Abstract
Congenital hydrocephalus, characterized by cerebral ventriculomegaly, is one of the most common reasons for paediatric brain surgery. Recent studies have implicated lin-41 (lineage variant 41)/TRIM71 (tripartite motif 71) as a candidate congenital hydrocephalus risk gene; however, TRIM71 variants have not been systematically examined in a large patient cohort or conclusively linked with an OMIM syndrome.
Through cross-sectional analysis of the largest assembled cohort of patients with cerebral ventriculomegaly, including neurosurgically-treated congenital hydrocephalus (totalling 2697 parent-proband trios and 8091 total exomes), we identified 13 protein-altering de novo variants (DNVs) in TRIM71 in unrelated children exhibiting variable ventriculomegaly, congenital hydrocephalus, developmental delay, dysmorphic features and other structural brain defects, including corpus callosum dysgenesis and white matter hypoplasia.
Eight unrelated patients were found to harbour arginine variants, including two recurrent missense DNVs, at homologous positions in RPXGV motifs of different NHL domains. Seven patients with rare, damaging, unphased or transmitted variants of uncertain significance were also identified. NHL-domain variants of TRIM71 exhibited impaired binding to the canonical TRIM71 target CDKN1A; other variants failed to direct the subcellular localization of TRIM71 to processing bodies. Single-cell transcriptomic analysis of human embryos revealed expression of TRIM71 in early first-trimester neural stem cells of the brain.
These data show TRIM71 is essential for human brain morphogenesis and that TRIM71 mutations cause a novel neurodevelopmental syndrome that we term ‘TRIM71-associated developmental disorders (TADD)’, featuring variable ventriculomegaly, congenital hydrocephalus and other structural brain defects.
Keywords: hydrocephalus, TRIM71, de novo variants, neural stem cells, brain development, structural brain disorders
Duy et al. provide evidence that mutations in TRIM71—a known regulator of stem cell fate and candidate congenital hydrocephalus risk gene—cause a novel form of syndromic congenital hydrocephalus with brain abnormalities and developmental delay.
Introduction
Hydrocephalus is characterized by expansion of the cerebral ventricles (ventriculomegaly) and is the most common reason for brain surgery in children.1 Hydrocephalus has been defined as the active, progressive distension of the cerebral ventricular system resulting from inadequate transport of CSF from its point of production to its point of absorption.2 This mechanism is evident in secondary (acquired) hydrocephalus associated with brain tumours, infection or haemorrhage, in which intracranial pressure is often elevated.3 However, when infantile hydrocephalus occurs without a known antecedent, it is classified as primary, developmental or congenital hydrocephalus (CH).1 CH can occur in the absence of obstruction to CSF flow (communicating hydrocephalus) or with complete/partial intraventricular obstruction (non-communicating hydrocephalus), most often due to aqueductal stenosis. Cases of communicating CH with normal or low intracranial pressure1,4 raise the question of whether the development of ventriculomegaly is a primary or a secondary passive process.5 Subsets of CH patients have cerebral ventriculomegaly and neurodevelopmental disorders (e.g. autism or epilepsy) that do not improve after neurosurgical CSF diversion.6-8 These findings implicate genetically encoded disruptions of brain development in CH pathogenesis.9
Genetic studies, including whole-exome sequencing (WES), of a few small CH cohorts have identified multiple candidate CH risk genes requiring further functional validation.9-21 Of utmost interest is the Tripartite motif 71 (Trim71)/lineage defective 41 (lin-41) (TRIM71), a target of the let-7 (lethal 7) miRNA in the heterochronic pathway in Caenorhabditis elegans that regulates stem cell fate.22 TRIM71 includes an N-terminal RING finger domain followed by two B-boxes, a coiled-coil domain and a C-terminal NHL domain. TRIM71 has both E3 ubiquitin ligase activity mediated by the N-terminal tripartite motif,23,24 and RNA-binding capability with transcriptional repression mediated by the C-terminal NHL domain.24,25 In mice, Trim71 is highly expressed during early embryonic development in stem cells of somatic, neural and germline lineage.26 Conditional deletion of Trim71 in mouse neural stem cells causes prenatal ventriculomegaly and cortical hypoplasia by promoting precocious neuroepithelial stem cell differentiation, thereby depleting available precursors for corticogenesis.11 Nonetheless, TRIM71 variants have not been systematically examined in a large CH cohort or conclusively linked with an OMIM disease. In consequence, the role of TRIM71 in human brain development and the phenotypic spectrum associated with TRIM71 variants remain poorly understood.
Here, we analysed the largest-ever compiled cohort of cerebral ventriculomegaly cases including those with neurosurgically-treated CH, comprising 8091 total exomes (2697 parent-proband trios). Our analysis identified 13 missense de novo variants (DNVs) in TRIM71, including three recurrent DNVs, in unrelated children with CH, developmental delay, dysmorphic features and other structural brain defects. Two recurrent DNVs in six patients defined TRIM71’s RNA-binding NHL domains as mutational hotspots. Experiments in cell lines showed NHL-domain variants of TRIM71 abrogated TRIM71 binding to its canonical targets, CDKN1A and EGR. Other TRIM71 variants altered the native subcellular localization of TRIM71 to processing bodies (P-bodies), potential sites of mRNA degradation. Our analyses of the human brain transcriptome revealed high TRIM71 expression in embryonic neural stem cells lining the developing neural tube during the first trimester of development. These results show recurrent missense DNVs in the RNA-binding NHL domain of TRIM71 cause a novel form of syndromic CH that we term ‘TRIM71-associated developmental disorders’. The data establish that TRIM71 is required for human brain morphogenesis, provide further evidence for a ‘neural stem cell’ paradigm of human CH pathogenesis, and suggest exome sequencing as a valuable adjunct in the management of CH patients.
Materials and methods
Patient cohort
All study procedures and protocols were guided by and in compliance with ‘Human Investigation Committee and Human Research Protection Program at Yale University and Massachusetts General Hospital (MGH). All participants provided written, informed consent to participate in accordance with the Declaration of Helsinki. For patients from the clinical laboratory GeneDx, denoted CHYDX, written informed consent for genetic testing was obtained from the guardians of all paediatric individuals undergoing testing. The Western Institutional Review Board waived authorization for the use of de-identified aggregate data for the purposes of this study. Criteria for inclusion in the study were congenital or primary cerebral ventriculomegaly, including those cases treated by neurosurgical CSF diversion. Patients and participating family members provided buccal swab samples (Isohelix SK-2S DNA buccal swab kits), medical records, neuroimaging studies, operative reports and phenotype data when available. Human phenotype ontology terms were used to aggregate relevant paediatric patients in the GeneDx database. The comparison control cohort consisted of 1798 unaffected siblings of people diagnosed with autism spectrum disorder and unaffected parents sourced from the Simons simplex consortium (SSC).27 Only unaffected siblings and parents (as designated by SSC) were included in the analysis and served as controls for this study. Permission was obtained to access the genomic data in the SSC on the National Institute of Mental Health Data Repository. Written and informed consent for all participants was provided by the Simons Foundation Autism Research Initiative.
Kinship analysis
Pedigree information and relationships between proband and parents were confirmed using pairwise PLINK identity-by-descent (IBD) calculation.28 The IBD sharing between the probands and parents in all trios was between 45% and 55%. Pairwise individual relatedness was calculated using KING.29 The ethnicity of each patient from the Yale and MGH cohorts was determined by single-nucleotide polymorphisms in cases, controls, and HapMap samples using EIGENSTRAT, as previously described.30 For the GeneDx cohort, kinship analysis was performed using an internally developed K-nearest neighbours/principal component analysis (KNN/PCA) pipeline.
Whole exome sequencing and variant calling
Patient genomic DNA samples derived from saliva or blood were subjected to exon capture using Roche SeqCap EZ MedExome Target Enrichment kit or IDT xGen target capture followed by 101 or 148 base-paired-end sequencing on Illumina platforms as described previously.9,10 Sequence reads were aligned to the human reference genome GRCh37/hg19 using Burrows–Wheeler Aligner-Maximal Exact Match (BWA-MEM). Single-nucleotide variants and small indels were called using a combination of the Genome Analysis Toolkit (GATK) HaplotypeCaller and Freebayes31,32 and annotated using ANNOVAR.33 Allele frequencies were annotated in the Exome Aggregation Consortium, gnomAD (v.2.1.1) and Bravo databases.34 Variant filtration and analysis were conducted following GATK best practices and consensus workflows.35 Meta-analytic support vector machine (MetaSVM) and Missense badness, PolyPhen-2 and Constraint (MPC) algorithms were used to predict the deleteriousness of missense variants (D-Mis, defined as MetaSVM-deleterious or MPC-score ≥2).36 Inferred loss-of-function (LoF) variants consisted of stop-gain, stop-loss, frameshift insertions/deletions, canonical splice site and start-loss. LoF and D-Mis variants were considered ‘damaging’. Analyses were conducted separately for each class of variant—DNVs and rare, heterozygous dominant variants—following previously established analytical methodologies.10,35 Firstly, DNVs from the Yale cohort were called from all CH parent-offspring trios using the established TrioDeNovo pipeline.37,38 GeneDx DNVs were called as previously defined.39 Candidate DNVs for all samples were further filtered based on whether the variants were called in the exonic or splice-site regions, the variant read depth (DP) was at least 10 in the proband as well as in both parents, and the global minor allele frequency was ≤4 × 10−4 in the Exome Aggregation Consortium database. Samples from the Yale cohort were subsequently filtered based on the following criteria: (i) proband’s alternate read depth ≥5; (ii) proband alternate allele ratio ≥28% for <10 alternate reads or ≥20% for ≥10 alternative reads; and (iii) alternate allele ratio in both parents ≤3.5%. Samples from the GeneDx cohort were additionally filtered based on the following criteria: (i) genotype quality (GQ) >40 for all family members; (ii) variant quality score log odds (VQSLOD) > −10; (iii) Phred-scaled P-value (Fisher's exact test) <30; (iv) proband alternate allele count >4; (v) proband alternate allele ratio >0.1; (vi) proband alternate allele ratio >0.15 if REF and ALT calls are of equal length; (vii) proband alternate allele ratio >0.25 if REF and ALT calls are of unequal length; (viii) proband alternate allele ratio <0.9 in proband if DNV is autosomal; (ix) DNV must be <100 bps in size for both the REF and ALT calls; (x) if the VQSLOD <7 and the alternate allele ratio in the proband <0.3, the variant was omitted; and (xi) DNVs were omitted if present in >2 unrelated probands. After filtering as above, in silico visualization was performed, applying in-house software to inspect each variant manually for false-positive calls. Variants found false-positive upon manual inspection were removed. TRIM71 variant annotations were then confirmed through manual cross-reference in the UCSC Genome Browser.33,40 Reported variants passing these filters and manual inspection in TRIM71 variants were further confirmed by Sanger sequencing.
De novo enrichment analysis
Enrichment for DNVs was performed using DenovolyzeR v.0.2.0.41 The expected number of DNVs in the case and control cohorts across each functional class was calculated by taking the sum of each functional class-specific probability multiplied by the number of probands in the study × 2 (diploid genomes). Then, the expected number of DNVs across functional classes was compared to the observed number in each study using a Poisson test. To examine whether any individual gene contains more protein-altering DNVs than expected, the expected number of protein-altering DNVs was calculated from the corresponding probability, adjusting for cohort size. The Poisson test was then used to compare the observed de novo mutations for each gene versus expected. As separate tests were performed for protein-altering, protein-damaging and LoF DNVs, the relevant Bonferroni multiple-testing threshold is therefore equal to α = 8.61 × 10−7 = [0.05 / (3 tests × 19 347 genes)].
Single-cell RNA-sequencing analysis
The preprocessing and clustering of the single cell dataset of early brain development has been previously described.42 Briefly, data from all samples were integrated using the standard Scanpy pipeline43. Cells expressing between 800 and 6000 genes and with <20% expression of mitochondrial genes were kept for downstream analysis, while genes expressed in <20 cells were removed. The filtered gene expression matrix was normalized and log-transformed. Highly variable genes were used for principal component analysis and nearest neighbour graphs were used for clustering using the Louvain algorithm. Raw data was downloaded from Gene Expression Omnibus and matched to cell metadata using Seurat.44 Counts were log-normalized using the NormalizeData command. Average expression of genes was calculated using the AverageExpression command. Differential expression was calculated using the Wilcoxon rank sum test by running FindAllMarkers.
In silico biophysical modelling
The mutations in E3 ubiquitin-protein ligase TRIM71 were modelled based on the structure described previously by Furey et al.9 To describe the C15G mutant, the model was downloaded from the alphafold protein structure database (entry Q2Q1W2). The free energy of change was calculated (ΔΔG) in silico using the ICM mutagenesis program45 (www.molsoft.com). We were unable to account for Q334R, K403N and R649Q due to the lack of structural resolution in our models.
Cell culture
HEK293T cells were cultured in DMEM supplemented with 10% fetal calf serum and 1% penicillin-streptavidin solution (all PAN Biotech). Cells were grown at 37°C in 5% CO2 at 95% relative humidity.
Cloning of TRIM71 point mutants via site-directed mutagenesis
Specific missense variants were introduced into prk5-Flag-TRIM71 and pN1-RFP-TRIM71 plasmids46 using completely self-complementary forward/reverse primers annealing with the region of interest. Primers (∼35 bp) contained the desired mutation in mid-sequence. Rolling-circle amplification of the whole plasmid using 2.5 U Pfu DNA polymerase (Agilent), 5 µl Pfu Ultra buffer (10×), 1 µl dNTPs (10 mM), 2 µl forward/reverse primers (10 µM), 2.5 µl DMSO and 100 ng plasmid DNA in a 50 µl PCR reaction generated amplified plasmid contained the desired mutation. Parental template plasmid was digested by adding to the PCR reaction 1 μl of DpnI (10 U/μl) restriction enzyme (competent to digest methylated DNA). Digested PCR product was directly transformed into bacteria, plasmid was isolated with the Xtra Maxi Kit (Macherey Nagel) and sequenced before use.
UV-crosslink RNA immunoprecipitation
Experiments were performed as previously described.11 Briefly, HEK293T cells were transfected with either prk5-Flag-TRIM71 [wild-type (WT), ΔNHL6, p.Asn701Lys, p.Arg655Gln, p.Asp624Asn, p.Arg608Cys, p.Lys403Asn, p.Gln334Arg, p.Gln228Ter, p.Cys15Gly] or prk5-Flag overexpression plasmids. Forty-eight hours after transfection, cells were washed with ice-cold PBS and irradiated with UV light at 300 mJ/cm2. Cells were harvested and lysed in RNA-immunoprecipitation (IP) buffer containing 120 U/ml of RNase inhibitor (RiboLock, Thermo Fisher Scientific). Twenty micrograms of total protein lysate were retained as input for immunoblotting, and 50 μl was retained for RNA input. One milligram of protein lysate was incubated with 10 μl of anti-FLAG M2 Magnetic Beads (Sigma-Aldrich) for 4 h at 4°C on a rotating wheel. After five washing steps with RNA-IP buffer, beads were divided for immunoblotting (20%) and quantitative real-time (qRT)-PCR (80%). Beads for qRT-PCR were incubated with Proteinase K (Peqlab) for 30 min at 37°C before RNA extraction. Immunoblotting, RNA extraction, qRT–PCR and calculation of specific target gene enrichment were performed as described by Duy et al.11
Cell imaging by confocal laser scanning microscopy
For P-body co-localization studies, HEK-293T cells were co-transfected with the P-body-specific marker DCP1A (pN1-GFP-DCP1A) and the desired pN1-RFP-TRIM71 construct in a 1:1 ratio and seeded on poly-L-lysine-coated coverslips 24 h post-transfection (hpt). At 48 hpt, cells were washed once with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature (RT). After three PBS washing steps, nuclear staining with DAPI was performed for 1 h at RT in a dark humid chamber. Cells were then washed three more times with PBS, and coverslips mounted on glass slides with FluoroShield mounting media supplemented with 50 mg/ml Dabco. Slides were imaged using an Olympus LSM FV-1000 microscope in confocal mode with a 60× oil immersion objective and laser wavelengths of 405, 488 and 594 nm for respective excitation of DAPI, GFP and RFP. At least 50 double-transfected (both GFP- and RFP-positive) cells per condition were observed for GFP-RFP co-localization, and the percentage of HEK-293T cells in which the given TRIM71 construct clearly localized to P-bodies was calculated, even though a diffuse cytoplasmic RFP background was detectable.
Primer sequences
Primers sequences (IDT) are provided in Supplementary Table 1.
Antibodies
The following antibodies were used: anti-FLAG M2 (mouse, F1804, Sigma-Aldrich), anti-GAPDH (mouse, ACR001P, Acris), anti-mouse IgG HRP-linked (Cell Signaling Technologies); TaqMan probe (Thermo Fisher Scientific):CDKN1A (Hs00355782_m1), EGR1 (Hs00152928_m1).
Results
TRIM71 de novo variants are highly enriched in patients with congenital hydrocephalus
The sequenced cohort consisted of 8091 exomes from an expanded cohort of 2697 proband-parent trios with cerebral ventriculomegaly, including cases of CH treated with neurosurgical CSF diversion (see the ‘Materials and methods’ section). The control cohort consisted of 1798 exomes from unaffected siblings of patients diagnosed with autism spectrum disorder and their unaffected parents sourced from the Simons simplex consortium.9,47 Genomic DNAs were subjected to WES, and variant calling was performed with GATK HaplotypeCaller and Freebayes followed by ANNOVAR annotation and confirmation by the Integrative Genomics Viewer.10,33,40 Variants were considered damaging if LoF variants or if predicted by MetaSVM as ‘deleterious’ and/or if MPC score ≥2 (see the ‘Materials and methods’ section). All reported variants were confirmed by Sanger sequencing.
We compared observed and expected numbers of non-synonymous DNVs in all genes in cases and controls using the DenovolyzeR algorithm41 (see the ‘Materials and methods’ section). TRIM71 surpassed genome-wide significance thresholds for DNV burden (multiple-testing correction threshold of 8.61 × 10−7 after correction for testing 19 347 RefSeq genes in triplicate using a one-tailed Poisson test (Fig. 1A). TRIM71 harboured a total of 13 missense or LoF DNVs in unrelated children, including two recurrent DNVs p.Arg608His and p.Arg796His (each in three patients),10 as well as novel DNVs p.Arg649Gln, p.Arg655Gln, p.Arg655*, c.1020+1G>C, p.Gly79Alafs*41 and p.Gln334Arg (Table 1). The probability of encountering 13 missense or LoF DNVs in TRIM71 in a cohort of this size is 4.47 × 10−19, an exceedingly unlikely chance event. No TRIM71 DNVs were observed in control trios. These data show TRIM71 DNVs are highly enriched in patients with cerebral ventriculomegaly, including those treated with neurosurgical CSF diversion.
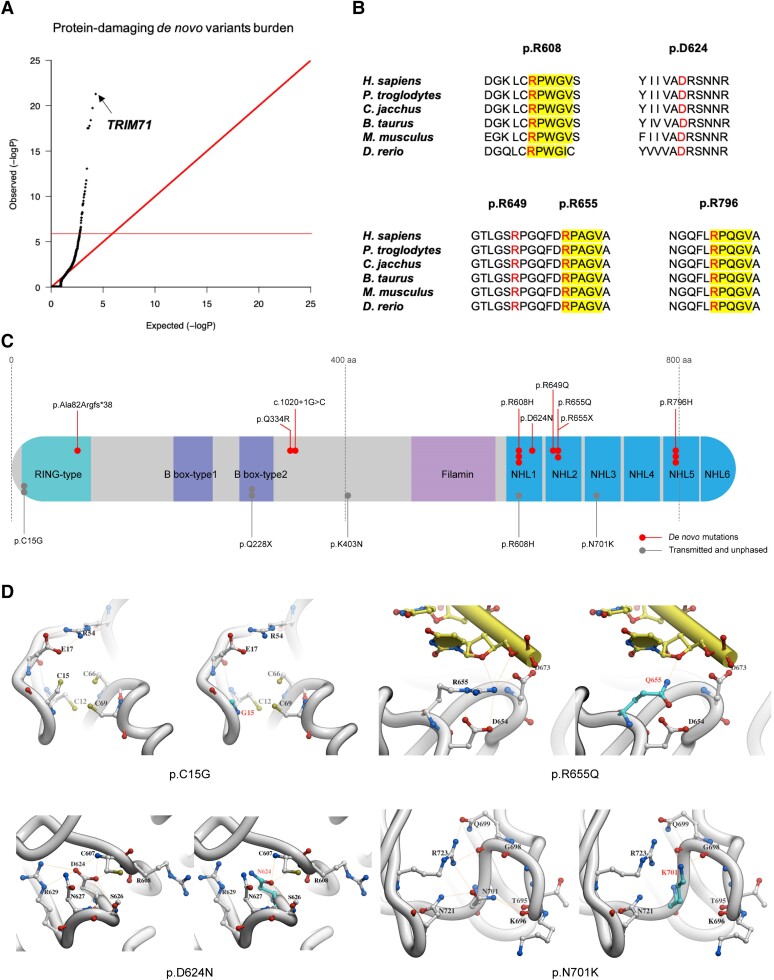
Figure 1.
De novo and transmitted variants in TRIM71 are highly enriched in patients with congenital hydrocephalus. (A) Quantile–quantile (Q-Q) plot of observed versus expected P-values for de novo mutations (DNMs) in each gene in 2697 trio cases. P-values were calculated by one-sided Poisson test (see the ‘Materials and methods’ section). For protein-damaging TRIM71 de novo variants (DNVs) [loss-of-function (LoF), meta-analytic support vector machine (MetaSVM) = deleterious and/or Missense badness, PolyPhen-2 and Constraint (MPC) > 2, P = 5.07 × 10−22]. (B) Damaging DNVs in TRIM71 mapped to highly conserved protein domains across different species. Locations of variants (p.Arg608His, p.Asp624Asn, p.Arg649Gln, p.Arg655Gln, p.Arg796His) are indicated in red. RPXGV motifs are highlighted in yellow. (C) Schematic diagram of variant locations in TRIM71 protein domains. Each filled circle represents a patient expressing a variant. DNVs are indicated with red circles. Transmitted and unphased variants are indicated with grey circles. (D) In silico modelling predicts modified protein structure of missense variants in TRIM71 (p.C15G, p.D624N p.R655Q and p.N701K), suggesting impaired function.
Table 1.
All de novo, transmitted and unphased TRIM71 variants
| Proband ID | Sex | Ethnicity | Inheritance | cDNA change | AA change | ACMG classification | Classification criteria | gnomAD frequency | Bravo frequency | MetaSVM | MPC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KCHYD154-1a | F | Caucasian | De novo | c.1823G>A | p.(Arg608His) | Likely pathogenic | PM6, PM2, PP5, PS4 | – | – | T | 2.33 |
| KCHYD79-1a | M | Mixed (half Japanese, half Caucasian) | De novo | c.1823G>A | p.(Arg608His) | Likely pathogenic | PM6, PM2, PP5, PS4 | – | – | T | 2.33 |
| 18CY000656a | F | Moroccan | De novo | c.1823G>A | p.(Arg608His) | Likely pathogenic | PM6, PM2, PP5, PS4 | – | – | T | 2.33 |
| KCHYD85-1a | M | Caucasian | De novo | c.2387G>A | p.(Arg796His) | Likely pathogenic | PM6, PM2, PP5, PS4 | – | – | T | 2.34 |
| KCHYD419-1a | F | Caucasian | De novo | c.2387G>A | p.(Arg796His) | Likely pathogenic | PM6, PM2, PP5, PS4 | – | – | T | 2.34 |
| KCHYD425-1a | F | Caucasian | De novo | c.2387G>A | p.(Arg796His) | Likely pathogenic | PM6, PM2, PP5, PS4 | – | – | T | 2.34 |
| KCHYD670-1a | M | Caucasian | De novo | c.1870G>A | p.(Asp624Asn) | Likely pathogenic | PM6, PM2, PP3 | – | – | D | 2.1 |
| KCHYD669-1 | M | Caucasian | De novo | c.1964G>A | p.(Arg655Gln) | VUS | PM6, PM2 | – | – | T | 2.26 |
| KCHYD548-1 | F | Caucasian | De novo | c.1020+1G>C | NA | Likely pathogenic | PM6, PM2, PVS1 | – | – | – | – |
| KCHYD575-1 | M | Mixed (half Hawaiian, half Caucasian) | De novo | c.1946G>A | p.(Arg649Gln) | VUS | PM6, PM2 | 0.0002 | 0.0002 | T | 2.37 |
| 3 413 261 | F | Caucasian | De novo | c.1963C>T | p.(Arg655*) | Likely pathogenic | PM6, PSV1, PM2 | – | – | . | . |
| KCHYD678-1 | F | Caucasian | De novo | c.1001A>G | p.(Gln334Arg) | VUS | PM6, PM2 | – | – | T | 1.07 |
| KCHYD679-1 | M | Caucasian | De novo | c.236_242dup | p.(Ala82Argfs*38) | Pathogenic | PM6, PM2, PVS1 | – | – | . | . |
| KCHYD154-5a | M | Caucasian | Transmitted (from mother KCHYD154-1) | c.1823G>A | p.(Arg608His) | Likely pathogenic | PM2, PP5, PS4 | – | – | T | 2.33 |
| KCHYD674-1 | F | Caucasian | Transmitted (from father) | c.43T>G | p.(Cys15Gly) | Likely Pathogenic | PM2, PS4 | – | – | T | 1.17 |
| KCHYD674-7 | F | Caucasian | Transmitted (from mother) | c.43T>G | p.(Cys15Gly) | Likely Pathogenic | PM2, PS4 | – | – | T | 1.17 |
| KCHYD675-1 | M | Haitian | Unknown | c.1209G>C | p.(Lys403Asn) | Vus | PM2 | – | – | T | 1.14 |
| KCHYD677-1 | M | Moroccan | Unphased | c.682C>T | p.(Gln288*) | Likely pathogenic | PM2, PVS1 | – | – | – | – |
| KCHYD677-2 | M | Moroccan | Unphased | c.682C>T | p.(Gln288*) | Likely pathogenic | PM2, PVS1 | – | – | – | – |
| KCHYD250-1a | F | African | Unphased | c.2103C>G | p.(Asn701Lys) | VUS | PM2 | – | – | T | 2.01 |
Changes in cDNA and protein sequence were respectively annotated per NM_001039111.3 and NP_001034200.1. AA = amino acid; ACMG = American College of Medical Genetics; F = female; M = male; MetaSVM = meta-analytic support vector machine; MPC = Missense badness, PolyPhen-2 and Constraint; VUS = variant of uncertain significance.
RNA-binding NHL domains are TRIM71 mutational hotspots
TRIM71 DNVs impacted highly conserved residues in key functional domains of the molecule. TRIM71 comprises an N-terminal RING finger domain followed by two B-boxes, a coiled-coil domain, and a C-terminal NHL domain. The NHL domain in TRIM71 consists of six repeats, each 40–50 residues long, that jointly comprise a six-bladed β-propeller.49 TRIM71 mediates post-transcriptional silencing of mRNAs via direct interactions of its NHL domain and the 5′ or 3′ untranslated regions (UTRs) of target genes. The de novo p.Arg608His (NHL1), p.Asp624Asn (NHL1), and p.Arg649Gln (NHL2), p.Arg655Gln (NHL2), p.Arg655* (NHL2), and p.Arg796His (NHL5) variants each localize to homologous positions in different blades of their respective NHL domains. Arginines at p.Arg608, p.Arg655 and p.Arg796 are conserved among orthologs across different species and reside in RPXGV motifs (Fig. 1B).
The NHL domain of TRIM71 is the closest homolog of the NHL domain of Drosophila melanogaster Brat,25,49 which has been co-crystallized bound to a target RNA.49 In this structure, the amino side chains of the mutated arginines form hydrogen bonds with either the phosphate backbone (p.Arg796), a uracil base (p.Arg608) or stabilizing the negative charge around the sugar-phosphate backbone of target RNA (p.Arg655; Fig. 1D). These interactions are all predicted to be altered by substitution with histidine11 or glutamine (Fig. 1D). While these variants were classified by MetaSVM as tolerated, they are judged as damaging by 43 of 45 other predictive algorithms, including the very conservative MPC-D algorithm (Table 1 and Supplementary Table 2).
Seven additional patients were identified with rare, damaging unphased or transmitted variants of uncertain significance located in highly conserved N-terminal domains of TRIM71 (Supplementary Fig. 1), including the novel recurrent RING-type domain variant p.Cys15Gly in Patients KCHYD674-1 and KCHYD674-7, and the novel B box-type two domain variant p.Gln228* in Patients KCHYD677-1 and KCHYD677-2. A fourth transmitted variant, NHL1 domain variant p.Arg608His was also identified, as well as the unphased novel NHL3 domain variants p.Lys403Asn and p.Asn701Lys (Fig. 1C and Table 1). Together, these data identify the NHL domain of TRIM71 as a mutational hotspot. Furthermore, it is of note that all of the TRIM71 mutations identified in this study have a very low allele frequency in gnomAD database. Moreover, the pLI score of 1 for TRIM71 indicates a high intolerance to LoF mutations. Collectively, these findings provide robust evidence supporting the pathogenicity of TRIM71.
TRIM71 variants cause a novel form of syndromic hydrocephalus
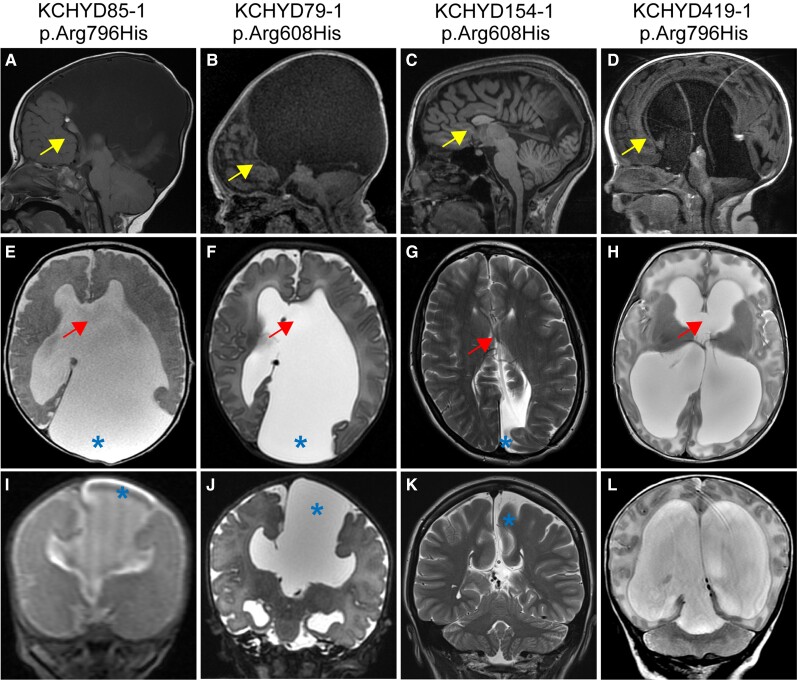
We examined the clinical phenotypes of probands harbouring TRIM71 DNVs and other rare, damaging transmitted or unphased CH-associated TRIM71 variants (Supplementary Table 3). Consistent with our ascertainment strategy, the majority of patients had cerebral ventriculomegaly, at least 11 of whom required neurosurgical CSF diversion by endoscopic third ventriculostomy or ventriculoperitoneal shunting. Eleven patients had known developmental delay or other neurobehavioural abnormalities (including attention-deficit hyperactivity disorder and autism), five patients exhibited limb defects, seven patients had craniofacial defects (including one with unilateral coronal craniosynostosis) and other dysmorphic features, four patients had hearing abnormalities and three patients had congenital cardiovascular defects (Fig. 2). Examination of patients with available advanced brain imaging showed other structural brain defects besides ventriculomegaly, including corpus callosum dysgenesis, white matter volume loss, absent/dehiscent septum pellucidum and cerebellar tonsillar ectopia. These data expand the phenotypic spectrum associated with TRIM71 variants and suggest variants in TRIM71 lead to a novel syndromic form of CH with other functional and structural CNS and systemic anomalies.
Figure 2.
TRIM71 variants cause a novel form of syndromic congenital hydrocephalus. Representative brain MRIs of patients with de novo and transmitted TRIM71 mutations showing ventriculomegaly and other structural brain defects, including dysgenesis of the corpus callosum (yellow arrow), absence/dehiscence of the septum pellucidum (red arrow), and interhemispheric cysts (blue asterisk). Sagittal (A–D), axial (E–) and coronal (I–L) MRIs are shown of proband Patients KCHYD85-1 (A, E and I), KCHYD79-1 (B, F and J), KCHYD154-1 (C, G and K) and KCHYD419-1 (D, H and L).
TRIM71 NHL-domain variants abrogate its RNA-binding capacity to canonical mRNA targets
We11 and others50,51 previously showed that the CH-associated TRIM71 NHL domain variants p.Arg608His and p.Arg796His impact RNA-binding capacity. To determine whether other novel CH-associated TRIM71 variants exhibit abnormal RNA-binding capacity, we overexpressed in HEK-293T cells FLAG-tagged human TRIM71-WT and the CH-associated variants p.Asn701Lys, p.Arg655Gln, p.Asp624Asn, p.Arg608Cys, p.Lys403Asn, p.Gln334Arg, p.Gln288Ter and p.Cys15Gly (see the ‘Materials and methods’ section). As controls, we transfected an empty FLAG-vector and a plasmid encoding the RNA-binding-deficient mutant TRIM71-ΔNHL6 lacking the C-terminal NHL repeat. We then performed a UV-crosslink-assisted RNA immunoprecipitation followed by qRT-PCR for the established TRIM71 target mRNAs, CDKN1A and EGR111 (Fig. 3).
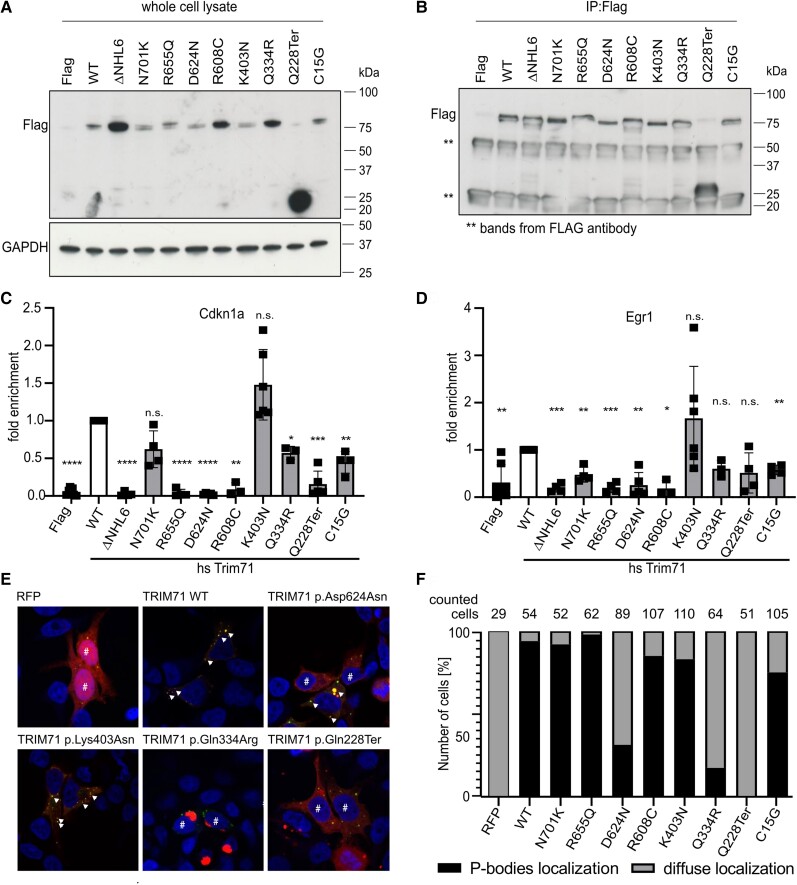
Figure 3.
TRIM71 variants disrupt the RNA-binding capacity of its NHL domain to CDKN1A and EGR1 or fail to localize TRIM71 to P-bodies. (A) Representative Flag immunoblot of HEK-293T total cell lysates showing the expression levels of the different constructs, i.e. Flag-control, Flag-TRIM71-WT (human) and Flag-TRIM71 variants ΔNHL6, -DN701 K, -R655Q, -D624N, -R608C, -K403N, -Q334R, -Q228Ter, -C15G. Predicted relative molecular mass (Mr) of TRIM71 Q228Ter is 24 kDa; Mr of wild-type (WT) and other variants is ∼80 kDa (predicted Mr 93 kDa). (B) Representative Flag immunoblot of anti-FLAG immunoprecipitates. Cross-linking and immunoprecipitation-quantitative real-time PCR enrichment of CDKN1A (C) and EGR1 (D) normalized to input and enrichment of housekeeping gene 18S RNA (means ± standard deviation; n ≥ 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s. (not significant): P > 0.05. One-sample t-test versus WT. (E) P-body localization of congenital hydrocephalus (CH)-associated TRIM71 variants was analysed by co-expression of an RFP empty vector, RFP-tagged human Trim71-WT or CH-associated mutations (TRIM71-p.Asp624Asn, TRIM71-p.Lys403Asn, TRIM71-p.Gln334Arg and TRIM71-p.Gln228Ter) (red) in HEK293T cells together with GFP-tagged P-body marker DCP1A (green). Shown are representative confocal images where some P-bodies with co-localized TRIM71 are marked with an arrow, while cells without co-localization are labelled with a number sign. (F) Percentage of cells exhibiting TRIM71-DCP1A co-localization. The number of cells counted for each condition was ≥ 50 (29 cells for RFP empty vector as negative control). Nuclei were stained with DAPI (blue).
As determined by western blot, TRIM71 protein stability was not affected by the variants tested. Although the transfection of TRIM71 constructs was not always even, all variants were robustly detected after the immunoprecipitation (Fig. 3A and B and Supplementary Fig. 2A). TRIM71 p.Gln228Ter was detectable at approximately 26 kDa. Like p.Arg608His and p.Arg796His,11 the three novel NHL domain TRIM71 variants p.Arg655Gln (NHL2), p.Asp624Asn (NHL1) and p.Arg608Cys (NHL1) exhibited complete impairment of CDKN1A RNA binding, comparable to that of TRIM71-ΔNHL6 (Fig. 3C). Likewise, the binding to EGR1 RNA was impaired for these three variants (Fig. 3D). As expected, the truncated (i.e NHL-deficient) TRIM71-p.Gln228Ter also showed strongly reduced CDKN1A RNA binding, whereas its binding to EGR1 was reduced by 50%. We attribute this to a higher background in EGR1 mRNA detection, which is also apparent when comparing the FLAG-vector control with the positive control (Fig. 3D). The novel unphased NHL3 domain variant p.Asn701Lys had a mild effect on CDKN1A and EGR1 RNA binding, respectively, with a reduced binding capacity of 40% (CDKN1A) and 50% (EGR1), respectively (Fig. 3C and D). CDKN1A and EGR1 binding by non-NHL mutants p.Gln334Arg and p.Cys15Gly was only slightly affected, while TRIM71 p.Lys403Asn showed a trend towards a gain-of-function. This effect was not significant, but is consistent with the findings of Liu et al.51 that Trim71 variants also could lead to increased RNA binding compared to WT TRIM71. These data confirm reduced RNA-binding capacity of most NHL-domain mutants of TRIM71, at least when employing the canonical mRNA targets CDKN1A or EGR1, respectively.
Some CH-associated variants of TRIM71 fail to localize to P-bodies
The coiled coil (CC) region of TRIM71 is considered essential for its localization to P-bodies.46 We speculated that some non-NHL domain TRIM71 variants might be pathogenic by altering subcellular localization of TRIM71. To test this hypothesis, we overexpressed RFP-tagged TRIM71-WT and various CH-associated TRIM71 variants in HEK293T cells with the GFP-tagged P-body marker DCP1A and evaluated their co-localization using confocal immunofluorescence microscopy (see the ‘Materials and methods’ section; Fig. 3F and Supplementary Fig. 2B). As expected, TRIM71-WT localized to P-bodies (Fig. 3E). TRIM71-p.Gln228Ter has no CC region due to a transcriptional stop in the first B-Box domain; consistent with this, TRIM71-p.Gln228Ter was evenly distributed throughout the cytoplasm, comparable to the negative control RFP-empty vector (Fig. 3E). The CC domain variant TRIM71-p.Lys403Asn clearly localized to P-bodies. Interestingly, TRIM71-p.Asp624Asn and TRIM71-p.Gln334Arg variants failed to localize to P-bodies (Fig. 3E). These results suggest some TRIM71 variants may alter cellular function by disrupting TRIM71 localization to P-bodies.
TRIM71 is expressed in early human embryonic neural stem cells
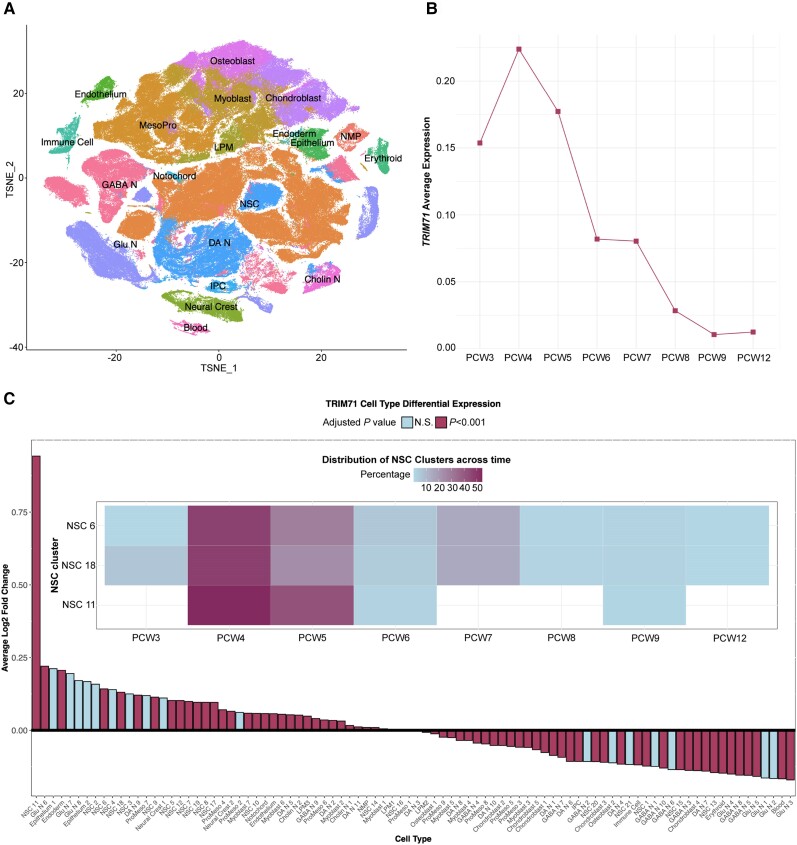
Trim71 is expressed in mouse ventricular zone neuroepithelial and neural progenitor cells during midgestation,26,51 a key epoch during which neurogenesis contributes to development of the diencephalon and telencephalon.52TRIM71 expression in human brain remains poorly characterized. We studied developmental expression of TRIM71 in the human brain using a single-cell RNA-sequencing database of 430 808 cells from human whole embryo, head and brain tissue spanning postconceptional weeks (PCW) 3–12 (see the ‘Materials and methods’ section; Fig. 4A).42 We found that TRIM71 expression peaked in PCW 4 and dropped off rapidly thereafter (Fig. 4B). TRIM71 was most highly differentially expressed in neural stem cell cluster 11 (log2 fold-change = 0.94, P = 1.5 × 10−5). This cluster was most predominant in PCW 4–5, as were other neural stem cell clusters with significant positive differential TRIM71 expression (Fig. 4C). These data show differential TRIM71 expression in human neural stem cells in early fetal neurogenesis.
Figure 4.
TRIM71 is expressed in neural stem cells during human fetal brain development. (A) A t-distributed stochastic neighbour embedding (t-SNE) plot of transcriptional dataset of developing human brain [postconceptual weeks (PCW) 3–12] coloured by main cell type: neural stem cells (NSC), intermediate progenitor cells (IPC), glutamatergic neurons (Glu N), GABAergic neurons (GABA N), dopaminergic neurons (DA N), cholinergic neurons (Cholin N), neuromesodermal progenitors (NMP), lateral plate mesoderm (LPM) and proliferating mesoderm (MesoPro). (B) Temporal gene expression profile of TRIM71 from PCW 3–12. (C) Differential TRIM71 expression across main cell type sub-clusters. Inset: Temporal distribution across PCW 3–12 of the top three significantly differentially expressed NSC clusters. Shading depicts the percentage of single cells in clusters found at each PCW.
Discussion
Unbiased genomic studies have identified mutations in TRIM71 as an important genetic cause of human CH.9-11 In this study, we provide further genetic and phenotypic evidence that recurrent missense DNVs in the RNA-binding NHL domain of TRIM71 cause a novel form of syndromic CH. These results highlight the importance of TRIM71 in human brain morphogenesis and provide further support for a ‘neural stem cell’ paradigm of human CH.11,53-55 These data demonstrate the power of trio-based WES for identifying pathogenic variants in sporadic structural brain disorders and suggest WES as a useful adjunct in the clinical evaluation and management of CH patients.
Our identification of multiple new patients with pathogenic TRIM71 variants suggests TRIM71 is a bona fide OMIM gene and expands the phenotypic spectrum of the disorder (TADD, TRIM71-associated developmental disorders). A core feature of the TADD is cerebral ventriculomegaly, which has often been treated with neurosurgical CSF diversion. This phenotype may be overrepresented in our patients, insofar as recruitment for our cohort was based on the presence of cerebral ventriculomegaly. Nonetheless, depletion of Trim71 in murine neural stem cells or knock-in of a homologous CH-associated TRIM71 variant in mice each elicits severe CH in utero.51 Continued identification of TRIM71 variants will likely further expand the TRIM71 phenotypic spectrum, including patients without ventriculomegaly, and may permit genotype-phenotype correlations.
As for other Mendelian forms of syndromic CH (e.g. L1CAM),19 most CH patients harbouring TRIM71 variants exhibit neurological findings and structural brain anomalies in addition to hydrocephalus, including developmental delay and other neurobehavioural abnormalities, limb defects, craniofacial defects and other dysmorphic features, hearing abnormalities, and congenital cardiovascular defects. Other structural brain defects were also common. In one striking example, a hydrocephalic mother and her hydrocephalic son with the same pathogenic TRIM71 variant both exhibited highly similar dysgenesis of the corpus callosum and interhemispheric cysts.9 These findings support the roles of TRIM71 in regulation of multiple fundamental aspects of anatomic and functional brain maturation, and in regulation of neural stem cell fate.46
Our results suggest incomplete penetrance and variable expressivity for some TRIM71 variants, a phenomenon recognized for other CH and autism spectrum disorder genes.9,10 The mechanistic drivers of incomplete penetrance and variable expressivity in the context of TRIM71 variation remain unclear, but likely include common genetic or environmental modifiers,56,57 as well as stochastic components resulting in mosaicism.58 Future genome sequencing focused on regulatory sequence variants (e.g. enhancers, promoters, and insulators adjacent to TRIM71 coding regions) and on other genes within TRIM71 biological pathways, would be particularly valuable for identification of genetic modifiers. Gene dosage-dependent mechanisms are also suggested by the phenotypic variability and lack of clear genotype-phenotype correlation characterizing the wide-ranging neurodevelopmental phenotypes associated with TRIM71 variants.59
TRIM71 regulates the balance between self-renewal and differentiation via the post-transcriptional silencing of its target mRNAs such as CDKN1A.22,23,60,61 TRIM71 mediates post-transcriptional silencing of mRNAs via direct interactions of its NHL domain and the 5′ or 3′ UTRs of target genes. A striking feature of most CH-associated TRIM71 variants is their localization to NHL-binding domains. The recurrence of DNVs at homologous positions across three different NHL domains suggest that TRIM71 mutations may not be simple loss-of-function mutations.51 In both previous11 and more recent studies,51TRIM71 p.Arg595His and p.Arg783His expedited differentiation and amplified commitment to the neural lineage in mouse embryonic stem cells. These variants also decreased binding to mRNAs targeted by wild-type TRIM71, consistent with previous reports. Notably, these two distinct TRIM71 mutants exhibit affinity for distinct target mRNAs. These data identify TRIM71’s NHL domain as a mutational hotspot and implicates dysregulation of the developing brain transcriptome in CH pathogenesis. HEK293T cells are a well-established surrogate system for these types of analyses. In a previous publication, we presented consistent data for the functional analysis of the TRIM71 variant R595H (mouse)/R608H (human) using either overexpressing HEK293T cells, or mutant murine embryonic stem cells.11 Therefore, we assume that principal features of TRIM71 can be robustly assayed using this cell type, but we cannot fully exclude cell specific effects. Effects of variants outside the NHL domain on the mRNA binding capacity and effects of variants outside the CC domain on the P-body localization could be potentially explained by changes to the 3D structure of TRIM71, but this needs further investigation.
Analogous to its heterochronic expression in C. elegans,62,63 mouse Trim71 expression significantly decreases during mouse development.64Trim71 is highly expressed in neural stem cells in mouse embryos, peaking at embryonic Day 9.5, but declines rapidly afterwards as neural differentiation proceeds.46,64 Although our study is limited by a small cohort size, our analysis suggests this heterochronic expression profile is conserved in human, with high TRIM71 expression in neuroepithelial stem cells through PCW 4, after which expression declines abruptly with continued maturation. Consistent with this robust and specific expression in the neural tube epithelium, Trim71 knockout in mice results in exencephaly (failure of closure of the cephalic end of the neural tube) and embryonic lethality by embryonic Day 10.65
Multiple ‘impaired brain plumbing’ mechanisms have been proposed to account for hydrocephalus, including increased CSF secretion from the choroid plexus, decreased intraventricular CSF transit reflecting ciliary dysfunction, and decreased CSF reabsorption associated with elevated venous pressure, arachnoid granulation immaturity, and lymphatic dysplasia.66 Many of these mechanisms may contribute especially to acquired forms of hydrocephalus in the context of inflammation and infection.67,68 However, accumulating genetic data9-11,69 suggest that impaired neurogenesis, rather than overactive CSF accumulation, may underlie multiple genetic forms of CH. Our TRIM71 findings support such a ‘neural stem cell’ paradigm of disease.9-11 In patients with TRIM71 variants, impaired post-transcriptional silencing of RNA targets, such as the cell cycle inhibitor CDKN1A, leads to decreased NSC proliferation at even earlier time points, resulting in severe cortical hypoplasia and secondary ventriculomegaly by altering biomechanical properties of brain parenchyma, with potential secondary CSF pooling.11,70
The diversity of CH genetic etiologies and underlying biochemical pathways supports implementation of routine clinical WES for newly diagnosed patients. Current recommendations for workup of fetal and neonatal ventriculomegaly include rapid commercial microarray testing for known chromosomal and copy-number abnormalities.71 However, this strategy does not address CH cases explained by recently detected variants in many new CH genes.9-11 Application of routine WES or whole genome sequencing could improve management of children with CH by aiding prognostication and treatment stratification (including when or when not to operate), increasing vigilance for medical screening of variant-associated conditions, and providing recurrence rates to increase reproductive confidence.
Supplementary Material
Contributor Information
Phan Q Duy, Department of Neurosurgery, University of Virginia School of Medicine, Charlottesville, VA 22908, USA; Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA; Department of Neuroscience, University of Virginia School of Medicine, Charlottesville, VA 22908, USA.
Bettina Jux, Molecular Immunology and Cell Biology, Life & Medical Sciences Institute (LiMES), University of Bonn, Bonn 53012, Germany.
Shujuan Zhao, Department of Genetics, School of Medicine, Washington University, St. Louis, MO 63110, USA; Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Kedous Y Mekbib, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA; Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Evan Dennis, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Weilai Dong, Laboratory of Human Genetics and Genomics, The Rockefeller University, New York, NY 10065, USA.
Carol Nelson-Williams, Department of Genetics, Yale University School of Medicine, New Haven, CT 06510, USA.
Neel H Mehta, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
John P Shohfi, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA; Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Jane Juusola, GeneDx, Gaithersburg, MD 20877, USA.
Garrett Allington, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA; Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Hannah Smith, Department of Neurosurgery, Yale University School of Medicine, New Haven, CT 06510, USA; Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Sandrine Marlin, Laboratory of Embryology and Genetics of Human Malformation, Imagine Institute, Paris Descartes, Sorbonne Paris Cité University, Paris 75013, France.
Kahina Belhous, Department of Radiology, Necker Children Hospital, Assistance Publique—Hôpitaux de Paris, University Paris 5, Paris 75004, France.
Berrin Monteleone, Division of Clinical Genetics, NYU Langone Health, Long Island, Mineola, NY 11501, USA.
G Bradley Schaefer, Section of Genetics and Metabolism, University of Arkansas for Medical Sciences, Little Rock, AR 77205, USA.
Margareta D Pisarska, Department of Obstretrics and Gynecology, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
Jaime Vásquez, Division of Clinical Genetics, NYU Langone Health, Long Island, Mineola, NY 11501, USA.
Juvianee I Estrada-Veras, Department of Surgery, Henry M Jackson Foundation for the Advancement of Military Medicine and Uniformed Services University of the Health Sciences, Bethesda, MD 20817, USA; Pediatric Subspecialty Genetics Walter Reed National Military Medical Center, Bethesda, MD 20889, USA.
Boris Keren, Department of Genetics, APHP, Sorbonne Université, Pitié-Salpêtrière University Hospital, Paris 75013, France.
Cyril Mignot, Department of Genetics, APHP, Sorbonne Université, Pitié-Salpêtrière University Hospital, Paris 75013, France; Centre de Référence Déficiences Intellectuelles de Causes Rares, Pitié-Salpêtrière University Hospital, Paris 75013, France.
Leigh A Flore, Division of Genetic, Genomic and Metabolic Disorders, Children's Hospital of Michigan, Detroit, MI 48201, USA; Central Michigan University College of Medicine, Mount Pleasant, MI 48858, USA.
Irene V Palafoll, Centre de référence Anomalies du développement, CHU Grenoble-Alpes, Grenoble 38700, France.
Seth L Alper, Division of Nephrology and Center for Vascular Biology Research, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA; Department of Medicine, Harvard Medical School, Boston, MA 02115, USA; Broad Institute of Harvard and MIT, Cambridge, MA 02142, USA.
Richard P Lifton, Department of Genetics, Yale University School of Medicine, New Haven, CT 06510, USA.
Shozeb Haider, School of Pharmacy, University College London, London WC1E 6BT, UK.
Andres Moreno-De-Luca, Department of Radiology, Neuroradiology Section, Kingston Health Sciences Centre, Queen’s University Faculty of Health Sciences, Kingston, ON K7L 3N6, Canada.
Sheng Chih Jin, Department of Genetics, School of Medicine, Washington University, St. Louis, MO 63110, USA; Department of Pediatrics, Washington University School of Medicine, St. Louis, MO 063110, USA.
Waldemar Kolanus, Molecular Immunology and Cell Biology, Life & Medical Sciences Institute (LiMES), University of Bonn, Bonn 53012, Germany.
Kristopher T Kahle, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; Broad Institute of Harvard and MIT, Cambridge, MA 02142, USA; Division of Genetics and Genomics, Manton Center for Orphan Disease Research, Department of Pediatrics, and Howard Hughes Medical Institute, Boston Children’s Hospital, Boston, MA 02115, USA; Harvard Center for Hydrocephalus and Neurodevelopmental Disorders, Massachusetts General Hospital, Boston, MA 02114, USA.
Data availability
We fully support the sharing of data and have already submitted the genomic and phenomic data from individuals recruited through Yale and Massachusetts General Hospital to the NCBI Database of Genotypes and Phenotypes. The data can be accessed under the accession number phs000744.v4.p2. However, it is important to note that due to consent restrictions, the raw genomic data for GeneDx patients cannot be shared. Furthermore, our in-house R and Python pipelines and codes are publicly available on our GitHub repository at https://github.com/Kahle-Lab.
Funding
This work was supported by the Yale-NIH Center for Mendelian Genomics (grant 5U54HG006504) and the University of Washington Center for Mendelian Genomics (grants UW-CMG, UM1HG006493, U24HG008956), National Institutes of Health (grants R01 NS111029-01A1, R01 NS109358, R01 NS127879-01) (K.T.K.); the Rudi Schulte Research Institute (K.T.K.), the Hydrocephalus Association Innovator Award (K.T.K., S.C.J.), the Clinical & Translational Research Funding Program Award (CTSA1405; (S.C.J.), the Children’s Discovery Institute Faculty Scholar Award (CDI-FR-2021-926) (S.C.J). W.K. is funded by the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy EXC2151–39087304.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Tully HM, Dobyns WB. Infantile hydrocephalus: A review of epidemiology, classification and causes. Eur J Med Genet. 2014;57:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rekate HL. The definition and classification of hydrocephalus: A personal recommendation to stimulate debate. Cerebrospinal Fluid Res. 2008;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387:788–799. [DOI] [PubMed] [Google Scholar]
- 4. Bret P, Chazal J. Chronic (“normal pressure”) hydrocephalus in childhood and adolescence. A review of 16 cases and reappraisal of the syndrome. Childs Nerv Syst. 1995;11:687–691. [DOI] [PubMed] [Google Scholar]
- 5. Alluhaybi AA, Altuhaini K, Ahmad M. Fetal ventriculomegaly: A review of literature. Cureus. 2022;14:e22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey AT, Kimmings EJ, Kleinlugtebeld AD, Taylor WA, Harkness WF, Hayward RD. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27:63–70. [DOI] [PubMed] [Google Scholar]
- 7. Hoppe-Hirsch E, Laroussinie F, Brunet L, et al. Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst. 1998;14:97–99. [DOI] [PubMed] [Google Scholar]
- 8. Lindquist B, Carlsson G, Persson EK, Uvebrant P. Learning disabilities in a population-based group of children with hydrocephalus. Acta Paediatr. 2005;94:878–883. [DOI] [PubMed] [Google Scholar]
- 9. Furey CG, Choi J, Jin SC, et al. De novo mutation in genes regulating neural stem cell fate in human congenital hydrocephalus. Neuron. 2018;99:302–314.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin SC, Dong W, Kundishora AJ, et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med. 2020;26:1754–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duy PQ, Weise SC, Marini C, et al. Impaired neurogenesis alters brain biomechanics in a neuroprogenitor-based genetic subtype of congenital hydrocephalus. Nat Neurosci. 2022;25:458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Dosari MS, Al-Owain M, Tulbah M, et al. Mutation in MPDZ causes severe congenital hydrocephalus. J Med Genet. 2013;50:54–58. [DOI] [PubMed] [Google Scholar]
- 13. Slavotinek A, Kaylor J, Pierce H, et al. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am J Hum Genet. 2015;96:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaheen R, Sebai MA, Patel N, et al. The genetic landscape of familial congenital hydrocephalus. Ann Neurol. 2017;81:890–897. [DOI] [PubMed] [Google Scholar]
- 15. Wallmeier J, Frank D, Shoemark A, et al. De Novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am J Hum Genet. 2019;105:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allocco AA, Jin SC, Duy PQ, et al. Recessive inheritance of congenital hydrocephalus with other structural brain abnormalities caused by compound heterozygous mutations in ATP1A3. Front Cell Neurosci. 2019;13:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hale AT, Bastarache L, Morales DM, Wellons JC 3rd, Limbrick DD Jr, Gamazon ER. Multi-omic analysis elucidates the genetic basis of hydrocephalus. Cell Rep. 2021;35:109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekici AB, Hilfinger D, Jatzwauk M, et al. Disturbed wnt signalling due to a mutation in CCDC88C causes an autosomal recessive non-syndromic hydrocephalus with medial diverticulum. Mol Syndromol. 2010;1:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenthal A, Jouet M, Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat Genet. 1992;2:107–112. [DOI] [PubMed] [Google Scholar]
- 20. Ito N, Riyadh MA, Ahmad SAI, et al. Dysfunction of the proteoglycan Tsukushi causes hydrocephalus through altered neurogenesis in the subventricular zone in mice. Sci Transl Med. 2021;13:eaay7896. [DOI] [PubMed] [Google Scholar]
- 21. Jacquemin V, Versbraegen N, Duerinckx S, et al. Congenital hydrocephalus: New Mendelian mutations and evidence for oligogenic inheritance. Hum Genomics. 2023;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ecsedi M, Grosshans H. LIN-41/TRIM71: Emancipation of a miRNA target. Genes Dev. 2013;27:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang HM, Martinez NJ, Thornton JE, Hagan JP, Nguyen KD, Gregory RI. Trim71 cooperates with microRNAs to repress Cdkn1a expression and promote embryonic stem cell proliferation. Nat Commun. 2012;3:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Connacher RP, Goldstrohm AC. Molecular and biological functions of TRIM-NHL RNA-binding proteins. Wiley Interdiscip Rev RNA. 2021;12:e1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loedige I, Gaidatzis D, Sack R, Meister G, Filipowicz W. The mammalian TRIM-NHL protein TRIM71/LIN-41 is a repressor of mRNA function. Nucleic Acids Res. 2013;41:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cuevas E, Rybak-Wolf A, Rohde AM, Nguyen DT, Wulczyn FG. Lin41/Trim71 is essential for mouse development and specifically expressed in postnatal ependymal cells of the brain. Front Cell Dev Biol. 2015;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krumm N, Turner TN, Baker C, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Purcell S, Neale B, Todd-Brown K, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stram DO. Software for tag single nucleotide polymorphism selection. Hum Genomics. 2005;2:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKenna A, Hanna M, Banks E, et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: The genome analysis toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11.10.1–11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang YC, Wu Y, Choi J, et al. Computational genomics in the era of precision medicine: Applications to variant analysis and gene therapy. J Pers Med. 2022;12:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong C, Wei P, Jian X, et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet. 2015;24:2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei Q, Zhan X, Zhong X, et al. A Bayesian framework for de novo mutation calling in parents-offspring trios. Bioinformatics. 2015;31:1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diab NS, King S, Dong W, et al. Analysis workflow to assess de novo genetic variants from human whole-exome sequencing. STAR Protoc. 2021;2:100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaplanis J, Samocha KE, Wiel L, et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature. 2020;586:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. Jun 2002;12:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ware JS, Samocha KE, Homsy J, Daly MJ. Interpreting de novo Variation in Human Disease using denovolyzeR. Curr Protoc Hum Genet. 2015;87:7.25.1–7.25.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng B, Liu Z, Lu Y, et al. The single-cell and spatial transcriptional landscape of human gastrulation and early brain development. Cell Stem Cell. 2023;30:851–866.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolf FA, Angerer P, Theis FJ. SCANPY: Large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hao Y, Hao S, Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abagyan R, Totrov M, Kuznetsov D. ICM—A new method for protein modeling and design: Applications to docking and structure prediction from the distorted native conformation. J Comput Chem. 1994;15:488–506. [Google Scholar]
- 46. Torres-Fernández LA, Jux B, Bille M, et al. The mRNA repressor TRIM71 cooperates with nonsense-mediated decay factors to destabilize the mRNA of CDKN1A/p21. Nucleic Acids Res. 2019;47:11861–11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duran D, Zeng X, Jin SC, et al. Mutations in chromatin modifier and ephrin signaling genes in vein of galen malformation. Neuron.. 2019;101:429–443.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jin SC, Dong W, Kundishora AJ, et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med. 2020;26:1754–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loedige I, Jakob L, Treiber T, et al. The crystal structure of the NHL domain in Complex with RNA reveals the molecular basis of Drosophila brain-tumor-mediated gene regulation. Cell Rep. 2015;13:1206–1220. [DOI] [PubMed] [Google Scholar]
- 50. Welte T, Tuck AC, Papasaikas P, et al. The RNA hairpin binder TRIM71 modulates alternative splicing by repressing MBNL1. Genes Dev. 2019;33(17–18):1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Q, Novak MK, Pepin RM, Maschhoff KR, Hu W. Different congenital hydrocephalus-associated mutations in Trim71 impair stem cell differentiation via distinct gain-of-function mechanisms. PLoS Biol. 2023;21:e3001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duy PQ, Rakic P, Alper SL, et al. A neural stem cell paradigm of pediatric hydrocephalus. Cereb Cortex. 2023;33:4262–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duy PQ, Rakic P, Alper SL, et al. Brain ventricles as windows into brain development and disease. Neuron. 2022;110:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodríguez EM, Guerra MM. Neural stem cells and fetal-onset hydrocephalus. Pediatr Neurosurg. 2017;52:446–461. [DOI] [PubMed] [Google Scholar]
- 56. Morrison AJ. Chromatin-remodeling links metabolic signaling to gene expression. Mol Metab. 2020;38:100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kenneth NS, Mudie S, van Uden P, Rocha S. SWI/SNF regulates the cellular response to hypoxia. J Biol Chem. 2009;284:4123–4131. [DOI] [PubMed] [Google Scholar]
- 58. D'Gama AM, Walsh CA. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci. 2018;21:1504–1514. [DOI] [PubMed] [Google Scholar]
- 59. Parenti I, Rabaneda LG, Schoen H, Novarino G. Neurodevelopmental disorders: From genetics to functional pathways. Trends Neurosci. 2020;43:608–621. [DOI] [PubMed] [Google Scholar]
- 60. Mitschka S, Ulas T, Goller T, et al. Co-existence of intact stemness and priming of neural differentiation programs in mES cells lacking Trim71. Sci Rep. 2015;5:11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Worringer KA, Rand TA, Hayashi Y, et al. The let-7/LIN-41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell Stem Cell. 2014;14:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kanamoto T, Terada K, Yoshikawa H, Furukawa T. Cloning and regulation of the vertebrate homologue of lin-41 that functions as a heterochronic gene in Caenorhabditis elegans. Dev Dyn. 2006;235:1142–1149. [DOI] [PubMed] [Google Scholar]
- 63. Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell. 2000;5:659–669. [DOI] [PubMed] [Google Scholar]
- 64. Schulman BR, Esquela-Kerscher A, Slack FJ. Reciprocal expression of lin-41 and the microRNAs let-7 and mir-125 during mouse embryogenesis. Dev Dyn. 2005;234:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maller Schulman BR, Liang X, Stahlhut C, DelConte C, Stefani G, Slack FJ. The let-7 microRNA target gene, Mlin41/Trim71 is required for mouse embryonic survival and neural tube closure. Cell Cycle. 2008;7:3935–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Govaert P, Oostra A, Matthys D, Vanhaesebrouck P, Leroy J. How idiopathic is idiopathic external hydrocephalus? Dev Med Child Neurol. 1991;33:274–276. [DOI] [PubMed] [Google Scholar]
- 67. Karimy JK, Zhang J, Kurland DB, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23:997–1003. [DOI] [PubMed] [Google Scholar]
- 68. Robert SM, Reeves BC, Kiziltug E, et al. The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus. Cell. 2023;186:764–785.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kundishora AJ, Singh AK, Allington G, et al. Genomics of human congenital hydrocephalus. Childs Nerv Syst. 2021;37:3325–3340. [DOI] [PubMed] [Google Scholar]
- 70. Duy PQ, Mehta NH, Kahle KT. Biomechanical instability of the brain-CSF interface in hydrocephalus. Brain. 2024;147:3274–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Etchegaray A, Juarez-Peñalva S, Petracchi F, Igarzabal L. Prenatal genetic considerations in congenital ventriculomegaly and hydrocephalus. Childs Nerv Syst. 2020;36:1645–1660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We fully support the sharing of data and have already submitted the genomic and phenomic data from individuals recruited through Yale and Massachusetts General Hospital to the NCBI Database of Genotypes and Phenotypes. The data can be accessed under the accession number phs000744.v4.p2. However, it is important to note that due to consent restrictions, the raw genomic data for GeneDx patients cannot be shared. Furthermore, our in-house R and Python pipelines and codes are publicly available on our GitHub repository at https://github.com/Kahle-Lab.