Abstract
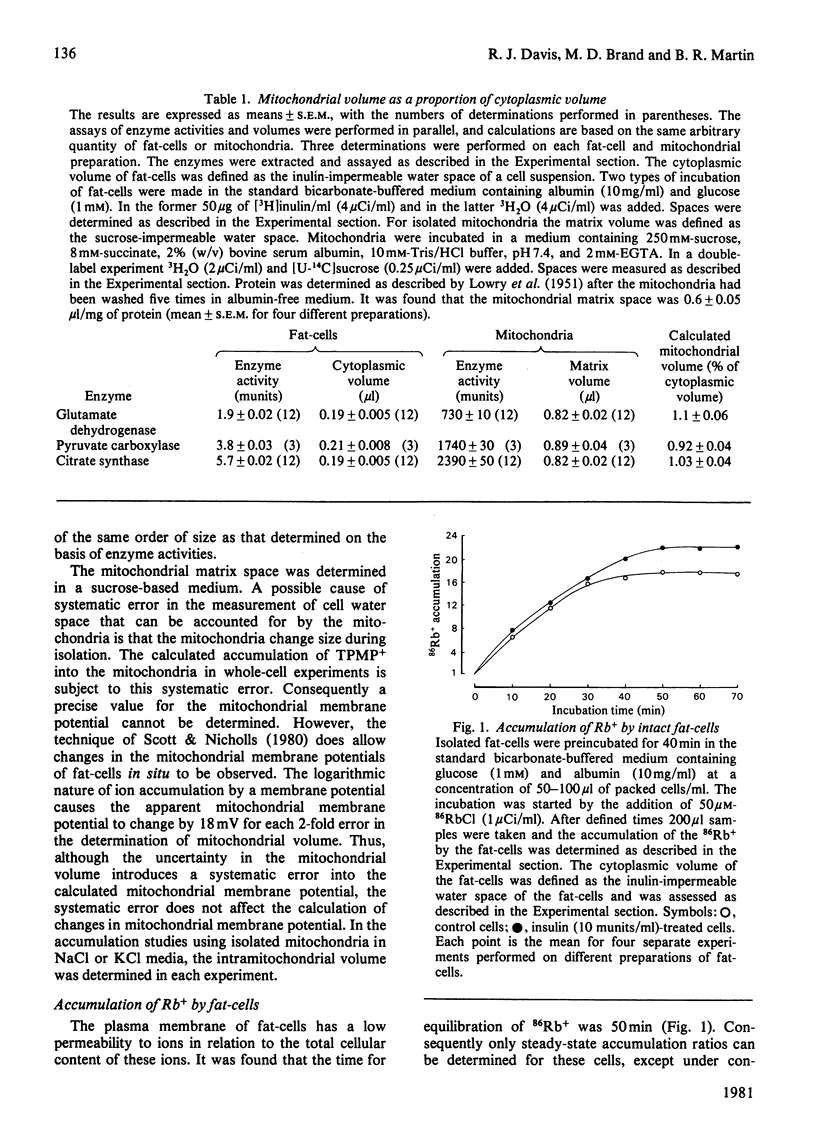
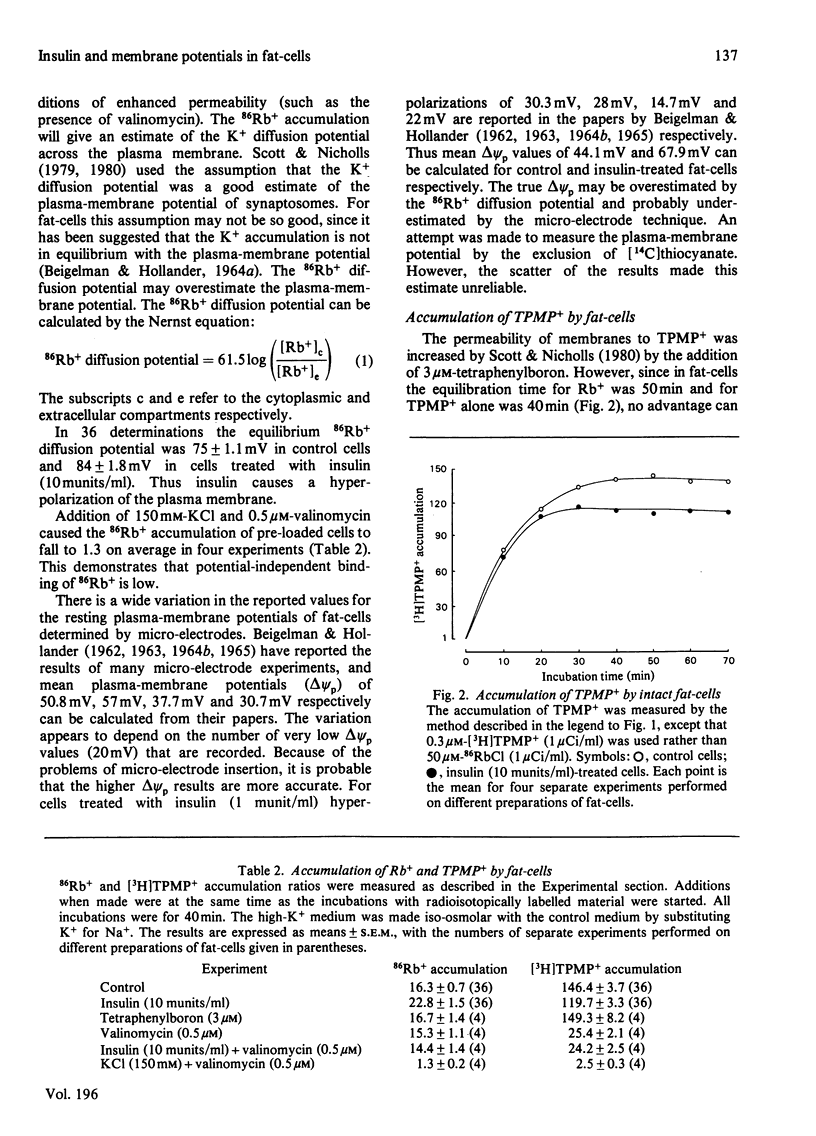
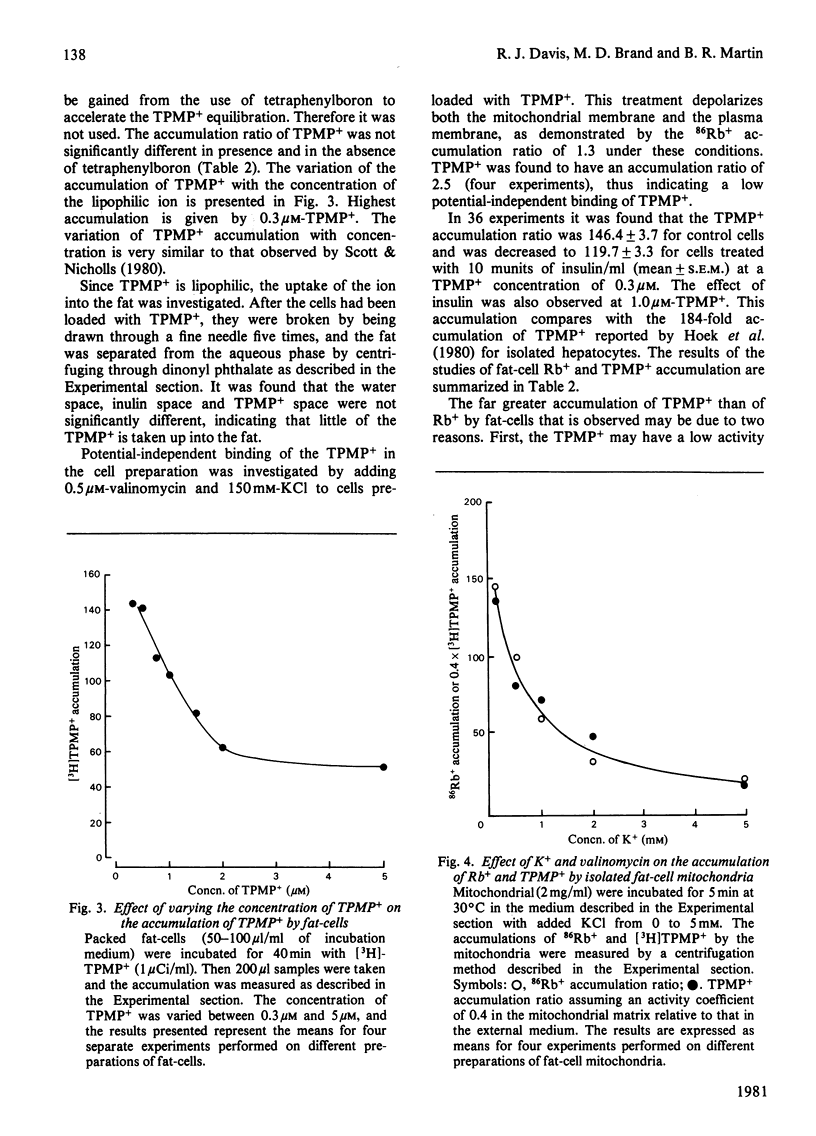
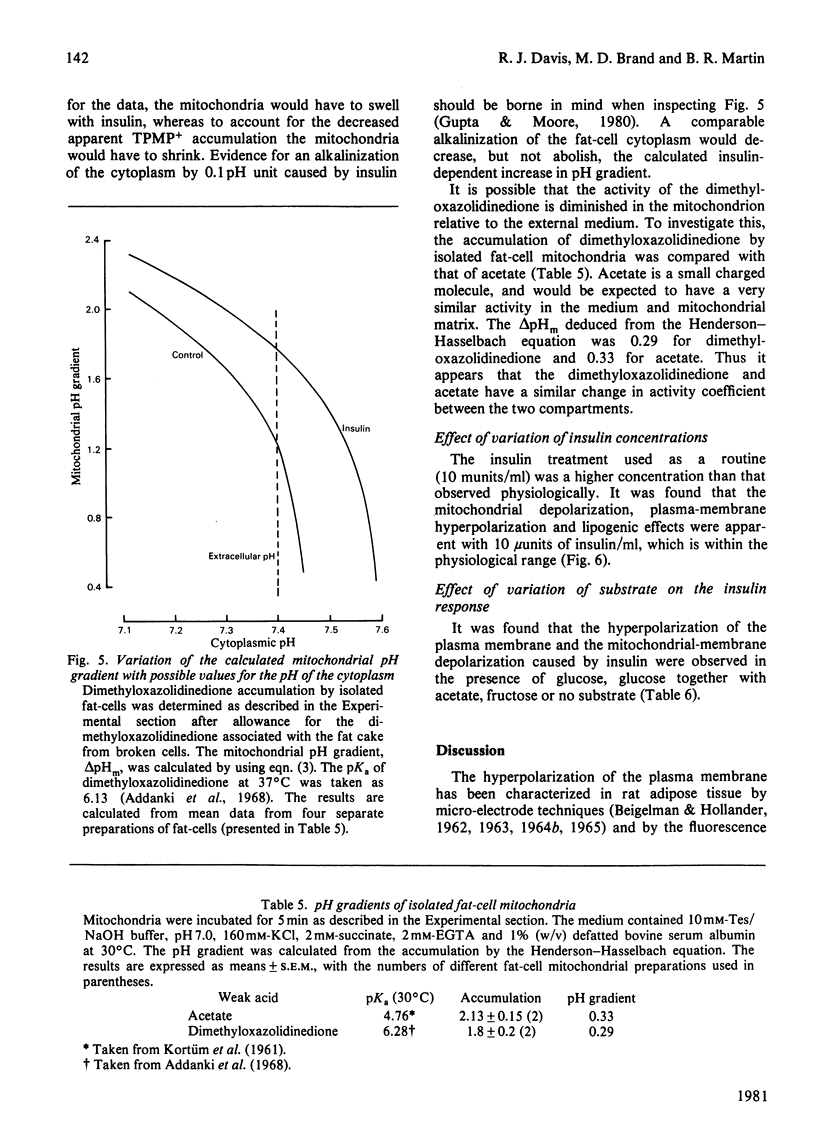
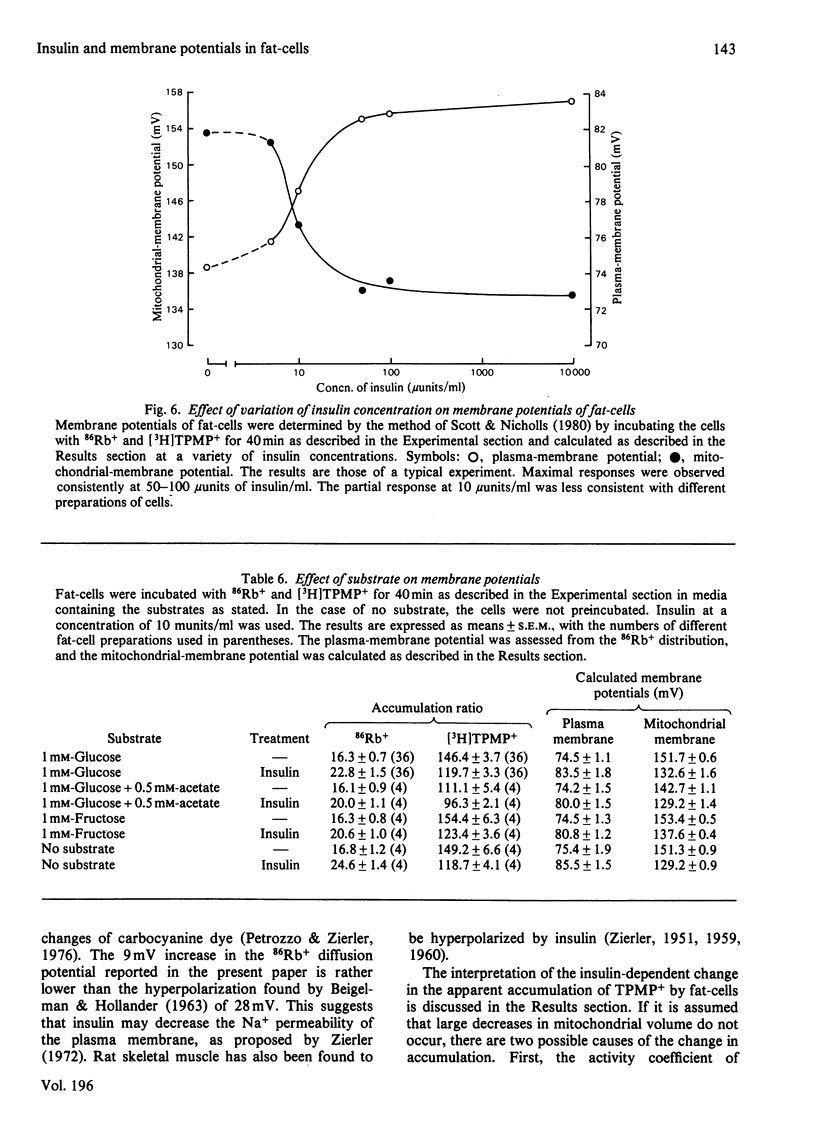
1. A recently developed technique for the measurement of plasma-membrane and mitochondrial-membrane potentials in intact cells by using the distribution of 86Rb+ and [3H]methyltriphenylphosphonium+ has enabled us to characterize a novel insulin effect on fat-cell mitochondria. For control cells the plasma-membrane and mitochondrial-membrane potentials were 75 mV and 152 mV respectively. Insulin (10 mu units/ml) caused a 9 mV hyperpolarization of the plasma membrane and a 19 mV depolarization of the mitochondrial membrane. 2. The insulin-dependent mitochondrial depolarization was observed at physiological insulin concentrations (10 mu units/ml) and was apparent when the cells metabolized a wide variety of substrates. 3. Evidence from the uptake of the weak acid 5,5-dimethyloxazolidine-2,4-dione by fat-cells was interpreted as indicating that the mitochondrial pH gradient was increased by insulin. 4. Insulin alters the balance between the electrical and pH-gradient components that form the mitochondrial protonmotive force. A model is proposed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. EFFECTS OF ELECTROLYTES UPON ADIPOSE TISSUE MEMBRANE ELECTRICAL POTENTIALS. Proc Soc Exp Biol Med. 1964 Jan;115:14–16. doi: 10.3181/00379727-115-28816. [DOI] [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. EFFECTS OF HORMONES UPON ADIPOSE TISSUE MEMBRANE ELECTRICAL POTENTIALS. Proc Soc Exp Biol Med. 1964 May;116:31–35. doi: 10.3181/00379727-116-29149. [DOI] [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. Effect of insulin and rat weight upon rat adipose tissue membrane resting electrical potential (REP). Diabetes. 1963 May-Jun;12:262–267. doi: 10.2337/diab.12.3.262. [DOI] [PubMed] [Google Scholar]
- BEIGELMAN P. M., HOLLANDER P. B. Effect of insulin upon resting electrical potential of adipose tissue. Proc Soc Exp Biol Med. 1962 Jul;110:590–595. doi: 10.3181/00379727-110-27588. [DOI] [PubMed] [Google Scholar]
- Beigelman P. M., Hollander P. B. Effect of insulin and insulin antibody upon rat adipose tissue membrane resting electrical potential (REP). Acta Endocrinol (Copenh) 1965 Dec;50(4):648–656. doi: 10.1530/acta.0.0500648. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Clausen T., Elbrink J., Dahl-Hansen A. B. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. IX. The role of cellular calcium in the activation of the glucose transport system in rat soleus muscle. Biochim Biophys Acta. 1975 Jan 28;375(2):292–308. doi: 10.1016/0005-2736(75)90197-2. [DOI] [PubMed] [Google Scholar]
- Clausen T., Elbrink J., Martin B. R. Insulin controlling calcium distribution in muscle and fat cells. Acta Endocrinol Suppl (Copenh) 1974;191:137–143. doi: 10.1530/acta.0.077s0137. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Del Boca J., Flatt J. P. Fatty acid synthesis from glucose and acetate and the control of lipogenesis in adipose tissue. Eur J Biochem. 1969 Nov;11(1):127–134. doi: 10.1111/j.1432-1033.1969.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Coore H. G., Martin B. R., Randle P. J. Insulin activates pyruvate dehydrogenase in rat epididymal adipose tissue. Nat New Biol. 1971 May 26;231(21):115–116. doi: 10.1038/newbio231115a0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Erecińska M., Werrlein R., Silver I. A. Cellular energy metabolism, trans-plasma and trans-mitochondrial membrane potentials, and pH gradients in mouse neuroblastoma. Proc Natl Acad Sci U S A. 1979 May;76(5):2175–2179. doi: 10.1073/pnas.76.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):389–398. doi: 10.1042/bj1720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- JUNGAS R. L., BALL E. G. Studies on the metabolism of adipose tissue. XII. The effects of insulin and epinephrine on free fatty acid and glycerol production in the presence and absence of glucose. Biochemistry. 1963 Mar-Apr;2:383–388. doi: 10.1021/bi00902a035. [DOI] [PubMed] [Google Scholar]
- Jacobs B. O., Krahl M. E. The effects of divalent cations and insulin on protein synthesis in adipose cells. Biochim Biophys Acta. 1973 Sep 7;319(3):410–415. doi: 10.1016/0005-2787(73)90181-0. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Clarke P., Vydelingum N., Hope-Gill H., Tulloch B., Fraser T. R. The role of calcium in insulin action. III. Calcium distribution in fat cells; its kinetics and the effects of adrenaline, insulin and procaine-HCl. Eur J Clin Invest. 1975 Jul 29;5(4):339–349. doi: 10.1111/j.1365-2362.1975.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Vydelingum N., Tulloch B. R., Hope-Gill H., Fraser T. R. The role of calcium in insulin action. I. Purification and properties of enzymes regulating lipolysis in human adipose tissue: effects of cyclic-AMP and calcium ions. Horm Metab Res. 1974 Jul;6(4):247–255. doi: 10.1055/s-0028-1093861. [DOI] [PubMed] [Google Scholar]
- Letarte J., Renold A. E. Ionic effects on glucose transport and metabolism by isolated mouse fat cells incubated with or without insulin. I. Lack of effect of medium Ca2+, Mg2+ or PO4 3(negative). Biochim Biophys Acta. 1969 Jul 15;183(2):350–356. doi: 10.1016/0005-2736(69)90091-1. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. M., Bruns D. E., Jarett L. Ability of insulin to increase calcium uptake by adipocyte endoplasmic reticulum. J Biol Chem. 1978 May 25;253(10):3504–3508. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Elevation of intracellular pH by insulin in frog skeletal muscle. Biochem Biophys Res Commun. 1979 Dec 14;91(3):900–904. doi: 10.1016/0006-291x(79)91964-8. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980 Mar 10;111(2):261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. Direct addition of insulin inhibits a high affinity Ca2+-ATPase in isolated adipocyte plasma membranes. Nature. 1979 Oct 11;281(5731):495–497. doi: 10.1038/281495a0. [DOI] [PubMed] [Google Scholar]
- Puskin J. S., Gunter T. E., Gunter K. K., Russell P. R. Evidence for more than one Ca2+ transport mechanism in mitochondria. Biochemistry. 1976 Aug 24;15(17):3834–3842. doi: 10.1021/bi00662a029. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. Electric charge stoichiometry of calcium translocation in mitochondria. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1273–1279. doi: 10.1016/s0006-291x(77)80117-4. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974 Nov 5;13(23):4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- Schweitzer E. S., Blaustein M. P. Calcium buffering in presynaptic nerve terminals. Free calcium levels measured with arsenazo III. Biochim Biophys Acta. 1980 Aug 14;600(3):912–921. doi: 10.1016/0005-2736(80)90493-9. [DOI] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980 Jan 15;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. The estimation of the electrical potential across the inner membrane of mitochondria within intact synaptosomes [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):969–970. doi: 10.1042/bst0070969. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Dunnett S. J. Calcium transport in mitochondria. FEBS Lett. 1970 Sep 18;10(1):1–5. doi: 10.1016/0014-5793(70)80402-1. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin B. G. The cytophysiology of mammalian adipose cells. Int Rev Cytol. 1972;33:297–334. doi: 10.1016/s0074-7696(08)61453-9. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on potassium efflux from rat muscle in the presence and absence of glucose. Am J Physiol. 1960 May;198:1066–1070. doi: 10.1152/ajplegacy.1960.198.5.1066. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Hyperpolarization of muscle by insulin in a glucose-free environment. Am J Physiol. 1959 Sep;197:524–526. doi: 10.1152/ajplegacy.1959.197.3.524. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Increase in resting membrane potential of skeletal muscle produced by insulin. Science. 1957 Nov 22;126(3282):1067–1068. doi: 10.1126/science.126.3282.1067. [DOI] [PubMed] [Google Scholar]


