Abstract
Introduction
To determine whether classification of accommodative insufficiency (AI) based on the subjective push‐up test is indicative of reduced amplitude measured objectively.
Methods
Monocular subjective accommodative amplitude was measured in participants 7–24 years of age with the push‐up test; a 0.9 mm letter was moved towards the eye until first sustained blur occurred. Monocular objective amplitude was measured with the same target and an autorefractor for demands from 2.5 to 30 D. The maximum response was termed the amplitude. Near point of convergence (NPC) was measured in a subset of participants. Participants were classified into groups using subjective amplitude: normal amplitude or AI (amplitude < ((15 – 0.25 × age) – 2)). Objective amplitude was plotted by age for each group and one‐way ANCOVA used to evaluate differences while controlling for age. For NPC measures, a t‐test compared the magnitude of the break between those with and without AI.
Results
Fifty‐five of 185 participants were classified as having AI. Objective amplitude decreased with age (0.20 D/year) and there was no significant difference in the age‐adjusted mean amplitudes for the two groups (AI: 7.62 D, CI = 7.19, 8.04; Normal: 7.86 D, CI = 7.58, 8.15; p = 0.11). For the subset with NPC measures, participants classified as having AI had significantly more receded break values than those without AI (7.7 ± 5 vs. 3.7 ± 3 cm, p < 0.001).
Conclusions
Factors other than accommodative ability may be contributing to lower subjective amplitude findings in individuals meeting the criterion for AI.
Keywords: accommodation, accommodative amplitude, near point of convergence, objective measures, subjective push‐up
Key points.
No difference in objectively measured accommodative amplitude was observed between individuals classified with accommodative insufficiency based on subjective testing versus those classified with age‐normal amplitudes.
For children and young adults, a receded push‐up value is not always indicative of an actual reduction in accommodative power.
The location at which the near point of convergence break occurs is strongly correlated with the monocular near point of clear vision in children and young adults.
PURPOSE
Accommodative amplitude is considered the maximum amount of dioptric change that the eye can make to focus on near targets. Accommodative amplitude is known to decrease with age until the fifth or sixth decade of life when little accommodative ability remains. 1 , 2 Measurement of accommodation in young patients is of interest, because a reduced ability in childhood (i.e., accommodative insufficiency [AI]) has been shown to result in symptoms of near blur or eyestrain. 3 , 4 , 5 In addition, conditions such as amblyopia are often accompanied by poor accommodative function, 6 , 7 as are genetic conditions such as Down syndrome, 8 , 9 and thus, it cannot be assumed that accommodation is fully functioning in all young individuals.
Clinically, accommodative amplitude is often measured using subjective tests that rely upon the patient's report of blur to determine the endpoint. A common test that has been used for over a century is the subjective push‐up test, whereby a small target is moved closer to the patient until a sustained blur is first reported. The distance at which sustained blur occurs is then expressed in dioptres and characterised as the accommodative amplitude. Large data sets from the 1800s and early 1900s provided the evidence for linear equations that are utilised to determine whether an individual's measured subjective amplitude falls within the expected range for a given age. 1 , 10 , 11 Based on those equations, a commonly used criterion to diagnose individuals with AI is a subjective amplitude that falls >2 D below the minimum age expected value (15 − 0.25 × age). 5 , 12 , 13 However, debate in the literature exists about the appropriateness of this single criterion for a diagnosis of AI, particularly in children under the age of 8 years, for whom the predictive age equations are extrapolated rather than based on actual data. 4 , 12 , 14
More recently, instruments have become available that provide objective measures of accommodative amplitude by measuring the refractive power of the eye directly. 2 , 15 , 16 , 17 These techniques demonstrate that the subjective push‐up technique grossly overestimates the true accommodative ability of the eye (particularly in young children) due to its inclusion of the depth of field in the measurement endpoint. 15 , 16 , 18 , 19 However, objective methods of accommodative amplitude have not been readily adopted in the clinical setting due to the required measurement time and the expensive equipment.
Given the continued use of the subjective push‐up test clinically, it would be useful to determine whether the subjective push‐up test can be used as a surrogate for objective measures. A previous study sought to identify a conversion factor between subjective and objective measurement techniques that would adjust subjective measurements to reflect the actual refractive power of the eye. 18 However, the accuracy of the conversion factor was only within 2 D, which still leaves a large margin of error. The present study takes a different approach of categorically binning participants to determine whether there are differences in objectively measured accommodative amplitude between children and young adults classified with AI by the subjective push‐up amplitude test. In other words, we sought to determine if a young participant who reports a receded near point of clear vision also demonstrates a true reduction in accommodative power.
To test this question, we utilised two data sets from previously conducted studies of objective accommodative amplitude, both conducted in the laboratory of the same principal investigator (HAA). The first was the aforementioned study conducted at the University of Houston to identify a conversion factor between subjective and objective accommodative amplitudes. 18 The more recent was a study conducted at The Ohio State University to compare different protocols to measure objective accommodative amplitude with an autorefractor. 18 , 20 Both studies included objective and subjective measures of accommodative amplitude using the same methods.
METHODS
The data analysed in this study were collected from the aforementioned studies 18 , 20 which both adhered to the tenants of the Declaration of Helsinki and were approved by the local institutional review board at either the Ohio State University or the University of Houston. Participants 18 years of age and older provided informed consent, whereas parental permission and participant assent were obtained from individuals <18 years of age.
Participants were recruited from university staff, faculty, students, family and friends and the data from those falling between 7 and 24 years was used for the present study. Eligibility criteria included visual acuity of 0.10 logMAR or better at distance and near (with or without correction) and an ability to sit for study measurements. Individuals with constant unilateral strabismus at either distance or near, or a history of lens extraction surgery were excluded from participation. Medical and ocular history, as well as medication use, was obtained from the participant or parent of the participant. Lensometry was performed to determine the spectacle power worn, if relevant, and contact lens powers were obtained from records or patient recollection for individuals wearing contacts. Visual acuity was used as a criterion to avoid having participants with large levels of uncorrected refractive error; however, a distance over‐refraction was also performed (as described in the objective amplitude testing below) to determine if any meaningful uncorrected refractive error was present in each study cohort (Table 1). Subjective and objective measures of accommodation were then performed on all participants at both sites as described below. For the Ohio State site, subjective amplitude measures were always performed first. For the University of Houston site, testing order was randomised.
TABLE 1.
Participant characteristics and test measurements shown as percent or mean values with standard deviations.
| Ohio State University Cohort (n = 94) | University of Houston Cohort (n = 91) | |||
|---|---|---|---|---|
| Classification | Age‐normal amplitude (n = 57) | Accommodative insufficiency (n = 37) | Age‐normal amplitude (n = 73) | Accommodative insufficiency (n = 18) |
| Age (years) | 15.5 ± 6 | 15 ± 5 | 16 ± 5 | 16 ± 5 |
| Sex | 51% female | 65% female | 57.5% female | 44% female |
| Race | ||||
| American Indian | 1.8% | 0% | N/A | N/A |
| Asian | 10.5% | 5.4% | ||
| Black | 7.0% | 2.7% | ||
| Mixed race | 12.3% | 5.4% | ||
| White | 68.4% | 86.5% | ||
| Hispanic ethnicity | 3.5% | 2.7% | N/A | N/A |
| Refractive correction type | ||||
| None | 63% | 70% | 53% | 61% |
| Glasses | 12% | 22% | 18% | 6% |
| Contact lenses | 25% | 8% | 29% | 33% |
| Over‐refraction (D) | 0.13 ± 0.37 | 0.20 ± 0.45 | 0.00 ± 0.41 | −0.03 ± 0.54 |
| CISS score | 13 ± 9 | 16 ± 12 | N/A | N/A |
| Met criterion for elevated CISS score a | 37% | 30% | N/A | N/A |
| NPC break (cm) | 3.7 ± 3 | 7.7 ± 5 | N/A | N/A |
| NPC break >6 cm | 18% | 73% | N/A | N/A |
Abbreviations: cm, centimetres; D, dioptres; NPC, near point of convergence.
A Convergence Insufficiency Symptom Score (CISS) of ≥16 for participants <18 years and ≥21 for participants ≥18 years was used as the criterion in the present analysis.
Monocular subjective accommodative amplitude
Subjective accommodative amplitude was measured using the push‐up test with a 0.9‐mm letter E (0.18 logMAR equivalent at 40 cm) as the accommodative target. The participant's left eye was occluded and a near point rod held against the participant's forehead, centred over the right eye. The target was steadily moved along the rod (1–2 cm per second) closer to the participant's eye until they first reported a sustained blur. The distance of the target to the eye was recorded and the test was repeated for a total of three measurements. The average of three measures expressed in dioptres was documented as the participant's subjective accommodative amplitude.
Monocular objective accommodative amplitude
Objective measures of accommodative amplitude were obtained with the Grand Seiko WAM‐5500 open‐field autorefractor (formerly manufactured by RyuSyo Industrial Co.). The instrument was set to measure at the corneal plane and measurements were taken on the participant's right eye with their left eye occluded. Participants wore their refractive correction, if they had one, and five repeated measures were taken at each demand tested. Any reading resulting in a cylinder measure of >2 dioptres was discarded as erroneous (likely due to off‐axis alignment), and additional measures were obtained. First, participants viewed a target at 3 m while a distance over‐refraction was performed to quantify any residual refractive error. Next participants were instructed to focus on the same 0.9 mm letter E positioned at 13 different dioptric demands (2.5, 3, 4, 5, 6, 7, 8, 10.5, 12, 15, 20, 25 and 30 D). Participants rested in between measurements as the investigator repositioned the target closer to the participant's eye in a stepwise fashion for each subsequent higher demand. Demand positions 2.5–8 D had the target mounted on the examiner's side of the autorefractor and positions 10.5–30 D had the target mounted on the participant's side due to the beamsplitter in the autorefractor limiting the demands available on the examiner's side. The target was mounted using custom‐built 3D printed attachments that were utilised in previous studies. 2 , 18 All measurements were taken with full room illumination; however, additional lighting was provided for positions 6–30 D in which the target was underneath the housing of the autorefractor by using a book lamp aimed at the target. The same 0.9‐mm letter ‘E’ was used for all measurements; however, the target on the examiner's side was printed in the centre of a blank piece of white paper, while the target on the participant's side was cut to have minimal borders and suspended from an open frame using clear thread in order to allow concurrent viewing of the target and measurement of the eye. Pictures of the target and custom mounts for the autorefractor have previously been published in Figure 1 from Anderson and Stuebing. 18
FIGURE 1.
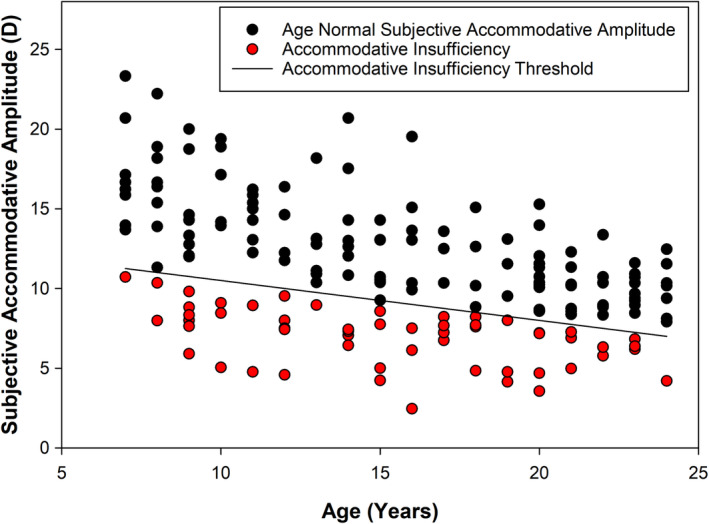
Subjective accommodative amplitude measures as a function of age.
For each demand tested, the spherical equivalents of the repeated measures were averaged with minus refraction expressed as accommodation in dioptres. If the participant was wearing spectacle lenses, the demand and response measurements were adjusted and referenced to the corneal plane using the effectivity formulae published by Mutti et al. 21 A stimulus response function was then plotted for each participant with accommodative demand on the x‐axis and accommodative response on the y‐axis. 18 The typical stimulus response function showed an increase in accommodative response with increasing accommodative demand until the maximum accommodative ability was reached, at which point the response plateaued or dropped off. The maximum accommodative response was identified from the stimulus response function and the spherical equivalent of the distance over‐refraction added to that response to account for any residual refractive error. This final value was termed the objective accommodative amplitude.
Classification with accommodative insufficiency
Subjective accommodative amplitude measures were used to classify participants as having AI or not. For this classification, the age‐based criterion of Hofstetter's minimum accommodative amplitude formula minus 2 D was used (Equation 1).
| (1) |
Additional study measures
For the participants at Ohio State University, additional testing was collected to provide the opportunity to identify study participants who may have symptoms consistent with binocular vision disorders as well as objectively identify individuals meeting the criteria for convergence insufficiency. The motivation for including these tests in the original study's protocol was to better characterise the participants included given the potential co‐existence of AI and other binocular vision complaints. These additional tests were performed prior to the accommodative testing described above. To document symptoms, the investigator administered the revised 15‐question symptom survey developed as part of the Convergence Insufficiency Treatment Trial. 22 , 23 For analysis, a score of 16 or higher was considered elevated in children <18 years of age and a score of 21 or higher considered elevated in participants 18 years of age and older. Near point of convergence was then measured on each of the participants using a vertical row of 0.18 logMAR‐sized letters. A near point rod was held against the participant's forehead and centred between the eyes as the letters were steadily moved (1–2 cm per second) towards the eyes in a slight downward gaze until the participant reported sustained diplopia, or the examiner observed a loss of fusion when an eye drifted out (termed break). The letters were then moved back along the rod until fusion was regained (termed recovery) and the target was reported as single by the participant. Three repeated measures were performed to obtain average break and recovery values.
Data analysis
All analyses were completed using SAS Version 9.4 (sas.com). An analysis of covariance controlling for age was used to compare the mean objective accommodative amplitude of those classified as having age‐normal subjective amplitude versus AI. For the cohort with near point of convergence (NPC) measures, a t‐test was used to compare the break values between participants classified with and without AI. This analysis was performed due to the similarity in subjective accommodative amplitude and NPC testing procedures. Both tests were performed with the same target increasing in proximity, albeit one is conducted under monocular viewing conditions with the participant reporting first sustained blur (subjective amplitude) and the other under binocular conditions with the participant reporting diplopia (NPC). Linear regression was then used to explore the relationship between the physical location of the target at which the subjective accommodative amplitude endpoint occurred (i.e., first sustained blur) as a function of the position at which the NPC break occurred.
RESULTS
Ninety‐four study participants were included from The Ohio State University and 91 from the University of Houston. Participant demographics, refractive error correction type, spherical equivalent over‐refraction, CISS scores and NPC findings are summarised in Table 1. From the cohort recruited at The Ohio State University, 62 participants were unaided for study measures, 14 wore a spectacle correction and 18 wore a contact lens correction. Of those wearing correction, two were hyperopic and the remainder had a myopic refractive error. Participants were well corrected with an average (SD) spherical equivalent distance over‐refraction of 0.16 (0.41) D for the right eye for the entire cohort. From the cohort recruited at the University of Houston, 50 participants were unaided for study measures, 14 wore a spectacle correction and 27 wore a contact lens correction. Of those wearing correction, one was hyperopic and the remainder had a myopic refractive error. Participants were well corrected with an average (SD) spherical equivalent distance over‐refraction of −0.01 (0.44) D in the right eye for the entire cohort.
Accommodative insufficiency
Subjective accommodative amplitude ranged from 2.4 to 23.3 D with a mean of 11.1 ± 4.1 D. Fifty‐five participants out of 185 (30%) met the criteria for classification with AI based on subjective accommodative amplitude findings (Figure 1). The sample from the University of Houston had a significantly lower percentage of participants classified with AI (20%) as compared with the sample from Ohio State University (39%; p = 0.004). As seen in Figure 1, participants that met the criterion spanned the entire age range of 7–24 years. Not surprisingly, the age‐adjusted mean subjective accommodative amplitude differed significantly between participants classified with AI and those with age‐normal subjective amplitude (p < 0.0001). In fact, the age‐adjusted mean subjective accommodative amplitude in participants with age‐normal findings (mean = 12.9 D; 95% CI 6.2, 7.5) was nearly twice that of those with AI (mean = 6.8 D; 95% CI 12.5, 13.3).
Objective accommodative amplitude
There was no significant difference in the adjusted means for objective accommodative amplitude between individuals classified with and without AI (p = 0.11, Table 2). A linear regression analysis found no significant difference in the slope estimates relating objective accommodative amplitude and age for the AI and normal subjective amplitude groups (p = 0.87; Figure 2). As age increases, objective accommodative amplitude declines by approximately 0.20 D per year for both those with and without AI. There was no significant effect of site on the comparison of adjusted means (p = 0.48) and of the slope estimates (p = 0.57).
TABLE 2.
Descriptive statistics for objective accommodative amplitude (D).
| Unadjusted | Adjusted for age | p‐Value a | ||||
|---|---|---|---|---|---|---|
| n | Mean | 95% CI | Mean | 95% CI | ||
| Age normal subjective amplitude | 130 | 7.86 | 7.58, 8.15 | 7.88 | 7.67, 8.10 | 0.11 |
| Accommodative insufficiency | 55 | 7.62 | 7.19, 8.04 | 7.57 | 7.24, 7.89 | |
Abbreviation: CI, confidence interval.
p‐Value from ANCOVA F‐test.
FIGURE 2.

Objective accommodative amplitude by age for those classified with normal subjective amplitude (solid black regression line) versus those classified with accommodative insufficiency (dashed red regression line).
NPC findings
Participants classified as having AI had significantly receded NPC break values (7.7 ± 4.9 cm) compared to participants with age‐normal subjective amplitudes (3.7 ± 2.7 cm, p < 0.001). Figure 3 shows a comparison of the physical location of the target when participants experienced the near point break versus the physical location of the target when they experienced sustained blur at near (subjective accommodative amplitude endpoint). Two male participants aged 16 and 17 years were identified as outliers (circled points in Figure 3); one with extremely receded subjective amplitude and the other with extremely receded NPC. Decreased (more distal) subjective accommodative amplitude was associated with more receded near point breaks both with (y = 0.54x + 8.5, 95% CI for slope = 0.29, 0.79, r 2 = 0.17) and without the outliers included (y = 0.82x + 6.9, 95% CI for slope = 0.57, 1.07, r 2 = 0.33). This relationship did not depend on age in either the full sample (p = 0.59) or after excluding the two outliers (p = 0.96). For the group of 57 with normal NPC (10 of whom were classified with AI), objective amplitude declined by approximately 0.33 D per year with age, and there was no significant effect of AI status (p = 0.19) on the slope estimate.
FIGURE 3.
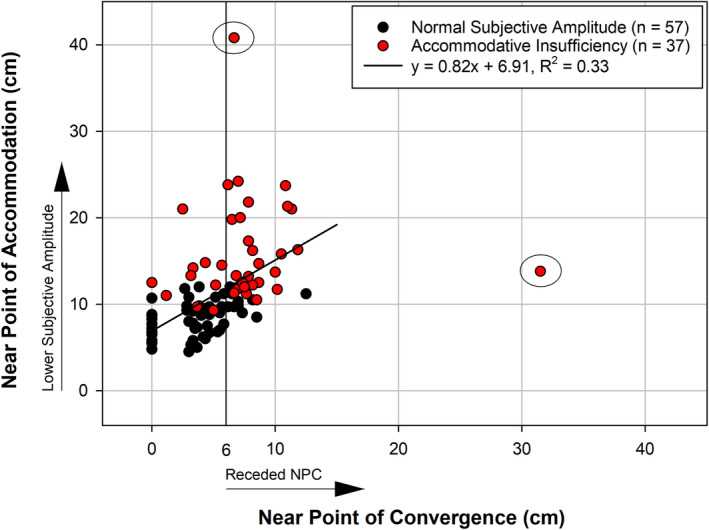
Correlation of the physical location of the target for the near point of convergence (NPC) break and the subjective monocular accommodative amplitude endpoint (for participants with both measures) after exclusion of two outliers (circled).
DISCUSSION
This study found no statistically significant difference in the age‐adjusted mean monocular objective accommodative amplitudes between participants with age‐normal monocular subjective accommodative amplitudes and those classified with AI (Figure 2). This demonstrates that individuals with poor subjective amplitude findings (i.e., meeting the threshold for AI) can still have similar objective accommodative amplitudes to those with better subjective amplitude performance.
A previous study found poor predictive ability of subjective monocular amplitude for objectively measured monocular accommodative amplitude. In the study, the predictive equation to convert subjective amplitude to objective was successful in predicting objective amplitude within ±2 D of the actual value for 92% of participants, but given that the participants had amplitudes of 9 D or less, missing the true amplitude by ±2 D still represents a 22% margin of error at best. 18 More recently, Leon et al. 24 examined 632 participants aged 8–19 years with a variety of accommodation tests and compared prevalence of AI across tests when applying the Hofstetter equation‐based criterion used in the present study. Their investigation demonstrated that this criterion is inappropriate to apply for amplitude tests other than the subjective push‐up due to the inherent differences between what is measured by subjective and objective tests. More importantly for the present study, their analysis found that the discriminatory capacity of accommodative amplitude measured with dynamic retinoscopy (an objective method) was poor at identification of individuals classified with AI by the subjective push‐up test. This supports the findings of the present study that subjective and objective amplitudes are not in agreement with respect to a classification of AI by subjective measurement techniques.
Approximately 30% of the participants included in this analysis were identified as having AI using a criterion of 2 D or more below the age expected minimum amplitude. We compared this prevalence to previously published studies, albeit there were differences in methodology, testing environments (e.g., school versus laboratory based settings) and overall participant age range. The prevalence rate found in the present study is similar to Wick and Hall who found a 25% prevalence of AI in 123 children attending grades 1–6; however, that investigation measured the subjective amplitude binocularly and used a cut‐off of the age‐expected minimum rather than 2 D below the age‐expected minimum which may have elevated their detection rate. 25 Borsting et al. found a prevalence for AI of 17% in a cohort of 392 children aged 7–14 years when using the same subjective testing method and classification criterion as the present study and Menjivar et al. found a prevalence of 23% for a cohort of 282 children age 9–14 years. 13 , 26 Most recently, Leon et al. 24 reported an 8% prevalence of AI when using the same testing method and classification criterion as the present study, although it should be noted that the target used to measure amplitude was larger (1.2 mm) than the present study's target (0.9 mm). With a larger target, individuals may not have noticed sustained blur until the target was closer, resulting in fewer meeting the criterion for AI.
One potential limitation to the analysis in this study is that we used a binary strategy to bin participants as having AI or not. As seen in Figure 1, multiple participants were close to the cut‐off criterion on each side; however, one could also argue that there were also many participants well below the threshold for classification with AI. We chose the criterion of 2 D below the age expected minimum due to its common use as a clinical indicator for AI. Leon et al. 24 also concluded that the subjective amplitude criterion was not adequate in identifying AI and suggested the inclusion of symptom surveys to identify individuals with true deficits. We did not observe a large difference in CISS score between our participants classified with and without AI (Table 1); however, it is important to note that we also did not screen participants for co‐existing binocular vision anomalies, and thus, we cannot rule out that other conditions may have impacted the CISS scores. In the absence of any co‐existing binocular vision anomalies, the present findings do agree with a recent study which found that children with AI did not report higher symptoms on the CISS than those without. 27 However, Chen and Borsting 28 previously reported a strong correlation between subjective accommodative amplitude findings and CISS scores when testing a group of 14 children with elevated CISS scores versus 12 children with normal scores. Alternate means of diagnosing AI have also been proposed by Chase et al., 29 who found a stronger association between visual symptoms and increased accommodative lag in adults when measured objectively over a 90‐s viewing period than the association between subjective amplitude and visual symptoms. Thus, there is evidence that substantiates a subjective report of a receded clear near point is not always predictive of the magnitude of objective accommodative ability in young individuals.
We did observe a strong relationship between the location of the NPC break and the location at which the participant experienced first sustained blur with the subjective, monocular push‐up test. It has been reported previously in the literature that AI and convergence insufficiency frequently co‐exist, 30 , 31 , 32 , 33 so this could explain the correlation between these measures. As seen in Figure 3, if a cut‐off of 6 cm is applied as the criterion for convergence insufficiency (CI), 27 individuals met the criterion for both AI and CI whereas only 10 individuals met the criterion for CI alone and 10 for AI alone. However, given that AI as classified by the subjective push‐up test was not indicative of true accommodative ability in this study, then the relationship between the endpoints of the subjective push‐up and NPC tests leads us to a new hypothesis that may be intriguing for future study. The protocol for these two tests is quite similar: A small target is moved along a near point rod until the participant reports sustained blur (subjective amplitude) or loss of fusion (NPC). The subjective amplitude test was performed monocularly whereas the NPC was performed binocularly; however, the physical location in space where the endpoints occurred was similar within most participants (Figure 3). Perhaps, despite the occlusion of an eye during the accommodative amplitude test, the visual system is accustomed to experiencing difficulty with a certain level of proximity of a near target due to fusional limitations rather than solely accommodative limitations. Although fusion is not required during the accommodative amplitude test, the eye under the cover is still converging as the target is moved closer due to the link between accommodation and convergence, and thus, it may be that once the target reaches the location of the convergence break, the individual is habituated to report difficulty with the proximity of the target irrespective of their ability to bring it into focus.
The present study provides strong evidence that using a subjective test to identify how close an observer perceives something as being clear before it blurs is not necessarily related to the actual ability of the eye to change refractive power, at least in children and young adults. Another limitation of this study is that we only included individuals aged 7–24 years, and thus, it is possible that the subjective push‐up test in middle‐aged adults nearing presbyopia may agree better with measures of objective accommodative amplitude. However, AI is a clinical diagnosis applied to children and young adults. Therefore, the subjective push‐up test's predictive ability is not as critical in older individuals. One might question whether applying a different equation to identify AI would have provided different results. Anything less strict would likely result in even more homogenous groups with respect to objective amplitude findings. We were unable to identify an alternate criterion that would provide greater separation of the objective measures. Ultimately, our decision to apply Hofstetter's minimum amplitude equation minus 2 D was based on the widely adopted use of this criterion clinically, as well as its use in previous studies of AI. 34 Lastly, we did not utilise randomisation of testing order at The Ohio State site since the original study protocol was designed to randomise different objective amplitude testing methods rather than both subjective and objective testing. That said, we did not observe any site differences in objective amplitude findings (University of Houston did randomise test order), and thus, it seems unlikely that this impacted the findings. It is a strength of this study that the findings were upheld at two different sites with data collected by different investigators recruiting separate cohorts.
CONCLUSIONS
The group of individuals identified with AI by the monocular push‐up technique did not have differences in monocular objective accommodative amplitude as compared to those with age‐normal monocular push‐up values. However, NPC was more receded in those classified with AI using the monocular subjective amplitude of accommodation. This suggests that factors other than total accommodative amplitude ability may be contributing to the lower subjective, monocular amplitude findings in the individuals meeting the criteria for AI.
AUTHOR CONTRIBUTIONS
Heather A. Anderson: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal); validation (equal); writing – original draft (equal). Sidney M. Parks: Data curation (equal); investigation (equal); writing – review and editing (equal). Marjean T. Kulp: Conceptualization (equal); formal analysis (equal); methodology (equal); project administration (equal); supervision (equal); writing – review and editing (equal). G. Lynn Mitchell: Formal analysis (equal); validation (equal); writing – review and editing (equal).
FUNDING INFORMATION
There is no funding to report for this study.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
Anderson HA, Parks SM, Kulp MT, Mitchell GL. Classification of accommodative insufficiency by monocular subjective push‐up test is poorly predictive of monocular objective amplitudes in children and young adults. Ophthalmic Physiol Opt. 2025;45:14–22. 10.1111/opo.13419
REFERENCES
- 1. Duane A. Normal values of the accommodation at all ages. JAMA. 1912;59:1010–1013. [Google Scholar]
- 2. Anderson HA, Hentz G, Glasser A, Stuebing KK, Manny RE. Minus‐lens‐stimulated accommodative amplitude decreases sigmoidally with age: a study of objectively measured accommodative amplitudes from age 3. Invest Ophthalmol Vis Sci. 2008;49:2919–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scheiman M, Wick B. Clinical Management of Binocular Vision. Philadelphia: Wolters Kluwer Health; 2019. [Google Scholar]
- 4. Sterner B, Gellerstedt M, Sjostrom A. Accommodation and the relationship to subjective symptoms with near work for young school children. Ophthalmic Physiol Opt. 2006;26:148–155. [DOI] [PubMed] [Google Scholar]
- 5. Marran LF, De Land PN, Nguyen AL. Accommodative insufficiency is the primary source of symptoms in children diagnosed with convergence insufficiency. Optom Vis Sci. 2006;83:281–289. [DOI] [PubMed] [Google Scholar]
- 6. Ciuffreda KJ, Hokoda SC, Hung GK, Semmlow JL, Selenow AR. Static aspects of accommodation in human amblyopia. Am J Optom Physiol Opt. 1983;60:436–449. [DOI] [PubMed] [Google Scholar]
- 7. Chen AM, Manh V, Candy TR. Longitudinal evaluation of accommodation during treatment for unilateral amblyopia. Invest Ophthalmol Vis Sci. 2018;59:2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woodhouse JM, Meades JS, Leat SJ, Saunders KJ. Reduced accommodation in children with Down syndrome. Invest Ophthalmol Vis Sci. 1993;34:2382–2387. [PubMed] [Google Scholar]
- 9. Anderson HA, Manny RE, Glasser A, Stuebing KK. Static and dynamic measurements of accommodation in individuals with Down syndrome. Invest Ophthalmol Vis Sci. 2011;52:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donders F. On the anomalies of accommodation and refraction of the eye, with a preliminary essay on physiological dioptrics. Br Foreign Med Chir Rev. 1864;34:443–445. [Google Scholar]
- 11. Hofstetter HW. A comparison of Duane's and Donders' tables of the amplitude of accommodation. Optom Vis Sci. 1944;21:345–363. [Google Scholar]
- 12. Cacho P, García A, Lara F, Seguí MM. Diagnostic signs of accommodative insufficiency. Optom Vis Sci. 2002;79:614–620. [DOI] [PubMed] [Google Scholar]
- 13. Borsting E, Rouse MW, Deland PN, Hovett S, Kimura D, Park M, et al. Association of symptoms and convergence and accommodative insufficiency in school‐age children. Optometry. 2003;74:25–34. [PubMed] [Google Scholar]
- 14. Sterner B, Gellerstedt M, Sjöström A. The amplitude of accommodation in 6–10‐year‐old children—not as good as expected! Ophthalmic Physiol Opt. 2004;24:246–251. [DOI] [PubMed] [Google Scholar]
- 15. Wold JE, Hu A, Chen S, Glasser A. Subjective and objective measurement of human accommodative amplitude. J Cataract Refract Surg. 2003;29:1878–1888. [DOI] [PubMed] [Google Scholar]
- 16. Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Res. 1989;29:1685–1692. [DOI] [PubMed] [Google Scholar]
- 17. Ostrin LA, Glasser A. Accommodation measurements in a prepresbyopic and presbyopic population. J Cataract Refract Surg. 2004;30:1435–1444. [DOI] [PubMed] [Google Scholar]
- 18. Anderson HA, Stuebing KK. Subjective versus objective accommodative amplitude: preschool to presbyopia. Optom Vis Sci. 2014;91:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamasaki D, Ong J, Marg E. The amplitude of accommodation in presbyopia. Am J Optom Arch Am Acad Optom. 1956;33:3–14. [DOI] [PubMed] [Google Scholar]
- 20. Parks SM, Kulp MT, Anderson HA. Comparison of proximal and minus lens autorefraction techniques to measure monocular accommodative amplitude. Optom Vis Sci. 2024;101:109–116. [DOI] [PubMed] [Google Scholar]
- 21. Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000;41:2469–2478. [PubMed] [Google Scholar]
- 22. Rouse MW, Borsting EJ, Mitchell GL, Scheiman M, Cotter SA, Cooper J, et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthalmic Physiol Opt. 2004;24:384–390. [DOI] [PubMed] [Google Scholar]
- 23. Borsting EJ, Rouse MW, Mitchell GL, Scheiman M, Cotter SA, Cooper J, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80:832–838. [DOI] [PubMed] [Google Scholar]
- 24. Leon A, Rosenfield M, Medrano SM, Durán SC, Pinzón CV. Objective and subjective assessment of accommodative insufficiency. Optom Vis Sci. 2024;101:44–54. [DOI] [PubMed] [Google Scholar]
- 25. Wick B, Hall P. Relation among accommodative facility, lag, and amplitude in elementary‐school‐children. Am J Optom Physiol Opt. 1987;64:593–599. [DOI] [PubMed] [Google Scholar]
- 26. Menjivar AM, Kulp MT, Mitchell GL, Toole AJ, Reuter K. Screening for convergence insufficiency in school‐age children. Clin Exp Optom. 2018;101:578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pang Y, Tan QQ, Gabriel H, Block SS, Wang J. Application of the convergence insufficiency symptom survey in oculomotor dysfunction and accommodative insufficiency. Optom Vis Sci. 2021;98:976–982. [DOI] [PubMed] [Google Scholar]
- 28. Chen AM, Borsting EJ. Near work symptoms and measures of accommodation in children. Clin Exp Optom. 2023;106:675–680. [DOI] [PubMed] [Google Scholar]
- 29. Chase C, Tosha C, Borsting E, Ridder WH 3rd. Visual discomfort and objective measures of static accommodation. Optom Vis Sci. 2009;86:883–889. [DOI] [PubMed] [Google Scholar]
- 30. Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, et al. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) Group. Optom Vis Sci. 1999;76:643–649. [DOI] [PubMed] [Google Scholar]
- 31. Mazow ML, France TD, Finkleman S, Frank J, Jenkins P. Acute accommodative and convergence insufficiency. Trans Am Ophthalmol Soc. 1989;87:158–168. [PMC free article] [PubMed] [Google Scholar]
- 32. Chen AM, Roberts TL, Cotter SA, Kulp MT, Sinnott LT, Borsting EJ, et al. Effectiveness of vergence/accommodative therapy for accommodative dysfunction in children with convergence insufficiency. Ophthalmic Physiol Opt. 2021;41:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scheiman M, Cotter S, Kulp MT, Mitchell GL, Cooper J, Gallaway M, et al. Treatment of accommodative dysfunction in children: results from a randomized clinical trial. Optom Vis Sci. 2011;88:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hussaindeen JR, Murali A. Accommodative insufficiency: prevalence, impact and treatment options. Clin Optom. 2020;12:135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]


