Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder of motor neurons in the brain and spinal cord. Accumulation of misfolded proteins is central to the pathogenesis of ALS and the glymphatic system is emerging as a potential therapeutic target to reduce proteinopathy. Using diffusion tensor imaging analysis along the perivascular spaces (DTI-ALPS) to assess glymphatic function, we performed a longitudinal analysis of glymphatic function in ALS and compared it to a disorder in the motor neuron disease spectrum, primary lateral sclerosis (PLS).
From a cohort of 45 participants from the Calgary site in the CALSNIC study (Canadian ALS Neuroimaging Consortium), including 18 ALS, 5 PLS and 22 control participants, DTI-ALPS was analysed and correlated to clinical features (age, sex, disease presentation, disease severity and progression rate) and white matter hyperintensity burden. This included longitudinal measurements at three time points, 4 months apart.
The DTI-ALPS index was reduced in ALS participants compared with PLS and control participants across all three time points. There was no association with clinical factors; however, the index tended to decline with advancing age. Our study suggests heterogeneity in glymphatic dysfunction in motor neuron diseases that may be related to the underlying pathogenesis.
Keywords: amyotrophic lateral sclerosis, primary lateral sclerosis, DTI-ALPS, glymphatic system, longitudinal
The glymphatic system, which controls waste clearance from the brain, is an emerging therapeutic target for proteinopathies. Sharkey et al. show that individuals with amyotrophic lateral sclerosis have reduced glymphatic function compared to age-matched controls and people with primary lateral sclerosis.
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common neurodegenerative disorder of motor neurons. The lifetime risk of ALS is approximately 1 per 400 of the population.1 It has an immense personal and societal impact due to its early age of onset (median 55 years) and rapid progression from severe disability to death. The pathophysiologic mechanisms of ALS are complex and not completely elucidated; however, as with other neurodegenerative disorders, ALS is associated with the accumulation of misfolded proteins in cells of the nervous system.2
The removal of misfolded proteins and other toxic products from the central nervous system occurs mainly via the recently discovered glymphatic system, a drainage system formed by astroglial endfeet along perivascular spaces that is primarily active during slow-wave sleep.3 Recent investigations implicate glymphatic dysfunction in neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases and its role in neurodegeneration has important lifestyle and public health implications.4 Glymphatic dysfunction is also increasingly recognized in neurological conditions with diverse aetiologies.5,6
One of the major challenges in studying the human glymphatic system is the difficulty in visualizing the glymphatic flux in living tissue.7 The perivascular spaces that make up the glymphatic system are too small to be easily viewed using conventional neuroimaging methods. The diffusion tensor image analysis along perivascular spaces (DTI-ALPS) method takes advantage of conventional magnetic resonance (MR) diffusion imaging analysis methods applied to the major white matter tracts, to calculate an indirect measure of glymphatic flow based on a ratio between tensors with and without glymphatic contributions. DTI-ALPS can be applied to commonly acquired MR diffusion images.8
A recent cross-sectional study using the DTI-ALPS index by Liu et al.9 identified reduced glymphatic function in patients with early-stage ALS. Using DTI-ALPS, our study presents a longitudinal analysis (three time points: baseline, 4 and 8 months) in a mixed cohort of patients with ALS having longer disease duration and in patients with primary lateral sclerosis (PLS), a disorder that affects only upper motor neurons (whereas ALS affects both upper and lower motor neurons).10 This sample, in addition to replicating the earlier finding, provides preliminary information about its relative specificity to ALS and information about the time course, which is crucial for understanding a degenerative disease.
Materials and methods
Subjects and data collection
Data were included from the Calgary participants enrolled in the Canadian ALS Neuroimaging Consortium (CALSNIC) study.11 A total of 45 participants, including 18 ALS, 5 PLS, and 22 healthy controls, were enrolled. As part of this prospective study, participants were followed longitudinally with standardized MR imaging performed at three time points (0, 4 and 8 months). Detailed clinical information was also collected as part of this study, and in our analysis, we examined age, sex, ALS Functional Rating Scale-Revised (ALSFRS-R) score, bulbar versus limb onset, progression rate (ALSFRS-R point loss per month) and disease duration (months from symptom onset until time of recruitment to the study) as clinical variables.
Neuroimaging
CALSNIC participants underwent a standardized 1-h MR protocol at three time points (baseline, 4 months and 8 months). MR scans were acquired on a 3 T GE Discovery 750 using a 12-channel phased-array head coil. The protocol included three-dimensional T1- and T2-weighted (fluid-attenuated inversion recovery, FLAIR) anatomical, diffusion, resting-state functional MR and susceptibility-weighted imaging.11 The DTI-ALPS analysis method used the T1-weighted and diffusion imaging data. Diffusion imaging acquisition used an echo planar imaging (EPI) sequence with repetition time (TR) = 9 s, echo time (TE) = 84.5 ms, field of view (FOV) = 256 × 256 mm, reconstructed isotropic voxel size of 2 mm, 70 axial slices, 30 diffusion directions with b = 1000 s/mm2 and phase encoding direction = posterior-anterior. T1-weighted structural imaging used a fast gradient echo sequence with TR = 7.4 ms, TE = 3 ms, flip angle = 11°, inversion time TI = 400 ms, FOV = 256 × 256 mm and reconstructed isotropic voxel size of 1 mm.
FLAIR imaging was used to assess the white matter hyperintensity (WMH) burden. FLAIR sequence parameters were TR = 9000 ms, TE = 140 ms, TI = 2250 ms, FOV = 256 × 256 mm and reconstructed isotropic voxel size of 1 mm.
DTI-ALPS processing
We followed the method described by Taoka and colleagues.8 Briefly, this method extracts the DTI Tensor information for each of two regions of interest in each hemisphere and calculates the ratio of the tensors parallel to known glymphatic channels (assumed to contain glymphatic signal) and tensors perpendicular to those channels (assumed to lack glymphatic signal). See the Supplementary material for more details of region of interest placement and representative image.
White matter hyperintensity burden
FLAIR images were scored for WMH burden using the scale developed by Fazekas et al.12,13 This semiquantitative scale ranges from 0–6 and is the sum of individual scores for periventricular white matter (0–3) and deep white matter (0–3), where a higher score indicates more severe lesion burden. FLAIR mages were reviewed by two raters (R.J.S. and G.P.), scored independently, and discordant scores were resolved by joint review of images to obtain a consensus score.
Mixed effects linear model
The DTI-ALPS data were entered into a series of linear mixed-effects models fit with REML, including the effect of diagnosis, age, sex, time point and the interaction between diagnosis and time with subject as a random effect. Model details are described in the Supplementary material. Analyses were repeated to consider the association with WMH burden as scored. For the ALS/PLS cases, the relationship of DTI-ALPS index to ALSFRS-R score and progression rate was tested. These subgroup experiments are detailed in the Supplementary material along with details of the statistical packages.
Results
Participant characteristics are summarized in Table 1. The age and sex distributions were similar between groups, and the measure of disability by ALSFRS-R was similar between ALS and PLS participants. ALS participants had an average disease duration of 37.4 months at the time of their baseline scan and an average progression rate of 0.52 ± 0.37 ALSFRS-R score point loss per month.
Table 1.
Participant characteristics
| ALS participants | PLS participants | Controls | Total | |
|---|---|---|---|---|
| Number of participants (female/male) | 18 (8/10) | 5 (2/3) | 22 (12/10) | 45 (23/22) |
| Age, mean (SD) | 57.6 (11.7) | 58.8 (2.6) | 56.6 (8.8) | 57.3 (9.6) |
| Limb onset: bulbar onset (for ALS cases) | 15:3 | – | – | – |
| ALSFRS-R score, mean (SD) | 34.53 (7.5) | 34.40 (3.2) | N/A | N/A |
| Estimated progression rate (ALSFRS-R point loss per month), mean (SD) | 0.52 (0.37) | 0.25 (0.14) | N/A | N/A |
| Disease duration in months from symptom onset, mean (SD) | 37.4 (27.0) | 86.9 (79.4) | N/A | N/A |
ALS = amyotrophic lateral sclerosis; ALSFRS-R = ALS Functional Rating Scale-Revised; PLS = primary lateral sclerosis; SD = standard deviation.
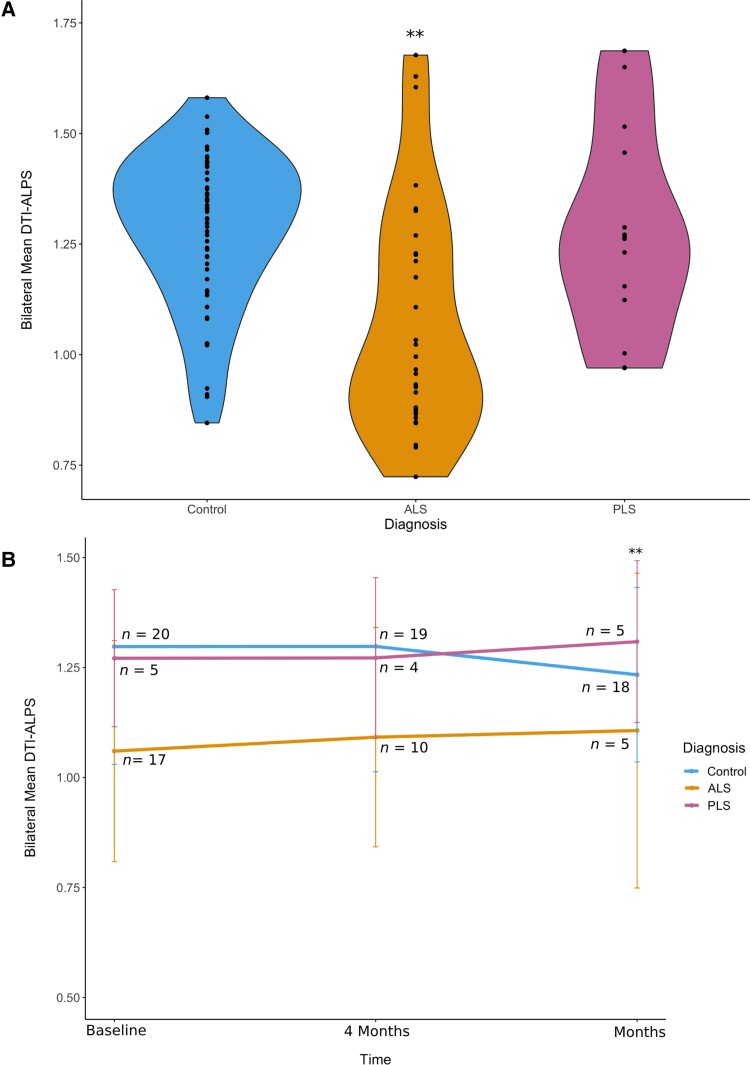
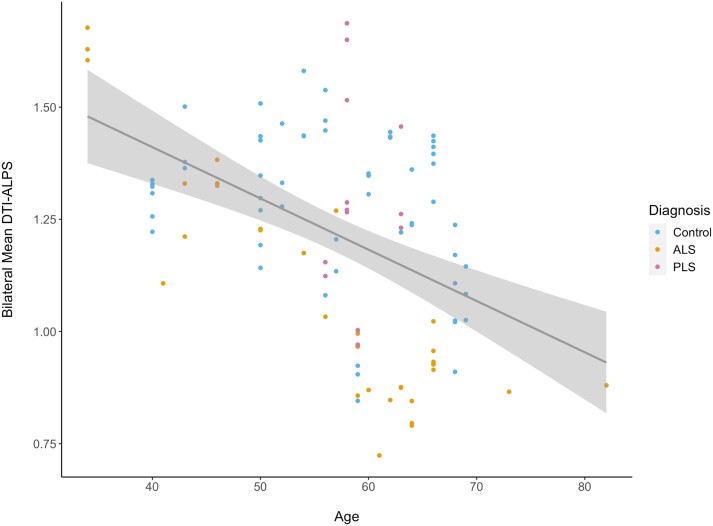
DTI-ALPS indices were significantly reduced in ALS participants in comparison to controls (P = 0.002) (Fig. 1A). This reduction was not identified amongst PLS participants; however, the sample size for this group was small (n = 5). When considering longitudinal data at the 4- and 8-month time points, the DTI-ALPS index showed a statistically significant difference in the pattern of change over time at 8 months for patients versus controls (Fig. 1B) (P = 0.05). We did not find any significant correlation with ALSFRS-R score (P = 0.4), progression rate (0.9) and sex (0.6). The DTI-ALPS index reduced significantly with the advancing age of participants (Fig. 2) (P = 0.0002).
Figure 1.
Diffusion tensor imaging analysis along the perivascular space (DTI-ALPS) index in amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS) and control participants and DTI-ALPS index over time. (A) ALS participants have significantly reduced DTI-ALPS index in comparison with both controls and PLS participants. This includes aggregated data from images at the baseline, 4-month and 8-month time points (P = 0.002). (B) Longitudinal representation of the data across the three time points (mean ± standard deviation). DTI-ALPS is reduced in ALS participants compared to PLS and control participants at all three time points. We observe a statistically significant difference in the pattern of change over time at the 8-month time point for cases versus controls (P = 0.05).
Figure 2.
Diffusion tensor imaging analysis along the perivascular space (DTI-ALPS) index in amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS) and controls according to participant age. DTI-ALPS index is shown according to participant age, with a trend toward lower values over time (P = 0.0002). The age of participants is their age at the time of their first scan in the Canadian ALS Neuroimaging Consortium (CALSNIC) study. Each scan is presented as a data-point in this plot (each participant had up to three scans in the study). A trendline shows the main effect of the overall decline in the DTI-ALPS index with age in this cohort and the confidence interval.
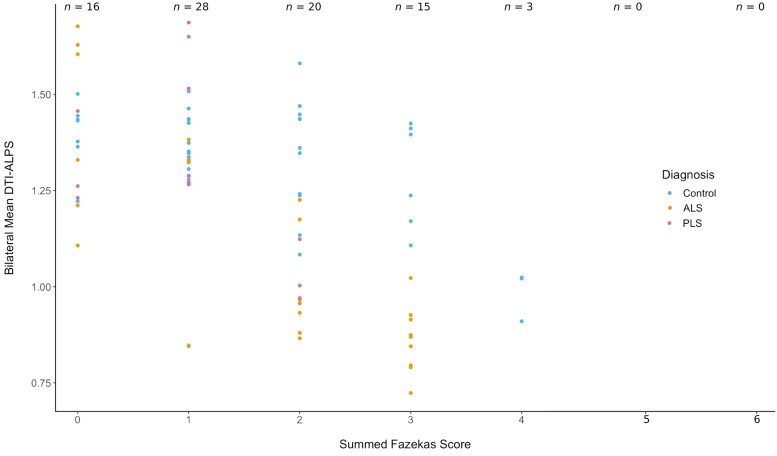
Based on the cerebral region of interest for the study, we investigated the association of lower DTI-ALPS with WMH burden. Most scans had Fazekas scores in the range of 0–2, suggesting a low burden (Fig. 3). No statistically significant associations with Fazekas scores were observed (P = 0.07), although only a single participant had a high Fazekas score (≥4).
Figure 3.
White matter hyperintensity burden and diffusion tensor imaging analysis along the perivascular space (DTI-ALPS). Using the scale developed by Fazekas et al.12,13 (0–6 points, with a higher score indicating a higher lesion burden), the majority of scans included in this study had a low lesion burden. There was no significant difference in the scores between controls and amyotrophic lateral sclerosis (ALS) participants. At a higher lesion burden (4 points and above), there are only three scans, which are all from the same participant. Because most participants in this longitudinal study had multiple scans, the number of scans exceeds the number of participants in the study; n indicates the number of scans.
Discussion
Using DTI-ALPS, we found reduced glymphatic function in ALS compared to controls and PLS participants. Our study confirms and extends the findings by Liu et al.9 This prior study included patients with early-stage ALS with average ALSFRS-R scores of 43.6 and average disease duration of only 9.0 months. In our study, disease duration was longer (average duration 37.4 months from symptom onset) and patients had more severe disability (average ALSFRS-R score 34.53; Table 1). Our study extends these findings by revealing the DTI-ALPS reductions in ALS persist as disease progresses, demonstrated by the longitudinal measurements in our participants after 4 and 8 months (Fig. 1B).
The glymphatic system is primarily active during slow-wave sleep.3 Altered glymphatic function is associated with sleep disruption in Alzheimer’s and Parkinson’s diseases4 that in many cases occurs prior to the cognitive and neuromotor symptoms.14 Whether glymphatic dysfunction is a cause, consequence or bystander in these proteinopathies remains to be determined. Sleep disruption is also recognized in ALS patients,15 although it is not clearly described as a presymptomatic feature for ALS, and its relationship to pathogenesis is unclear.16 Given that clinically apparent neurodegeneration is often restricted to the motor system and glymphatic alterations are observed throughout disease progression (Fig. 1), ALS may be a disease of choice to study glymphatic alterations in neurodegeneration and amenable to therapeutic intervention.17
The glymphatic flow depends on vascular pulsations from the cardiovascular and respiratory systems, and both systems can be impaired early in ALS by autonomic dysfunction and weakness of bulbar and respiratory musculature.18 Aquaporin-4 (AQP4), the water channel expressed in central nervous system tissues, is critical to the flow of fluid across astrocytic endfeet, and is known to be over-expressed in ALS pre-clinical models and patients.19 This observation may be compensatory or protective as reduction of AQP4 function in pre-clinical ALS animal models has been shown to cause earlier onset and death.20
In the longitudinal analyses, there is a qualitative increase in the DTI-ALPS index at the 8-month time point for ALS participants (Fig. 1B). This is probably related to drop-out of more severely affected ALS patients as the study continued. Amongst the ALS participants, 10 were able to complete the 4-month scan, and only five were able to complete the 8-month scan. This may be informative on its own and suggests that patients with the most severe disease also had the most severe glymphatic dysfunction as measured with DTI-ALPS. It raises the potential for DTI-ALPS as a possible prognostic biomarker for ALS, which will be an important question for future study. When evaluating evidence from studies of DTI-ALPS in other neurodegenerative disorders, the DTI-ALPS index declines in correlation with cognitive function in Parkinson’s disease5 and appears to be a predictive marker of the development of dementia in patients with cerebral small vessel disease.6 Further longitudinal studies with larger cohorts of ALS participants will be needed to determine whether similar associations exist in ALS.
The longitudinal analysis also showed a different pattern of change between disease participants and controls (Fig. 1B). To our knowledge, there is no current literature on normal fluctuations in glymphatic function on this timescale, so the exact interpretation of the pattern of fluctuation in the controls is unclear. Additional longitudinal studies that include healthy control populations will help to better inform future research.
PLS participants did not show the same reductions in DTI-ALPS index compared to controls, and this remained the case longitudinally. PLS participants in this study had a longer disease duration (average 86.9 months), but given the slower progression compared to ALS, studies of PLS likely require longer follow-up.
The finding that the DTI-ALPS index is similar between PLS participants and controls is intriguing; it suggests that the DTI-ALPS reduction in ALS participants is not simply the result of neurological disability (since ALS and PLS participants have similar ALSFRS-R scores in this study, 34.53 compared to 34.40; Table 1). Although ALS and PLS are both motor neuron disorders, there are clear differences in clinical presentation, disease pathogenesis, and neuropathology.21 The differing DTI-ALPS indices between these disorders may be an indication of differing risk factors, pathogenic and/or neuroprotective mechanisms. The number of PLS participants in this study was small (n = 5) and future studies with a larger sample size will consolidate or refute this idea.
Some limitations of this study include the relatively small numbers of patients and characteristics of our cohort. The cohort is composed of participants with generally slow disease progression of 0.52 points/month in the ALSFRS-R score, which is lower than the usual average ALS progression rate of 1 point/month.22 As the CALSNIC study involved longitudinal assessments, this may have favoured the recruitment of participants with relatively more stable disease. As such, this study may not be representative of the complete ALS disease spectrum and further studies should also investigate more rapidly progressing disease. Within our study, no correlation was observed between disease progression rate and DTI-ALPS index. However, it is important to note that there was attrition of participants in the ALS group over time (n = 17, 10 and 5, respectively, at the three time points) which may have affected these results, and again may have favoured participants with milder disease. To better stratify patients based on their disease severity, future study may consider staging patients using ALS staging methods such as King’s and/or Milano-Torino systems.23 Glymphatic function may also disproportionately affect specific neurologic systems in ALS; therefore, we also recommend that future studies with larger sample sizes should consider analyses of cognitive function as well as measures of upper motor neuron dysfunction. Similarly, given the relationship of glymphatic function to vascular pulsations from the cardiovascular and respiratory systems, any correlation of DTI-ALPS index to autonomic, cardiac, or respiratory dysfunction should be considered in the future.
Another possible study limitation is the use of DTI-ALPS as a measure of glymphatic function. DTI-ALPS is an emerging technique that has shown significant promise in ALS and other disorders. There may, however, be advantages to contrast-based imaging approaches that provide more regional specificity.24 In contrast, DTI-ALPS provides a single measure of glymphatic function in each hemisphere. While reductions in the DTI-ALPS index have been reproducibly linked to multiple conditions associated with glymphatic impairment, it is an indirect measure; true confirmation of direct glymphatic contributions is difficult.25
The DTI-ALPS index has also been correlated with several other imaging markers of vascular and perivascular health. Extracellular free water analysis, another diffusion measure, provides information about interstitial fluid accumulation, which relates to glymphatic drainage.25,26 Vessel pulsatility analysis measures arterial pulsation, which is one of the main drivers of glymphatic flow.27 Future imaging studies combining these measures with DTI-ALPS might provide additional physiological clarity. For robustness, future glymphatic imaging studies of ALS participants would benefit from inclusion of additional, more invasive, methods to confirm these findings, which may also include studies of CSF tracer clearance28 or biomarkers from plasma or CSF.29
Because the regions used for the DTI-ALPS analysis may contain WMH, we included an analysis of WMH burden to examine for correlation between reduced DTI-ALPS and white matter abnormalities, which has been identified in earlier studies25 (Fig. 3). We did not identify any association between WMH burden and DTI-ALPS scores. However, it should be emphasized that most participants had low lesion burden (Fig. 3), and therefore, this sample was poorly suited to identify the impact of WMHs in this population. Future studies should consider any impact of WMHs or other structural changes associated with DTI-ALPS in ALS. Our method for quantifying the WMH burden is semi-quantitative, whereas more quantitative approaches may have greater reproducibility.30
In summary, this study demonstrates the finding of reduced glymphatic function in ALS participants compared to controls in a more advanced cohort and with longitudinal scans across an up to 8-month time period. The lack of a similar association in PLS participants is of great interest and requires further study. The association of glymphatic alteration with ALS is crucially important for future study. This would help inform the disease pathogenesis and identify targets for future therapies. There is also biomarker potential for diagnosis (in cases with UMN presentation to potentially discriminate PLS from ALS) or for prognosis (given the possibility that ALS patients with lower DTI-ALPS were lost to follow-up, suggesting more severe disease). Moreover, because easily implemented health interventions (e.g. improved sleep hygiene) can modify this system, therapeutic development targeting glymphatic function offers translational benefit to patients.
Supplementary Material
Contributor Information
Rachel J Sharkey, Department of Radiology, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB T2N 4N1, Canada; Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Filomeno Cortese, Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB T2N 4N1, Canada; Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Bradley G Goodyear, Department of Radiology, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB T2N 4N1, Canada; Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Lawrence W Korngut, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Sarah M Jacob, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Keith A Sharkey, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Physiology and Pharmacology, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Sanjay Kalra, Division of Neurology, Department of Medicine, University of Alberta, Edmonton, AB T6G 2G3, Canada; Neuroscience and Mental Health Institute, University of Alberta, Edmonton, AB T6G 2G3, Canada.
Minh Dang Nguyen, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Cell Biology and Anatomy, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Biochemistry and Molecular Biology, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Richard Frayne, Department of Radiology, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB T2N 4N1, Canada; Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Gerald Pfeffer, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada; Department of Medical Genetics, Cumming School of Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Data availability
The original data used to perform this study are available to qualified investigators via the CALSNIC study (www.calsnic.org/contact) following the execution of appropriate data transfer agreements.
Funding
This work was supported by grants from the ALS Society of Canada (M.D.N., G.P.), the Barry Barrett Foundation (M.D.N., G.P.), the Rose Family Foundation (M.D.N., G.P.), the Hotchkiss Brain Institute (HBI) (R.F.) and the Canadian Institutes of Health Research (CIHR) (K.A.S.). CALSNIC is funded by the Canadian Institute of Health Research, ALS Society of Canada and Brain Canada Foundation. R.J.S. was an Eyes High Scholar at the University of Calgary and is supported by a fellowship from the CIHR-funded Vascular Training (VAST) program. R.F. is the HBI Hopewell Professor of Brain Imaging. S.K. is the Henri M. Toupin Chair in Neurological Sciences.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Alonso A, Logroscino G, Jick SS, Hernán MA. Incidence and lifetime risk of motor neuron disease in the United Kingdom: A population-based study. Eur J Neurol. 2009;16:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parakh S, Atkin JD. Protein folding alterations in amyotrophic lateral sclerosis. Brain Res. 2016;1648(Pt B):633–649. [DOI] [PubMed] [Google Scholar]
- 3. Reddy OC, van der Werf YD. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020;10:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buccellato FR, D'Anca M, Serpente M, Arighi A, Galimberti D. The role of glymphatic system in Alzheimer's and Parkinson's disease pathogenesis. Biomedicines. 2022;10:2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Si X, Guo T, Wang Z, et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson's disease. NPJ Parkinsons Dis. 2022;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong H, Tozer DJ, Markus HS. Relationship of perivascular space markers with incident dementia in cerebral small vessel disease. Stroke. 2024;55:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naganawa S, Taoka T. The glymphatic system: A review of the challenges in visualizing its structure and function with MR imaging. Magn Reson Med Sci. 2022;21:182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35:172–178. [DOI] [PubMed] [Google Scholar]
- 9. Liu S, Sun X, Ren Q, et al. Glymphatic dysfunction in patients with early-stage amyotrophic lateral sclerosis. Brain. 2024;147:100–108. [DOI] [PubMed] [Google Scholar]
- 10. Yedavalli VS, Patil A, Shah P. Amyotrophic lateral sclerosis and its mimics/variants: A comprehensive review. J Clin Imaging Sci. 2018;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalra S, Khan M, Barlow L, et al. The Canadian ALS Neuroimaging Consortium (CALSNIC)—a multicentre platform for standardized imaging and clinical studies in ALS. medRxiv. [Preprint] 10.1101/2020.07.10.20142679 [DOI]
- 12. Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 13. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 14. Lajoie AC, Lafontaine AL, Kaminska M. The spectrum of sleep disorders in Parkinson disease: A review. Chest. 2021;159:818–827. [DOI] [PubMed] [Google Scholar]
- 15. Boentert M. Sleep and sleep disruption in amyotrophic lateral sclerosis. Curr Neurol Neurosci Rep. 2020;20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucia D, McCombe PA, Henderson RD, Ngo ST. Disorders of sleep and wakefulness in amyotrophic lateral sclerosis (ALS): A systematic review. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22:161–169. [DOI] [PubMed] [Google Scholar]
- 17. Eisen A, Nedergaard M, Gray E, Kiernan MC. The glymphatic system and amyotrophic lateral sclerosis. Prog Neurobiol. 2024;234:102571. [DOI] [PubMed] [Google Scholar]
- 18. Oprisan AL, Popescu BO. Dysautonomia in amyotrophic lateral sclerosis. Int J Mol Sci. 2023;24:14927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou S, Lan YL, Wang H, Zhang B, Sun YG. The potential roles of aquaporin 4 in amyotrophic lateral sclerosis. Neurol Sci. 2019;40:1541–1549. [DOI] [PubMed] [Google Scholar]
- 20. Hirose M, Asano M, Watanabe-Matsumoto S, et al. Stagnation of glymphatic interstitial fluid flow and delay in waste clearance in the SOD1-G93A mouse model of ALS. Neurosci Res. 2021;171:74–82. [DOI] [PubMed] [Google Scholar]
- 21. Marzoughi S, Pfeffer G, Cashman N. Primary lateral sclerosis. Handb Clin Neurol. 2023;196:89–99. [DOI] [PubMed] [Google Scholar]
- 22. Atassi N, Berry J, Shui A, et al. The PRO-ACT database: Design, initial analyses, and predictive features. Neurology. 2014;83:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang T, Al Khleifat A, Stahl DR, et al. Comparison of the king's and MiToS staging systems for ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siow TY, Toh CH, Hsu JL, et al. Author response: Association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology. 2023;100:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taoka T, Ito R, Nakamichi R, Nakane T, Kawai H, Naganawa S. Diffusion Tensor Image Analysis ALong the Perivascular Space (DTI-ALPS): Revisiting the meaning and significance of the method. Magn Reson Med Sci. 2024;23:268–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andica C, Kamagata K, Takabayashi K, et al. Neuroimaging findings related to glymphatic system alterations in older adults with metabolic syndrome. Neurobiol Dis. 2023;177:105990. [DOI] [PubMed] [Google Scholar]
- 27. Xie L, Zhang Y, Hong H, et al. Higher intracranial arterial pulsatility is associated with presumed imaging markers of the glymphatic system: An explorative study. Neuroimage. 2024;288:120524. [DOI] [PubMed] [Google Scholar]
- 28. Eide PK, Vinje V, Pripp AH, Mardal KA, Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain. 2021;144:863–874. [DOI] [PubMed] [Google Scholar]
- 29. Eide PK, Lashkarivand A, Pripp A, et al. Plasma neurodegeneration biomarker concentrations associate with glymphatic and meningeal lymphatic measures in neurological disorders. Nat Commun. 2023;14:2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright DK, Symons GF, O’Brien WT, et al. Diffusion imaging reveals sex differences in the white matter following sports-related concussion. Cereb Cortex. 2021;31:4411–4419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data used to perform this study are available to qualified investigators via the CALSNIC study (www.calsnic.org/contact) following the execution of appropriate data transfer agreements.