Abstract
Objective
Hyperglycemia in preterm infants is usually treated with adjustment of glucose intake and, if persistent, with continuous insulin infusion. However, hypoglycemia is a well-known complication of intravenous (iv) insulin treatment. The aim of this study was to evaluate the feasibility of continuous subcutaneous insulin infusion (CSII) in extremely preterm infants.
Methods
Clinical data from extremely premature infants (< 28 weeks of gestation) undergoing CSII treatment for severe hyperglycemia in the neonatal intensive care unit were included. Blood glucose levels during CSII, as well as the nutritional intake and insulin intake were recorded. Data were analyzed and compared to a control group of very preterm infants receiving iv insulin therapy.
Results
Normoglycemia rates were best in the iv insulin-cohort (n=22, 50.3%) compared to the CSII group (n=15, 15.6%). Hypoglycemia was very rare in both groups (0.4% vs. 0.0%). CSII therapy appears to require higher insulin doses compared to continuous iv therapy to achieve a similar effect. Subcutaneous Insulin therapy in extremely preterm infants is feasible, at least for prevention of hypoglycemia. However, dose control needs to be improved.
Conclusion
The results justify further model validation and clinical trial research to explore a model-based protocol and the use of CSII in this population.
Keywords: Continuous subcutaneous insulin infusion, extremely preterm infants, hyperglycemia
What is already known on this topic?
Hyperglycemia is common in preterm infants and can affect long-term outcomes, especially regarding the neurological outcome. However, there are no treatment guidelines and there are a lot of controversies. Insulin is mostly administered via continuous intravenous infusion in preterm infants. Continuous subcutaneous insulin infusion (CSII) in preterm infants is only described in a few case reports and clinical research papers.
What this study adds?
This study adds clinical expertise in the application of CSII in extremely preterm infants. The comparison of CSII and intravenously given Insulin in preterm infants allows to draw conclusions on the feasibility and dose control of CSII in preterm infants. To our knowledge it is the largest, described cohort of extremely preterm infants receiving CSII.
Introduction
In terms of long-term neurocognitive development, prevention of hypo- and hyper-glycemia plays a major role in the care of premature infants (PI). Persistent hyperglycemia occurs mostly in very low birth weight infants (VLBW) in the first days to weeks of life (1, 2). There is a negative correlation between gestational age (GA), birth weight, and the occurrence of hyperglycemic episodes (1). In this respect, an isolated blood glucose (BG) level >10 mmol/L within the first 28 days of life in VLBW is associated with a more than two-fold increase in 28-day mortality (3). Furthermore, hyperglycemia within the first 24 hours of life is associated with reduction in brain white matter structure on magnetic resonance imaging (4).
Thresholds for managing hyperglycemia vary considerably across clinical settings (5, 6, 7, 8, 9). Due to varying definitions and methodological differences for BG level assessment, the incidence of hyperglycemia in studies varies between 40-80% (1, 3, 10, 11). The prevalence of hyperglycemia is highest at the end of the second week of life with approximately 30% of preterm infants below 1500 g presenting with BG levels >10 mmol/L (10, 11). Management of hyperglycemia starts with the adjustment of glucose intake. with the clinical aim of a reduction to a basic requirement of 5-6 g/kg/day. Insulin treatment has been introduced using continuous intravenous (iv) infusion (3, 12, 13, 14, 15). However, increased catheter-associated infections have been described with iv treatment (2). In addition, the use of iv insulin infusion involves the fear of a resulting, iatrogenic hypoglycemia. Although continuous subcutaneous insulin infusion (CSII) therapy is regarded as a standard treatment of diabetes management in the pediatric population, very few data exist for using CSII in neonatal hyperglycemia. Available data are limited to a few studies in connection with neonatal diabetes mellitus (16, 17), case reports, or “close loop“ monitoring in neonates (6, 7, 8). Hence, there is a gap in knowledge, which might offer benefit.
In the neonatal intensive care unit (NICU) of the University of Oldenburg, CSII in the management of hyperglycemia of extremely preterm infants was introduced as part of standard care since 2015. The aim of this study was to review the management of hyperglycemia following a standard CSII protocol in view of feasibility and safety. Data are compared with a cohort of preterm infants, treated for hyperglycemia within the first weeks of life using iv insulin from the NICU in Christchurch Hospital, New Zealand.
Methods
In a retrospective multi-center observational study 15 extremely preterm infant, receiving CSII treatment for severe hyperglycemia in the first weeks of life during the period 01/01/2015 to 01/04/2021, were identified. All infants were inborn patients treated at the level 3 NICU of the University of Oldenburg. Data on patient characteristics, BG test results, insulin medication, enteral nutritional intake, administration of parenteral iv infusion and individual drug medication were collected from patient records. Enteral glucose was supplied either as breast milk or preterm formula. For breast milk, a carbohydrate content of 70 mg per liter was estimated. The carbohydrate content of preterm formula was calculated according to the manufacturer’s instructions.
All BG measurements were performed by rapid Accu-Chek BG test (Roche Diabetes Care Inc., Indianapolis, Indiana, USA). The definition of hyper- and hypo-glycemia, as well as the therapeutic intervention in the management of transient hyperglycemia, followed the local NICU guideline: Hypoglycemia, <4 mmol/L; life-threatening hypoglycemia, <2.6 mmol/L; normoglycemia, 4-10 mmol/L; and hyperglycemia, >10 mmol/L. For BG values >16.65 mmol/L repeat measurement was performed within 12 hours, reduction of parenteral glucose infusion rate in 1-1.5 g/kg/day steps to a minimum of 5-6 mg/kg/hour was performed initially. The indication for continuous insulin therapy was when BG values >16.65 mmol/L persisted over a period of 12 hours, despite adjustment of the iv glucose rate to a minimum of 5-6 g/kg/d. According to our guideline, the initial dosing of CSII was 0.01-0.05 IU/kg/hour, increased in small increments to a maximum rate of 0.1 IU/kg/hour, depending on measured BG values. Insulin dosing was re-evaluated and modified at the bedside with each 3-hour BG measurement. A BG level between 8.3-11.2 mmol/L (150-200 mg/dL) was the clinical goal for the BG range during CSII.
All preterm infants used an Accu-Chek Combo insulin pump (Roche Diabetes Care Inc., Indianapolis, Indiana, USA) with rapid-acting (2-5 hour action duration) Humalog insulin (Eli Lilly Co., Indianapolis, Indiana, USA). The insulin pumps were not designed for use in preterm infants, so the standard insulin concentration of 100 IU/mL would have limited delivery in PI. Therefore, the insulin was diluted 1/10 with “Sterile Diluent for Humalog U-100” to achieve a concentration of 10 IU/mL. The subcutaneous needle was placed in the thigh of the patients and was routinely changed every 48-72 hours, following guidelines. The insulin in the pump was routinely changed every seven days.
Results were compared with a retrospective, iv insulin-treated cohort of preterm infants, consisting of 22 VPI at the NICU in Christchurch, New Zealand between 2005-2009. Analogous to the procedure in Oldenburg, a reduction in glucose intake was carried out in the corresponding study period in the case of persistent hyperglycemia. With two values above 10 mmol/L, intravenous insulin therapy with 0.05 IU/kg/hour was started. In the course, a fixed adjustment was made depending on the BG value.
Ethics approval to conduct this study was obtained from the Medical Ethics Committee of the University of Oldenburg (no: 2021-024, date: 11.02.2021).
Statistical Analysis
For statistical analysis, descriptive tests were used. The distribution of values was non-Gaussian. Statistical analyses were processed using Statistical Package for the Social Sciences statistical software, version 26.0 for Mac (IBM Inc., Armonk, NY, USA).
Results
Patient characteristics are presented in Table 1. The SCII group (n=15) of VPI presented with a median birth-weight of 620 [interquartile range (IQR): 560-700] g and GA of 24 (24+0 - 24+6) weeks. CSII was initiated at a median of 77 (61-141) hours of life. The median duration of insulin therapy using CSII was 191 (71-244) hours in the cohort. A total of 2.736 hours of insulin therapy by CSII and 803 glucose readings were included. No major complications requiring treatment occurred. Local redness at needle insertion site was observed twice, and both healed spontaneously.
Table 1. Cohorts and demographic data for the CSII (Oldenburg, Germany) cohort and the iv insulin treated cohort (Christchurch, New Zealand). Data are shown as median value and (range) or (IQR) as shown.
|
CSII cohort (Oldenburg) |
iv cohort (Christchurch) |
|
|
n patients (n male) |
15 (8) |
22 (7) |
|
Total hours of treatment |
2736 |
2946 |
|
Amount of BG measurements |
803 |
902 |
|
Duration of insulin application, hours [IQR] |
191 [71-249] |
86 [32.5-184] |
|
Gestational age*, weeks [IQR] |
24 [24-24] |
27 [26-27] |
|
Body weight*, gram [IQR] |
620 [570-735] |
840 [800-900] |
|
Postnatal age**, days [IQR] |
3 [2-5] |
3 [1-7] |
|
BG measurement interval, hours [IQR] |
3.3 [2.9-3.9] |
3.4 [2.8-3.9] |
|
Insulin rates, IU/kg/h [IQR] |
0.02 [0.02-0.05] |
0.03 [0.02-0.05] |
|
Total glucose input, mg/kg/min [IQR] |
8.0 [6.8-9.6] |
8.5 [5.5-9.2] |
|
BG median [IQR] |
11.2 [9.9-12.5] |
7.9 [6.6-9.2] |
|
Median % BG between 4.0-8.0 mmol/L [IQR] (mean) |
15.6 [5.6-21.1] (13.2) |
50.3 [42.1-66.3] (50.9) |
|
Median % BG >10 mmol/L [IQR] (mean) |
62.8 [50.0-77.5] (65.0) |
8.1 [4.9-21.4] (17.2) |
|
Median % BG <4.0 mmol/L [IQR] (mean) |
0.0 [0.0-1.3] (0.5) |
0.4 [0.0-3.0] (2.1) |
|
Median % BG <2.6 mmol/L [IQR] (mean) |
0.0 [0.0-0.0] (0.1) |
0.0 [0.0-0.0] (0.1) |
|
Number of patients with BG level <2.6 mmol/L |
1 |
1 |
*At birth.
**At the start of insulin treatment.
IQR: interquartile range, CSII: continuous subcutaneous insulin infusion, iv: intravenous, BG: blood glucose
The iv-cohort was composed of 22 preterm infants (7 male, 31.8%) who received continuous iv insulin in the NICU at the Christchurch Women’s Hospital. Median GA was 27 (26-27) weeks with a median birth weight of 840 (800-900) g. Median duration of therapy was 86 (32.5-184) hours. Table 1 depicts patient and therapeutic characteristics of both cohorts.
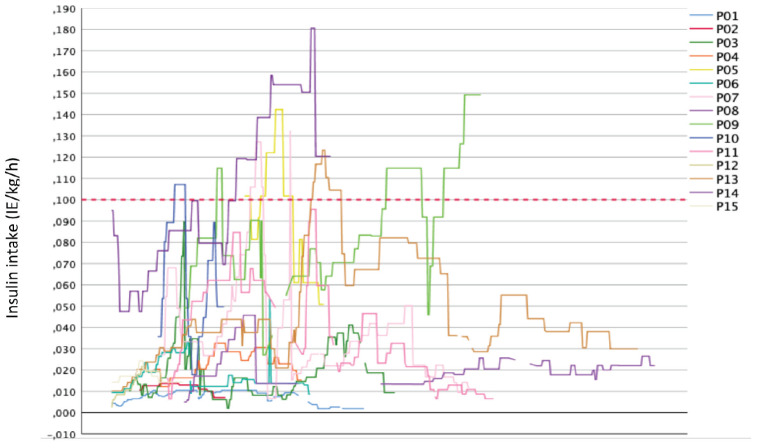
Table 1 lists key delivery and outcome glycemia results for both cohorts. For the Oldenburg CSII cohort, the starting dose used was a minimum of 0.002 IU and a maximum of 0.102 IU/h/kg, with a median of 0.014 (0.036-0.010) IU/h/kg. The minimum insulin intake in this cohort ranged from 0.002 to 0.051 IU/h/kg, spanning 0.049 IU/h/kg body weight. The maximum insulin dose ranged from 0.011 to 0.181 IU/h/kg. A total of 40% (6/15 VPI) received insulin doses above the maximum recommended dose of 0.1 IU/h/kg (Figure 1), where all infants had highly variable administration rates. The interquartile range of insulin intake of the 15 VPI of 0.02-0.05 IU/kg/h illustrates a high variability of the insulin dose used around the median of 0.02 IU/h/kg. In 3 (20%) the starting dose was below the recommended minimum of 0.01 IU/h/kg at baseline. In contrast, 6 (40%) VPI received an insulin supply above the recommended maximum of 0.1 IU/h/kg. The median insulin intake for the entire duration of therapy was below the possible recommended maximum starting dose of 0.05 IU/h/kg in 11/15 (73.3%) preterm infants.
Figure 1.
Insulin intake of VPI (n=15) suffering from persistent hyperglycemia
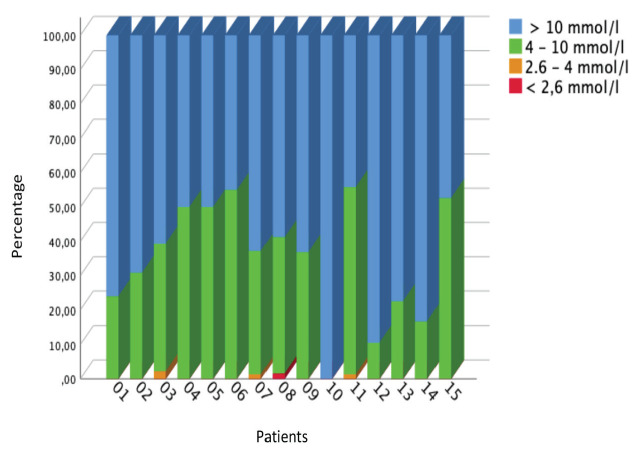
The 22 patients in the comparison group receiving iv insulin as a continuous insulin infusion had a median insulin dose of 0.03 IU/h/kg (Table 1). Insulin delivery rates had a higher median value, but similar range and IQR. Glucose administration was similar between the two cohorts. The evaluation of BG using CSII was based on the percentage of measured values in a defined range for normo-, hypo- and hyper-glycemia. Only values measured during CSII insulin administration (>0.0 IU/kg/h) were analyzed. The percentages refer to the number of glucose readings of the individual n=15 patients. Figure 2 shows the percentages of individual patients results. In 10/15 (66.7%) infants of the CSII cohort, contrary to expectations, the greater proportion of BG readings (58.8-100%) were above the defined reference range. In 5 (33%) of this cohort, 50% to a maximum of 55% of the BG readings were within the reference range. The maximum proportion of normoglycemia for each preterm infant considered individually was not very high, at 55%. This outcome may be clarified when considering all 803 glucose readings. Overall, across the CSII cohort, only 34.5% were between 4.0-10.0 mmol/L and only 13.2% between 4.0-8.0 mmol/L. Results and time in the normoglycemic range were relatively much higher for the iv insulin treated cohort (Table 1).
Figure 2.
Percentage of glucose measured in plasma for each of the n=15 preterm infants
Discussion
Severe hyperglycemia in extremely preterm infants is associated with numerous comorbidities, which may persist into adulthood (4, 8, 18, 19). This highlights the need for adequate therapy, which should compensate for the metabolic instability and/or functional insufficiency of compensating mechanisms during the first weeks of life of extremely preterm infants (1, 2, 3, 11). The causes of dysregulation of glucose metabolism in preterm infants are diverse and include inadequate insulin production, low glycogen stores, and possible insulin resistance (7). In addition, treatment of hyperglycemia with continuous iv insulin is difficult and requires close monitoring to avoid associated hypoglycemia. Hyperglycemia has been shown as a first sign of cerebral intraventricular hemorrhage and at the same time being attributed to its development (20). As early as 1986, Ostertag et al. (21) provided evidence that extremely preterm infants may benefit from insulin pump therapy. Previous findings provide evidence for lower glucose fluctuations due to insulin pump therapy with continuous glucose measurement, compared to continuous intravenous insulin administration (6, 8).
In the present study, 15 extremely preterm infants who received insulin subcutaneously via an insulin pump were studied. During the therapy period of more than 2.700 hours, no local or systemic infections requiring treatment were observed. Furthermore, only one episode of severe hypoglycemia was observed in more than 800 BG measurements. Thus, the therapeutic use of subcutaneous insulin therapy in VPI can be considered safe. However, in this study no CGM was used and thus hypoglycemia might have gone undetected.
The preterm infants in the CSII cohort and a comparator iv insulin cohort received similar insulin administration rates (CSII: 0.02 IU/h/kg; iv insulin: 0.03 IU/h/kg). However, the iv insulin cohort had a significantly higher proportion of normoglycemic readings. Thus, the glycemic control of the CSII cohort appears inadequate at similar insulin rates. However, the birth weight, as well as the GA of the CSII cohort was lower than the iv cohort. The results suggest the different kinetics of iv insulin versus subcutaneous insulin therapy, and particularly the potential for subcutaneous insulin losses, which may explain the differences. Hence, the results suggest CSII in these cohorts may require a higher insulin dose, especially at start of the treatment, compared to iv insulin.
In the cohort studied, the median starting CSII insulin dose was within the in-house recommended range of 0.01-0.05 IU/h/kg. However, there was marked variability. The median value also corresponds to the dosage of insulin used in previous studies (9, 22). Compared with the cohort of continuous intravenous insulin delivery, the insulin rate of the CSII cohort was lower, even though possible insulin losses may reduce its impact. This difference may also be attributed to differences in protocol between the units and a different level of acceptance regarding safe insulin dosing levels. The “hesitant” use of insulin contrasts with the high BG values before the start of therapy and the high proportion of hyperglycemia during therapy. The reason for this may be the risk associated with hypoglycemia and a desire to avoid this, which is certainly of high priority for preterm infants. However, to achieve continuous normoglycemia, adequate insulin dosing is essential. Moreover, persistent hyperglycemia (>180 mg/dL or 10 mmol/L) is also associated with worse outcome in preterm infants (13, 23). However, the lack of treatment recommendations when using CSII makes adequate glycemic control difficult. Avoiding hyperglycemia by means of adequate insulin delivery should be seen as important as avoiding hypoglycemia.
In the 15 preterm infants studied, adequate glycemic control could not be achieved using a CSII with the insulin rates used. Overall, this study is a comparison of cohorts with differences in sample number, GA, and birth weight. Nevertheless, descriptive comparisons can be made because the number of glucose measurements and the total duration of insulin administration are similar. The importance of adequate insulin dosing is evident when considering the high proportion of hyperglycemia in the cohort studied. Severe hyperglycemia is associated with worse outcome in preterm infants (23). For example, Kao et al. (23) demonstrated a significant association between hyperglycemia (mean 7-day glucose >180 mg/dL or 10 mmol/L) and the occurrence of necrotizing enterocolitis II°-III°. In addition, hyperglycemia >8 mmol/L in extremely preterm infants appears to be associated with delayed motor development and lower intelligence quotients at 6.5 years of age. Insulin therapy, on the other hand, appears to have no effect on either outcome (24). Current data suggest model-based insulin administration has the potential to improve therapy management. STAR-GRYPHON is a metabolic model that already improves the control of continuous iv insulin therapy, considering factors such as enteral and parenteral glucose intake, weight and age. In the NICU in Christchurch, New Zealand, it has been used in clinical practice for some time (25). In a recent study, Zhou et al. (26) demonstrated model-based subcutaneous insulin therapy may allow for better control to achieve the goal of normoglycemia more rapidly and persistently.
Study Limitations
The small number of cases in the study limits the conclusions that can be drawn from this analysis. Furthermore, the iv insulin cohort comparator is not randomized nor matched and was born around ten years before the CSII Group. In the last ten years there have been many changes in the practice of neonatology, which might also affect the outcome. The CSII cohort received insulin in a neonatal center with different in-hospital standards and protocols. The iv treatment protocol had, for example, a lower treatment threshold, which has to be considered when looking at the results. In addition, the preterm infants in the iv insulin treated cohort had a higher median GA and birth weight and a higher case load. Due to the small number of cases the calculation of p values to compare continuous non-parametric groups of values was waived. However, the differences and similarities in glycemic outcomes allow conclusions to be drawn on the safety and potential efficacy of CSII in these cohorts and the need to better account for differences in insulin kinetics between delivery routes.
Conclusion
Overall, the comparison of the two cohorts allows an indication of inadequate glycemic control and insulin rates in the CSII cohort. This study shows CSII in extremely preterm infants is feasible but, compared with the retrospective iv insulin treated cohort, the current insulin regime leads to insufficient control of hyperglycemia. In terms of hypoglycemia, as well as local infections, CSII in extremely preterm infants appears quite safe. However, in view of different kinetics compared to iv therapy, there is still considerable potential for improvement in dosing. CSII required higher dosing of insulin compared to iv administered insulin. Ideally, the mode of administration should be model-based to best account for inter- and intra-patient variability in kinetics and dynamics of insulin action. Randomized studies with an adequate number of cases are necessary once safe, effective treatment protocols have been established.
Ethics
Ethics Committee Approval: Ethics approval to conduct this study was obtained from the Medical Ethics Committee of the University of Oldenburg (no: 2021-024, date: 11.02.2021).
Informed Consent: Retrospective study.
Footnotes
Authorship Contributions
Concept: Axel Heep, Design: Axel Heep, Matthias Lange, Data Collection or Processing: Merle Böettger, Tony Zhou, Analysis or Interpretation: Merle Böettger, Jennifer Knopp, J. Geoffrey Chase, Axel Heep, Michael von Vangerow, Eva Cloppenburg, Matthias Lange, Literature Search: Merle Böettger, Writing: Merle Böettger, J. Geoffrey Chase, Axel Heep, Matthias Lange.
Conflict of interest: None declared.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, vanWeissenbruch M, Midgley P, Thompson M, Thio M, Cornette L, Ossuetta I, Iglesias I, Theyskens C, de Jong M, Gill B, Ahluwalia JS, de Zegher F, Dunger DB. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. J Pediatr. 2010;157(5):715–719. doi: 10.1016/j.jpeds.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Ditzenberger GR, Collins SD, Binder N. Continuous insulin intravenous infusion therapy for VLBW infants. J Perinat Neonatal Nurs. 1999;13(3):70–82. doi: 10.1097/00005237-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Zamir I, Tornevi A, Abrahamsson T, Ahlsson F, Engström E, Hallberg B, Hansen-Pupp I, Sjöström ES, Domellöf M. Hyperglycemia in Extremely Preterm Infants-Insulin Treatment, Mortality and Nutrient Intakes. J Pediatr. 2018;200:104–110. doi: 10.1016/j.jpeds.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrou G, Skiöld B, Karlén J, Tessma MK, Norman M, Adén U, Vanpée M. Early hyperglycemia is a risk factor for death and white matter reduction in preterm infants. Pediatrics. 2010;125(3):584–591. doi: 10.1542/peds.2009-0449. [DOI] [PubMed] [Google Scholar]
- 5.Segerer H, Bührer C, Kapellen T, Mattern E, Ramsauer B, Somville T, Trotter A. Betreuung von Neugeborenen diabetischer Mütter. Leitlinie der GNPI, DGPM, DDG, DGHWi, dem DHV, der DGKJ und DGGG. (2k-Level, AWMF-Leitlinien-Register Nr. 024/006, Juli 2017). Z Geburtshilfe Neonatol. 2018;222(3):107–114. doi: 10.1055/a-0628-0873. [DOI] [PubMed] [Google Scholar]
- 6.Beardsall K, Thomson L, Elleri D, Dunger DB, Hovorka R. Feasibility of automated insulin delivery guided by continuous glucose monitoring in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2020;105(3):279–284. doi: 10.1136/archdischild-2019-316871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogilvy-Stuart AL, Beardsall K. Management of hyperglycaemia in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):126–131. doi: 10.1136/adc.2008.154716. [DOI] [PubMed] [Google Scholar]
- 8.Beardsall K, Ogilvy-Stuart AL, Ahluwalia J, Thompson M, Dunger DB. The continuous glucose monitoring sensor in neonatal intensive care. Arch Dis Child Fetal Neonatal Ed. 2005;90(4):307–310. doi: 10.1136/adc.2004.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Lugt NM, Smits-Wintjens VE, van Zwieten PH, Walther FJ. Short and long term outcome of neonatal hyperglycemia in very preterm infants: a retrospective follow-up study. BMC Pediatr. 2010;10(1):52. doi: 10.1186/1471-2431-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymońska I, Jagła M, Starzec K, Hrnciar K, Kwinta P. The Incidence Of Hyperglycaemia In Very Low Birth Weight Preterm Newborns. Results of A Continuous Glucose Monitoring Study--Preliminary Report. Dev Period Med. 2015;19(3 Pt 1):305–312. [PubMed] [Google Scholar]
- 11.Fernández-Martínez MDM, Gómez-Llorente JL, Momblán-Cabo J, Martin-González M, Calvo-Bonachera M, Olvera-Porcel M, Bonillo-Perales A. Monitoring the incidence, duration and distribution of hyperglycaemia in very-low-birth-weight newborns and identifying associated factors. J Perinat Med. 2020;48(6):631–637. doi: 10.1515/jpm-2020-0074. [DOI] [PubMed] [Google Scholar]
- 12.Ng SM, May JE, Emmerson AJ. Continuous insulin infusion in hyperglycaemic extremely-low- birth-weight neonates. Biol Neonate. 2005;87(4):269–272. doi: 10.1159/000083863. [DOI] [PubMed] [Google Scholar]
- 13.Alsweiler JM, Harding JE, Bloomfield FH. Tight glycemic control with insulin in hyperglycemic preterm babies: a randomized controlled trial. Pediatrics. 2012;129(4):639–647. doi: 10.1542/peds.2011-2470. [DOI] [PubMed] [Google Scholar]
- 14.Hewson M, Nawadra V, Oliver J, Odgers C, Plummer J, Simmer K. Insulin infusions in the neonatal unit: delivery variation due to adsorption. J Paediatr Child Health. 2000;36(3):216–220. doi: 10.1046/j.1440-1754.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson D, Mc Clure G. Continuous Insulin Infusion is Safe and Efficacious in the Hyperglycemic ELBW Infant: Results of a RCT † 1587. Pediatr Res. 1999;43(Suppl 4):271. [Google Scholar]
- 16.Wintergerst KA, Hargadon S, Hsiang HY. Continuous subcutaneous insulin infusion in neonatal diabetes mellitus. Pediatr Diabetes. 2004;5(4):202–206. doi: 10.1111/j.1399-543X.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 17.Beardsall K, Pesterfield CL, Acerini CL. Neonatal diabetes and insulin pump therapy. Arch Dis Child Fetal Neonatal Ed. 2011;96(3):223–224. doi: 10.1136/adc.2010.196709. [DOI] [PubMed] [Google Scholar]
- 18.Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E, Embleton N, Hewer O, Johnson S, Juszczak E, Leaf A, Linsell L, McCormick K, McGuire W, Omar O, Partlett C, Patel M, Roberts T, Stenson B, Townend J, SIFT Investigators Group. Controlled Trial of Two Incremental Milk-Feeding Rates in Preterm Infants. N Engl J Med. 2019;381(15):1434–1443. doi: 10.1056/NEJMoa1816654. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen ME, Brown SJ, Satrom KM, Scheurer JM, Ramel SE, Rao RB. Long-Term Outcomes after Early Neonatal Hyperglycemia in VLBW Infants: A Systematic Review. Neonatology. 2021;118(5):509–521. doi: 10.1159/000517951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldas JP, Braghini CA, Mazzola TN, Vilela MM, Marba ST. Peri-intraventricular hemorrhage and oxidative and inflammatory stress markers in very-low birth weight newborns. J Pediatr (Rio J) 2015;91(4):373–379. doi: 10.1016/j.jped.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Ostertag SG, Jovanovic L, Lewis B, Auld PA. Insulin pump therapy in the very low birth weight infant. Pediatrics. 1986;78(4):625–630. doi: 10.1542/peds.78.4.625. [DOI] [PubMed] [Google Scholar]
- 22.Meetze W, Bowsher R, Compton J, Moorehead H. Hyperglycemia in extremely- low-birth-weight infants. Biol Neonate. 1998;74(3):214–221. doi: 10.1159/000014027. [DOI] [PubMed] [Google Scholar]
- 23.Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;26(12):730–736. doi: 10.1038/sj.jp.7211593. [DOI] [PubMed] [Google Scholar]
- 24.Zamir I, Stoltz Sjöström E, Ahlsson F, Hansen-Pupp I, Serenius F, Domellöf M. Neonatal hyperglycaemia is associated with worse neurodevelopmental outcomes in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2021;106(5):460–466. doi: 10.1136/archdischild-2020-319926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knopp Nee Dickson JL, Lynn AM, Shaw GM, Chase JG. Safe and effective glycaemic control in premature infants: observational clinical results from the computerised STAR-GRYPHON protocol. Arch Dis Child Fetal Neonatal Ed. 2019;104(2):205–211. doi: 10.1136/archdischild-2017-314072. [DOI] [PubMed] [Google Scholar]
- 26.Zhou T, Boettger M, Knopp JL, Lange M, Heep A, Chase JG. Model-based subcutaneous insulin for glycemic control of pre-term infants in the neonatal intensive care unit. Comput Biol Med. 2023;160:106808. doi: 10.1016/j.compbiomed.2023.106808. [DOI] [PubMed] [Google Scholar]