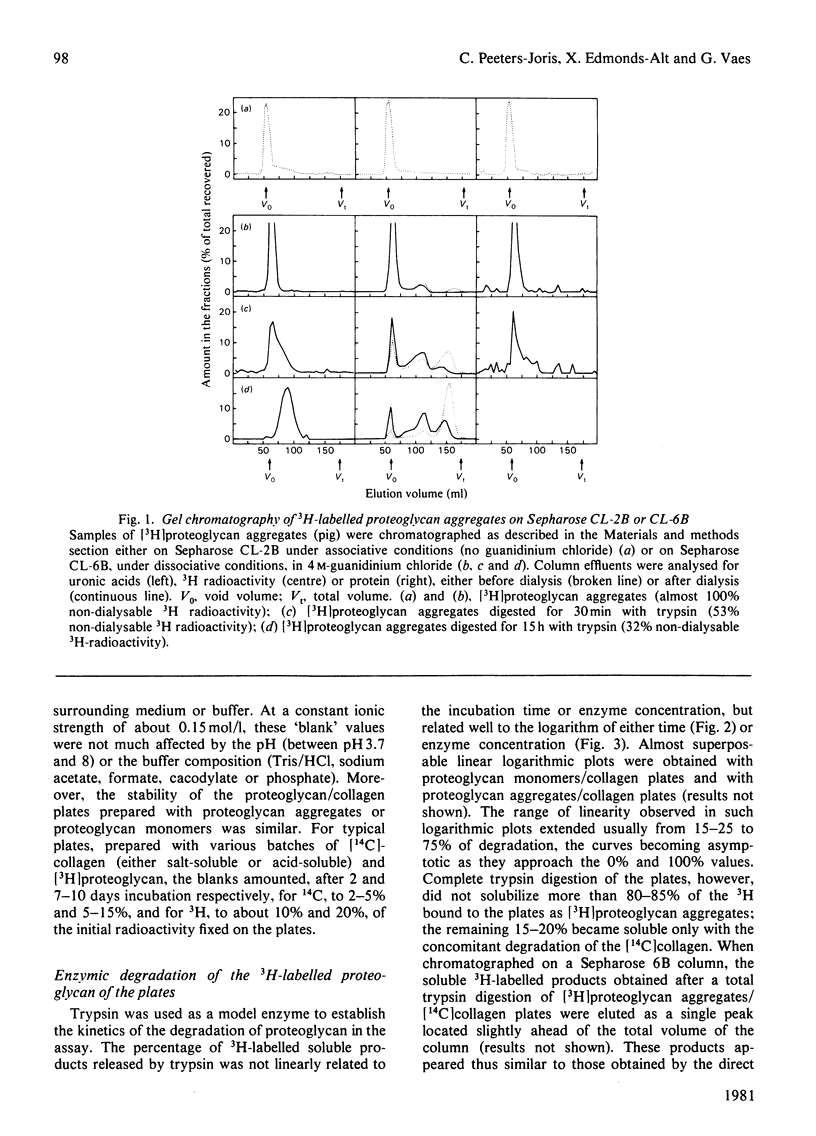
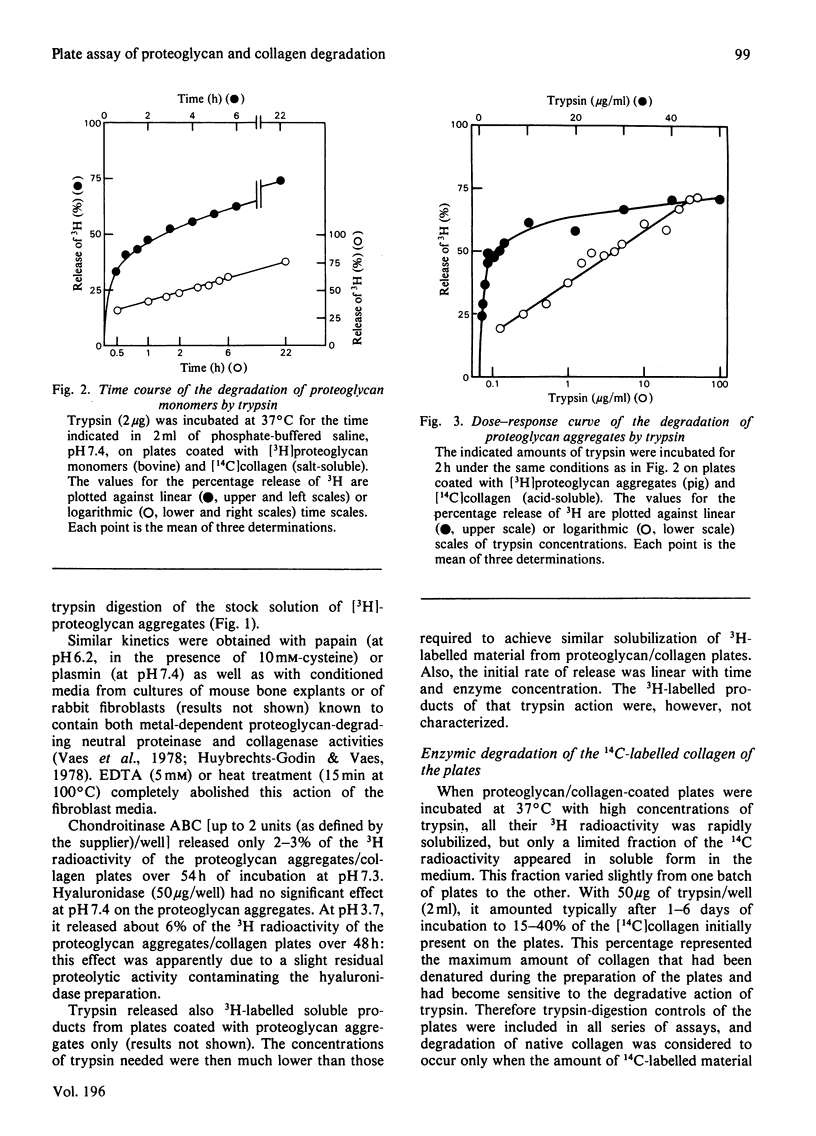
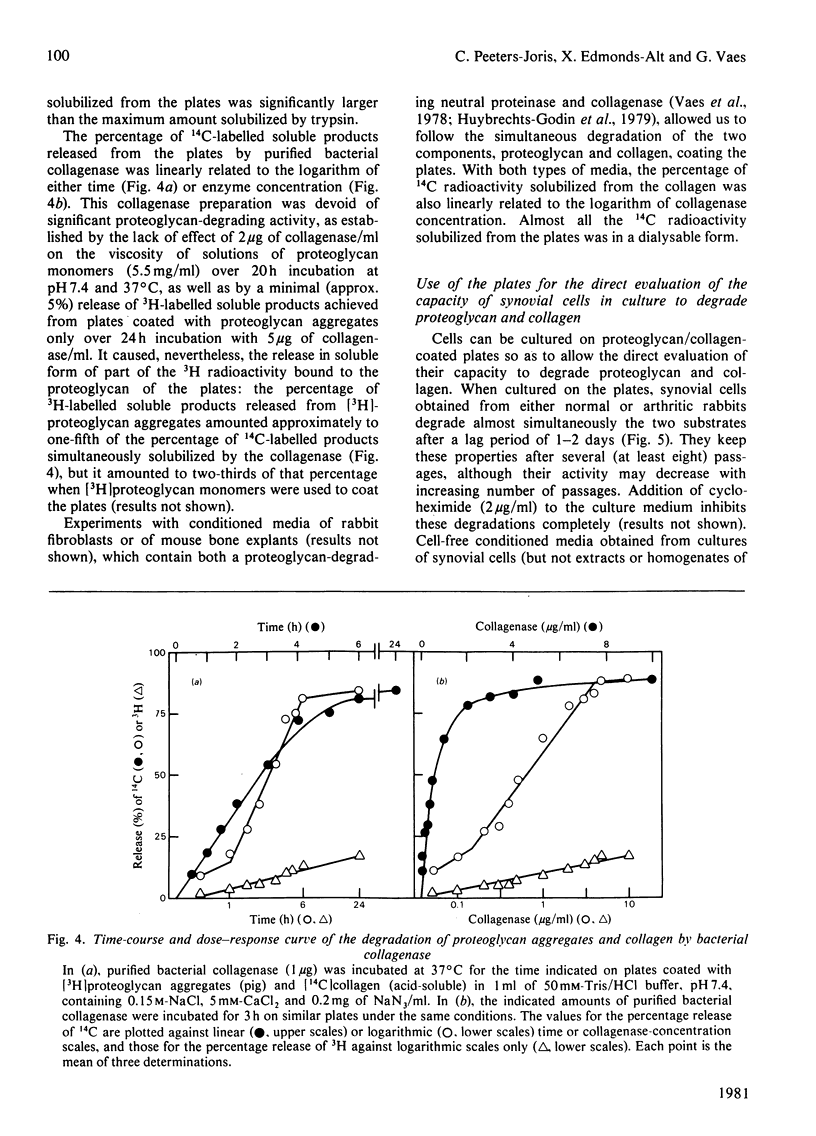
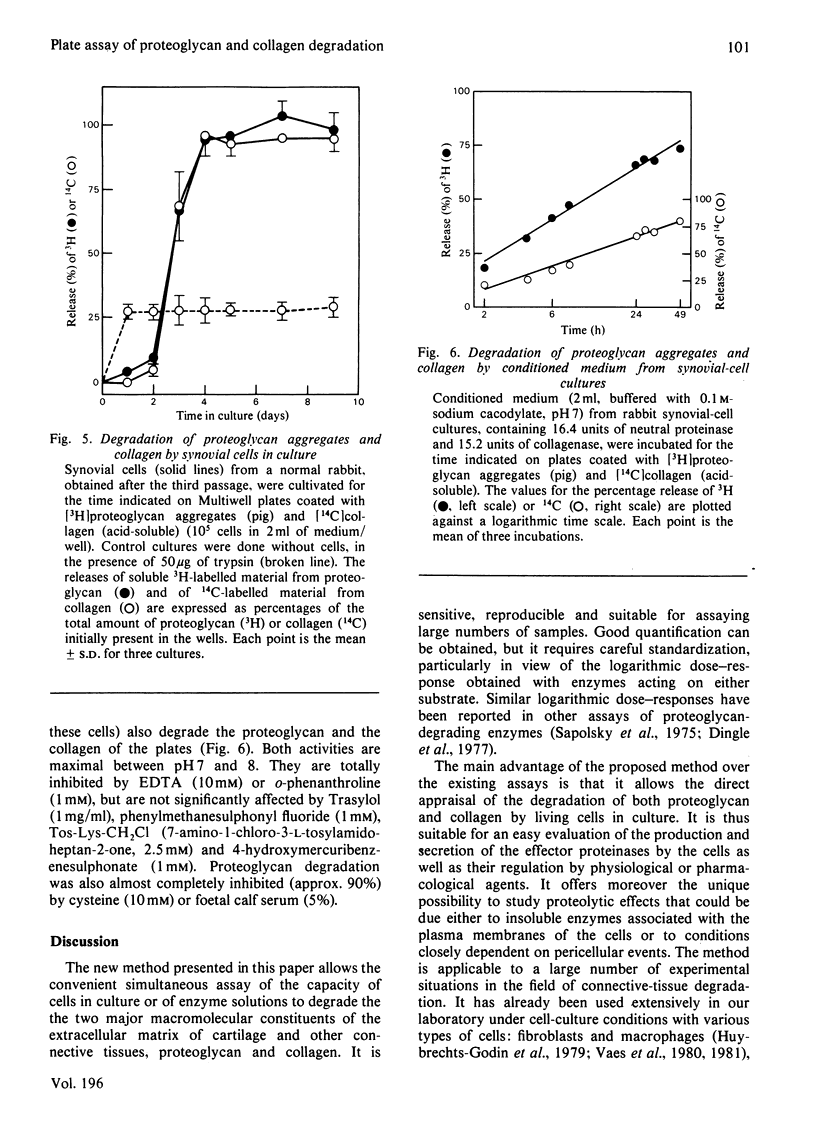
Abstract
1. A radiochemical plate assay is presented that allows a simultaneous evaluation of the capacity of cells in culture to degrade proteoglycan and collagen. Its principle consists of monitoring the release of soluble radioactive degradation products from Multiwell culture plates coated with dried reconstituted 3H-labelled-proteoglycan/14C-labelled-collagen mixed gels. The plates can also be used for the assay of proteolytic activities within enzyme solutions. 2. When cultured on the plates, rabbit synovial cells degrade collagen and proteoglycan almost simultaneously, owing to the secretion of collagenase and of a proteoglycan-degrading metal-dependent neutral proteinase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Cooke T. D., Jasin H. E. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. I. The role of antigen on the local immune response. Arthritis Rheum. 1972 Jul-Aug;15(4):327–337. doi: 10.1002/art.1780150402. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Blow A. M., Barrett A. J., Martin P. E. Proteoglycan-degrading enzymes. A radiochemical assay method and the detection of a new enzyme cathepsin F. Biochem J. 1977 Dec 1;167(3):775–785. doi: 10.1042/bj1670775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonds-Alt X., Quisquater E., Vaes G. Proteoglycan- and fibrin-degrading neutral proteinase activities of Lewis lung carcinoma cells. Eur J Cancer. 1980 Sep;16(9):1257–1261. doi: 10.1016/0014-2964(80)90186-3. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hauser P., Vaes G. Degradation of cartilage proteoglycans by a neutral proteinase secreted by rabbit bone-marrow macrophages in culture. Biochem J. 1978 May 15;172(2):275–284. doi: 10.1042/bj1720275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Huybrechts-Godin G., Hauser P., Vaes G. Macrophage-fibroblast interactions in collagenase production and cartilage degradation. Biochem J. 1979 Dec 15;184(3):643–650. doi: 10.1042/bj1840643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts-Godin G., Vaes G. Secretion of a latent neutral proteinase that degrades cartilage proteoglycans by skin and synovial fibroblasts in culture. FEBS Lett. 1978 Jul 15;91(2):242–245. doi: 10.1016/0014-5793(78)81182-x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Oronsky A. L., Perper R. J. Breakdown of noncollagenous chondromucoprotein matrix by leukocyte lysosome granule lysates from guinea pig, rabbit, and human. Clin Immunol Immunopathol. 1973 Nov;2(1):36–51. doi: 10.1016/0090-1229(73)90034-2. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Means G. E., Feeney R. E. Reductive alkylation of amino groups in proteins. Biochemistry. 1968 Jun;7(6):2192–2201. doi: 10.1021/bi00846a023. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Laidlaw J., Hascall V. C., Dziewiatkowski D. D. The effect of proteoglycans on the formation of fibrils from collagen solutions. Arch Biochem Biophys. 1975 Oct;170(2):698–709. doi: 10.1016/0003-9861(75)90167-8. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Sapolsky A. I., Woessner J. F., Jr, Howell D. S. A photometric assay for protease digestion of the proteoglycan subunit. Anal Biochem. 1975 Aug;67(2):649–654. doi: 10.1016/0003-2697(75)90339-5. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Binding and precipitation of soluble collagens by chick embryo cartilage proteoglycan. J Biol Chem. 1976 Feb 10;251(3):895–897. [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. The effect of chondroitin sulphate-protein on the formation of collagen fibrils in vitro. Biochem J. 1968 Oct;109(5):857–866. doi: 10.1042/bj1090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G., Eeckhout Y., Lenaers-Claeys G., François-Gillet C., Druetz J. E. The simultaneous release by bone explants in culture and the parallel activation of procollagenase and of a latent neutral proteinase that degrades cartilage proteoglycans and denatured collagen. Biochem J. 1978 May 15;172(2):261–274. doi: 10.1042/bj1720261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973 Mar 10;248(5):1634–1642. [PubMed] [Google Scholar]
- Woolley D. E., Evanson J. M. Effect of cartilage proteoglycans on human collagenase activities. Biochim Biophys Acta. 1977 Mar 29;497(1):144–150. doi: 10.1016/0304-4165(77)90147-7. [DOI] [PubMed] [Google Scholar]


