging enzymes and glutathione concentrations in human and rat liver and their relationship to the pathogenesis of tissue damage in iron overload. Clin Sci (Lond) 1980 Mar;58(3):211–219. doi: 10.1042/cs0580211. [DOI] [PubMed] [Google Scholar]
- Stein J. A., Tschudy D. P., Corcoran P. L., Collins A. Delta-aminolevulinic acid synthetase. 3. Synergistic effect of chelated iron on induction. J Biol Chem. 1970 May 10;245(9):2213–2218. [PubMed] [Google Scholar]
- Taljaard J. J., Shanley B. C., Deppe W. M., Joubert S. M. Prophyrin metabolism in experimental hepatic siderosis in the rat. II. Combined effect of iron overload and hexachlorobenzene. Br J Haematol. 1972 Oct;23(4):513–519. doi: 10.1111/j.1365-2141.1972.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Waxman A. D., Collins A., Tschudy D. P. Oscillations of hepatic delta-aminolevulinic acid synthetase produced in vivo by heme. Biochem Biophys Res Commun. 1966 Sep 8;24(5):675–683. doi: 10.1016/0006-291x(66)90377-9. [DOI] [PubMed] [Google Scholar]


