Abstract
Background:
We aimed to determine the relative risk of pedal vessel calcification (PVC) on major adverse foot events (MAFEs) and chronic kidney disease (CKD) stage in patients with diabetes mellitus (DM) and peripheral neuropathy (PN).
Methods:
We retrospectively reviewed electronic medical records of 152 patients with diagnoses of DM, PN, and CKD stages one to five who had at least one foot radiograph obtained. PVC was scored (from 0–4) based on foot anatomic location and radiology reported MAFEs, which includes foot fracture, Charcot neuroarthropathy, foot ulcer, osteomyelitis, or minor amputation. Risk ratios (RR) with 95% confidence intervals (95% CI) and Poisson regressions were performed assessing the risk of sustaining MAFEs with number of PVCs and stage of CKD.
Results:
The risk of any MAFE increased as PVC score increased (RR = 1.23); the risk of any MAFE increased as CKD stage increased (RR = 1.35); and risk of any PVC increased as CKD stage increased (RR = 1.71).
Conclusions:
Pedal vessel calcification on a foot radiograph increases the risk of any MAFE and increases with progressive stage of CKD. Pedal vessel calcification may serve as a gateway to prompt investigation, treatment, or referral for at-risk diabetic neuropathic, nephropathic patients. (J Am Podiatr Med Assoc 114(5), 2024; doi:10.7547/23–233)
Vessel calcification refers to the pathologic deposition of minerals into the walls of blood vessels and is recognized as a general cardiovascular risk factor.1,2 Vessel calcification is a common complication and potentially serious problem in patients with diabetes mellitus (DM) and chronic kidney disease (CKD). Commonly found in the coronary and carotid anatomical regions, vessel calcification can also be identified in the peripheral musculoskeletal system, including the pedal (foot) region.
Pedal vessel calcification (PVC) is a well-known phenomenon associated with diabetes-related foot disease, which results in a major global burden for patients and the healthcare system.3 Upwards of 19% to 34% of patients with diabetes will develop a neuropathic or ischemic ulcer in their lifetime, with plain film radiography often used as the initial imaging tool of choice to rule out infection or osteomyelitis.4,5 Pedal vessel calcification is often identified incidentally on a foot radiograph, has a high positive predictive value for diabetes, and is also observed paradoxically in patients with CKD and decreased bone mineral density.6,7 Given its strong association with adverse outcomes, vessel calcification may serve as a proxy for cardiovascular morbidity and mortality, including stroke, myocardial infarction, and death.8
Although analytical models exist that predict the risk of foot ulcer or amputation in patients with diabetes mellitus, the prognostic value and utility of PVC remains elusive.9 The estimated global prevalence of amputations in individuals with diabetes mellitus is more than 7 million.10 Moreover, the highest rate of nontraumatic lower-extremity amputation occurs in individuals with diabetes and peripheral neuropathy who have end-stage CKD, with diabetic foot ulceration (DFU) indisputably the most prevalent precursor.4,11 Diabetic foot ulcers are a part of a number of sequelae that we have termed major adverse foot events (MAFEs), which also includes Charcot neuroarthropathy, foot fractures, osteomyelitis, and minor foot amputations and based off the nomenclature that is used as a composite endpoint in cardiovascular disease research.12 The purpose of our retrospective study was to explore the relationships between PVC identified on a plain foot radiograph, MAFEs, and CKD stage in a limited group of patients with DM and PN. We hypothesized that a higher PVC score would be strongly associated with an increased prevalence of MAFEs and deteriorating kidney function (ie, advancing CKD stage).
Methods
Study Design
This study involved a retrospective electronic medical record (Epic platform; Epic Systems Corporation, Verona, Wisconsin) review of patients in the Atrium Health Wake Forest Baptist health system diagnosed with both diabetes mellitus (DM), peripheral neuropathy (PN), and chronic kidney disease stage 1 to 5 from 2012 to 2021. The aim was to capture at least 25 individual medical records of patients with diabetes mellitus in each of the five stages of CKD. Moreover, the inclusion criteria called for all study participants to have had at least one radiograph of the foot, which would inevitably serve as the surrogate marker for assessing the relative risk of having one or more PVC locations on the presence of any MAFE and CKD stage. Following the search analysis, a confidential participant identification number was assigned to the medical record number of each patient, and this information was then recorded in a REDCap database (REDCap Consortium; Vanderbilt University, Nashville, Tennessee). The study protocol was reviewed and approved by Wake Forest University School of Medicine’s Institutional Review Board (IRB00056905) and given exempt status.
Demographics and Participants
Patient demographic characteristics were extracted including age (determined at time of the foot radiograph), weight and height, body mass index, sex, race, diabetes diagnosis (type 1 or 2), peripheral neuropathy (yes or no), and dates performed of both the comprehensive metabolic panel (CMP) and foot radiographs (Table 1).
Table 1.
Demographic Characteristics of Patient Records with Diabetes Mellitus (DM) and Chronic Kidney Disease (CKD) By Stage from Electronic Medical Record
| Stage 1 (n = 34) | Stage 2 (n = 42) | Stage 3 (n = 26) | Stage 4 (n = 25) | Stage 5 (n = 25) | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 53 ± 11 | 63 ± 9 | 69 ± 10 | 68 ± 9 | 62 ± 10 |
| Weight (kg) | 93 ± 26 | 94 ± 23 | 92 ± 25 | 96 ± 20 | 86 ± 24 |
| BMI (kg/m2) | 33 ± 10 | 32 ± 8 | 32 ± 8 | 33 ± 9 | 31 ± 8 |
| Sex | |||||
| Male | 13 | 20 | 11 | 13 | 12 |
| Female | 20 | 20 | 14 | 12 | 13 |
| Race | |||||
| White | 21 | 37 | 22 | 14 | 15 |
| Black | 9 | 3 | 3 | 11 | 10 |
| Asian | 1 | 0 | 0 | 0 | 0 |
| Othera | 2 | 0 | 0 | 0 | 0 |
| Neuropathy | |||||
| Yes | 13 | 21 | 18 | 21 | 24 |
| No | 16 | 14 | 6 | 4 | 1 |
| Not reported | 4 | 5 | 1 | 0 | 0 |
| eGFR (mL/min/1.73 m2)b | 104 ± 11 | 77 ± 8 | 48 ± 9 | 24 ± 4 | 9 ± 3 |
| HbA1c (mean %) | 7.8 | 7.6 | 8.2 | 7.6 | 7.7 |
| No. of days from foot radiographs to comprehensive metabolic panel blood draw | 116 ± 467 | 6 ± 170 | 28 ± 143 | 72 ± 193 | 8 ± 89 |
Other is Hawaiian, Pacific Islander, or not specified.
eGFR, estimated glomerular filtration rate from serum creatinine using CKD-EPI equation.13
Note: Data are presented as value ± SD unless otherwise indicated.
Comprehensive Metabolic Panel
The serum creatinine concentration obtained from the CMP was then used in the CKD-Epi equation to estimate the glomerular filtration rate (eGFR).13 The eGFR was used to stage the CKD severity (ie, diabetic nephropathy) (Table 1).14
Peripheral Neuropathy
Each patient’s electronic medical record was searched for evidence of having peripheral neuropathy. It was recorded as yes/present or no/absent. Typically, the presence or absence of peripheral neuropathy was found in the past medical history section of the electronic medical record or, when not clear, in the actual provider’s noted objective assessment (eg, documented as a loss of protective sensation.
Foot Radiography
Standard three-view (ie, anteroposterior, oblique, and lateral) plain foot radiographs obtained in routine clinical care by either podiatry services, primary-care providers, hospitalists, or other members of the health-care team were noted. Foot radiographs were most often ordered for complaints of foot pain or other symptoms, such as to rule out foot fracture or deep infection. Radiographs were most often interpreted by radiology (68%) or podiatry services (19%). From the medical records reviewed, the majority of foot radiographs were obtained with the study participants in a non-weightbearing stance; 65% were nonweightbearing; 35%, weightbearing. Pedal vessel calcification and any major adverse foot events were noted from these radiographs.
Pedal Vessel Calcification
The presence of PVC was noted from the foot radiographs by an expert clinician (M.A.J.) with more than 20 years of experience viewing foot radiographs. The clinician documented the presence (yes/no) of any pedal vessel that showed calcification and noted its location within the foot (Fig. 1). Pedal vessel calcification was recorded via the following four anatomic landmarks: digital if it was located distal to the metatarsophalangeal joints (MTPJs), metatarsal if located in the intermetatarsal spaces (proximal to the MTPJs and distal to the tarsometatarsal joints), and both ankle/hindfoot (anterior) and ankle/hindfoot (posterior) if calcification was found anywhere in between the tarsometatarsal joints and tibiotalar joint. Both the digital and metatarsal PVCs were identified from the anteroposterior radiograph and the ankle/hindfoot (anterior and posterior) locations were recorded from the lateral radiograph, with the bisection of the distal tibia at the ankle (ie, tibiotalar joint) serving as a coronal plane divider between the anterior and posterior anatomic regions. A PVC score ranging from 0 to 4 was then tallied from the anatomical locations, where 0 = no PVC and 4 = all 4 locations with PVC (Fig. 1). Scores 1 to 3 may have had any variation (eg, ankle/hindfoot [anterior] and digital PVCs noted, but none for ankle/hindfoot [posterior] or metatarsal, for a PVC score of 2).
Figure 1.
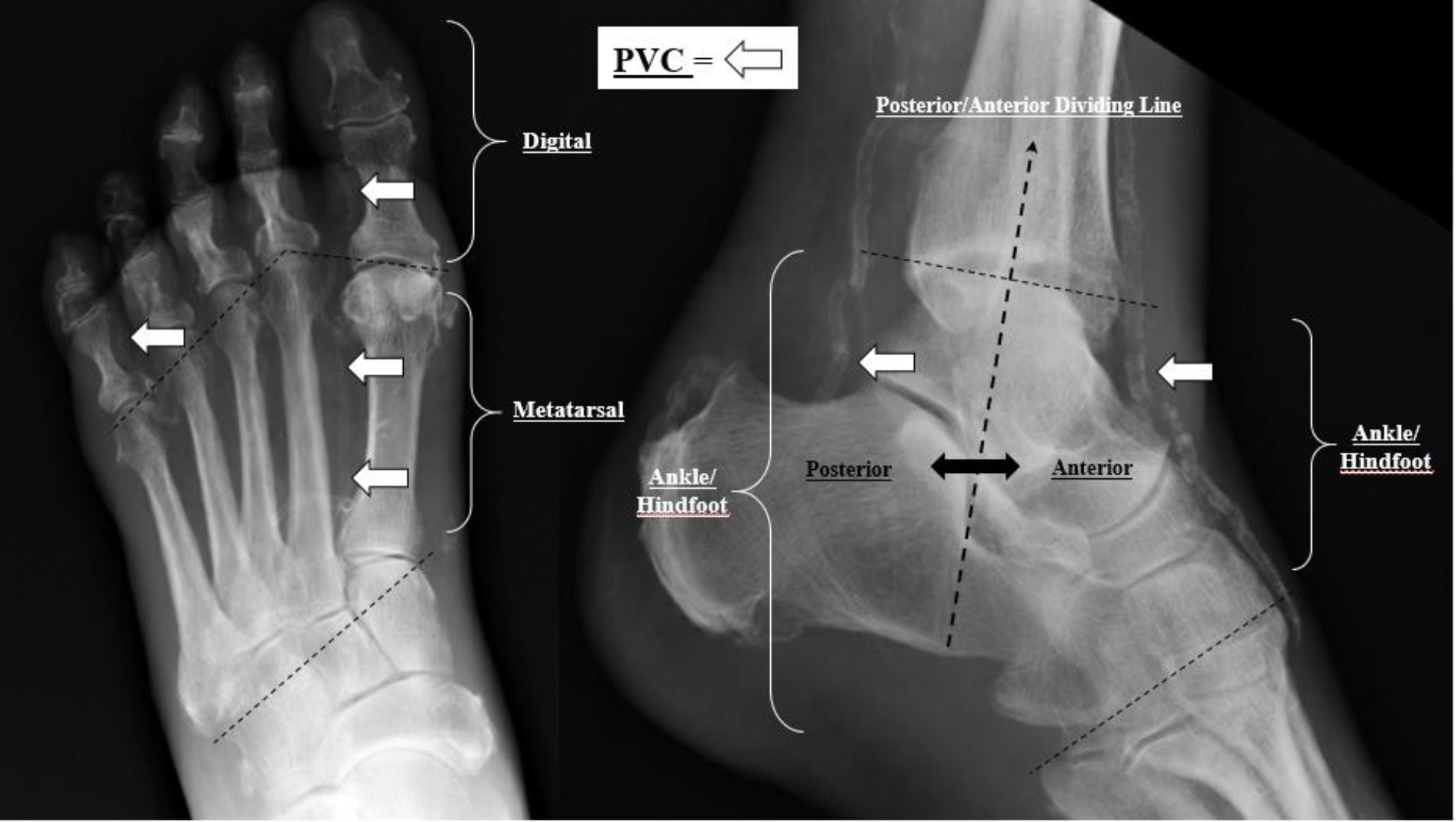
Pedal Vessel Calcification (PVC) Scoring System 0 to 4 and anatomic location (digital, metatarsal, ankle/hindfoot anterior, and ankle/hindfoot posterior). PVC = 0 if NO calcified vessels identified. PVC =1 if ANY anatomic location identified as calcified, PVC = 2 if ANY 2/4 anatomic regions show calcification, PVC = 3 if ANY 3/4 anatomic locations show calcification, and PVC = 4 if ALL anatomic locations show calcification.
 Anatomic region divider
Anatomic region divider 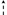 Posterior/Anterior dividing line.
Posterior/Anterior dividing line.
Major Adverse Foot Events
The clinician reviewer (M.A.J.) also recorded any foot fracture, Charcot neuroarthropathy, ulceration, and osteomyelitis, as well as any minor foot amputation, such as partial/complete toe amputation, partial/complete ray amputation, or transmetatarsal amputation, identified via plain film radiography, which was reported verbatim from the radiology report in the electronic medical record. The presence of any MAFE was used to analyze the relationships between both PVC score and CKD stage.
Statistical Analyses
Risk ratios were calculated to assess the risk of sustaining any MAFE by the presence of one or more PVCs and in relation to progressive stages of CKD. A series of Poisson regressions were performed to assess the relative risk of MAFEs (single or combination of) by both PVC score and CKD stage. All risk models were adjusted for age and body mass index. All models are reported as risk ratios with 95% confidence intervals (95% CI) to assess the relationship and strength of association between each of the following combinations:
MAFEs by PVC score
MAFEs by CKD stage
PVC score with CKD stage
Statistical significance was determined using a P value threshold of < 0.05. Statistical analysis was performed using R 4.2.1 (R Core Team/R Foundation for Statistical Computing, Vienna, Austria). The fmsb package was used for risk ratio analyses and the glm function was used for Poisson regressions.
Data and Resource Availability
The datasets generated during or analyzed in the current study are available from the senior author (D.R.S.) upon reasonable request.
RESULTS
Major Adverse Foot Events by Pedal Vessel Calcification Score
The unadjusted relative risk (RR) and 95% CIs are shown in Table 2. The age- and BMI-adjusted relative risk of the association between PVC score and the number of MAFEs was significant at 1.23 (95% CI: 1.10, 1.38; P < .001).
Table 2.
Unadjusted Risk Ratios (RR) and 95% Confidence Intervals (CI) for Any Major Adverse Foot Event (MAFE) by Pedal Vessel Calcification (PVC) Score and Chronic Kidney Disease (CKD) Stage, and Any PVC by CKD Stage
| Any MAFE by PVC score | RR (95% CI) |
|---|---|
|
| |
| PVC score 0 versus PVC score 1 | 2.11 (1.2–3.6) |
| PVC score 0 versus PVC score 2 | 1.95 (1.1–3.3) |
| PVC score 0 versus PVC score 3 | 2.05 (1.1–3.7) |
| PVC score 0 versus PVC score 4 | 2.54 (1.7–3.9) |
| Any MAFE by CKD stage | RR (95% CI) |
|
| |
| Stage 1 versus Stage 2 | 0.97 (0.58–1.63) |
| Stage 1 versus Stage 3 | 1.13 (0.66–1.94) |
| Stage 1 versus Stage 4 | 1.27 (0.76–2.12) |
| Stage 1 versus Stage 5 | 1.72 (1.11–2.67) |
| Any PVC by CKD stage | RR (95% CI) |
|
| |
| Stage 1 versus Stage 2 | 1.16 (0.50–2.72) |
| Stage 1 versus Stage 3 | 2.62 (1.24–5.54) |
| Stage 1 versus Stage 4 | 2.72 (1.29–5.74) |
| Stage 1 versus Stage 5 | 4.87 (2.51–9.40) |
Major Adverse Foot Events by Chronic Kidney Disease Stage
The unadjusted RR and 95% CIs are shown in Table 2. The age- and BMI-adjusted analysis of the association between CKD stage and the number of MAFEs revealed a significant association (adjusted RR: 1.35; 95% CI: 1.18, 1.54; P < .001). Moreover, there was a statistically significant interstage difference in MAFEs between CKD stages 5 (end-stage renal disease) and 1 (normal or baseline kidney function) (adjusted RR: 1.72; 95% CI: 1.11, 2.67; P = .015).
Pedal Vessel Calcification Score with Chronic Kidney Disease Stage
The unadjusted RR and 95% CIs are shown in Table 2. The age- and BMI-adjusted analysis of the association between PVC score and increasing CKD stage also revealed a significant relative risk (adjusted RR: 1.71; 95% CI: 1.52, 1.92; P < .001).
Table 3 presents the raw frequency counts and overview of 152 patients with at least one MAFE, categorized by both PVC score and CKD stage, serving as a clear depiction of how PVC and CKD stage interplay throughout the disease progression with a notable increased prevalence in CKD stages 4 and 5.
Table 3.
Number of Participants with at Least One Major Adverse Foot Event (MAFE) Reported in the Electronic Medical Record by Pedal Vessel Calcification (PVC) Score and Chronic Kidney Disease (CKD) Stage
| Pedal Vessel Calcification (PVC) Score | Chronic Kidney Disease (CKD) Stage |
|||||
|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Total | |
|
| ||||||
| 0 | 27 | 31 | 12 | 11 | 0 | 81 |
| 1 | 4 | 2 | 3 | 5 | 1 | 15 |
| 2 | 1 | 4 | 8 | 2 | 4 | 19 |
| 3 | 2 | 1 | 2 | 3 | 4 | 12 |
| 4 | 0 | 4 | 1 | 4 | 16 | 25 |
| Total | 34 | 42 | 26 | 25 | 25 | 152 |
DISCUSSION
Vascular calcification is highly associated with cardiovascular disease mortality, particularly in high-risk patients with DM and CKD.1 This retrospective study explored the relationship between PVCs, MAFEs, and CKD stage in patients with DM and PN. To our knowledge, this investigation is the first to report findings that reveal significant associations between PVC score, MAFEs, and CKD stage, underscoring the potential value of a single, plain film radiograph of the foot as a surrogate marker for assessing the relative risk of MAFEs from increasing locations of PVC and nephropathy in patients with DM and PN.
To classify our outcomes in a comprehensive manner, DFUs, Charcot neuroarthropathy, foot fractures, osteomyelitis, and minor foot amputations were placed into the broad category of major adverse foot events, as each are known individually and collectively to contribute to major nontraumatic lower-extremity amputation, a harbinger of death.15–18 Similar to how myocardial infarction and stroke, among other major adverse cardiovascular events, are employed in research related to heart disease outcomes, major adverse foot events, like DFUs and osteomyelitis, may hold value in outcomes research examining major (ie, transtibial or transfemoral) lower-extremity amputations, which is effectively the consequence of end-stage foot disease.12 In our study, a PVC scoring system was employed as a gauge to examine the association between these MAFEs and CKD stage.
The study’s significant association (RR = 1.23) between PVC score and MAFEs suggests that PVC severity may be a predictive marker for adverse foot outcomes in patients with DM and PN. These findings suggest that a higher PVC score is associated with an increased risk of MAFEs and thus may have implications for risk assessment, prevention, and management of foot complications, particularly in populations with CKD or DM. Prior research has already demonstrated the clinical significance of PVC and diabetic foot disease.3,6,19 This is especially true in the diabetic neuropathic patient, where it has previously been established as a known specific complication in the potential dysregulation of calcium and phosphate minerals. As a result, the loss of vasomotor control due to underlying autonomic denervation presumably then creates an environment conducive to PVC.20–21 Thus the presence of PVCs on foot radiographs may indicate underlying neuropathy and vasculopathy, serving as the proverbial canary in a coal mine for identifying patients at an increased risk of foot ulceration, osteomyelitis, and other MAFEs. By incorporating a PVC scoring system into routine clinical practice, health-care providers can potentially enhance risk stratification and guide treatment decisions for foot-related issues in patients with DM, PN, and CKD.
Our study also uncovered a significant association between MAFEs and CKD stage, underscoring the impact of kidney function on foot health in patients with DM. Individuals with advanced CKD stage are at an increased risk of experiencing foot-related complications.22 Our retrospective analysis revealed a 35% increased risk of experiencing MAFEs for each unit increase in CKD stage. Scrutinizing the data further, when utilizing stage 1 CKD as essentially normal or baseline kidney function and comparing it against their subsequent declining statuses, stage 5 CKD showed a significant 1.72 relative risk in comparison to the norm (Table 2). These findings reinforce the importance of monitoring kidney function in patients with DM and PN to identify those at higher risk for foot complications and potentially implementing preventative measures effectively. Addressing CKD as a potential risk factor for MAFEs can guide clinical management and improve preventative strategies, potentially reducing the burden of diabetic foot disease.
We also observed a direct association between PVC score and advancing CKD stage, indicating that PVC severity on a radiograph of the foot may serve as a marker of declining kidney function. This aligns with existing evidence on the relationship between vascular calcification and CKD, though admittedly the etiology of PVC is likely multifactorial, still poorly understood in CKD, and somewhat complex involving several metabolic pathways regulating phosphate and calcium in vascular smooth muscle cells, which have phenotypic plasticity (including the ability to differentiate into a chondrocytic-like or osteocytic-like cell with the right stimuli/environment).23–24 Notably, patients with DM who have impaired kidney function are at a higher risk of developing foot ulcers, infections, and other MAFEs due to vascular and metabolic abnormalities. Our study’s findings suggest that individuals with calcified pedal vessels are 1.71 times more likely to be associated with a higher stage of kidney disease compared to those without calcification. The correlation between PVCs and CKD progression highlights the importance of early detection and intervention to manage both foot complications and renal disease effectively.
The clinical implications of these findings are noteworthy. Although there is currently no proven therapy to definitively treat or prevent PVC (or vascular calcification altogether), its finding on a plain film foot radiograph provides a window into cardiovascular health, including coronary and/or carotid artery disease.8 Pedal vessel calcifications are frequently discovered incidentally, yet they are readily identifiable on radiographs of the foot. Additionally, early identification of seemingly inconsequential PVCs on a foot radiograph may actually have usefulness, as health-care providers can leverage this unexpected information as an opportunity to prompt further evaluation of cardiovascular risk and renal health in patients with DM, potentially leading to timely management and appropriate referrals.
The number of individuals in this retrospective study noted with at least one MAFE, categorized by both PVC score and CKD stage, underscores the significance of considering PVC score in assessing its impact on renal health. For instance, the majority of persons with a PVC score of 0 occurred in CKD stages 1 to 2, while the majority of those with a PVC score of 3 or 4 occurred in CKD stages 4 to 5 (Table 3). Though purely speculative, identification of PVC and knowing its relative risk at various stages of CKD may allow for employment of management strategies that mitigate the risk and prevent advancement of cardiovascular disease including stringent lifestyle modifications, blood pressure control, lipid management, and the use of other medications as deemed appropriate. In summary, the utility of a PVC scoring system from a plain foot radiograph may improve risk assessment in patients with DM and PN, thereby guiding clinical decision making and resource allocation for patients at high risk of major adverse foot events.
Our study had several limitations. First, our investigation is a retrospective design, performed at a single institution/health system, and partially reliant on ICD-9 or ICD-10 coding systems (typically obtained for third-party reimbursement reasons). Our retrospective analysis is dependent on the available medical records, which may have inaccurate or discordant data and introduce inherent biases, and thus may not establish causality between PVCs, CKD stage, and MAFEs. The study’s sample size is limited to 152 patients from a specific population, which may of course affect the generalizability of our study’s findings. Further prospective studies with larger sample sizes and diverse patient populations are warranted to validate any of the associations observed from our particular study.
Additionally, arterial calcification can affect both the arterial intima and arterial media, the latter of which is often referred to as Mönckeberg sclerosis or medial arterial calcification.6 When observed on plain film radiography, calcification in the arterial intima produces a patchy, discontinuous appearance of comparatively large calcific deposits, while calcification of the arterial media creates a more diffuse appearance usually affecting the whole circumference of the vessel (often described as a “pipe-stem” or “tramline” appearance, typically ≥ 1 cm of the vessels length) and thus distinguishing the two on plain film radiographs.6,25 Admittedly, when documenting the aberrant PVC, the identifying clinician (M.A.J.) did not make any differentiation between the two, and thus it is certainly plausible that PVC was perhaps either incorrectly noted or mistaken for other heterotopic soft-tissue calcifications by the single assessor in our study. Further scrutiny and fine-tuning of our PVC scoring system is necessary before there can be certainty in reproducibility for future studies.
Moreover, our study is potentially limited by the absence of noninvasive arterial studies, such as ankle-brachial indices, within our small cohort. Ankle-brachial indices values exceeding 1.3 have been established as an indicator of medial arterial calcification.25 Although there have been previous studies evaluating PVC as a predictor of either peripheral arterial disease or nontraumatic lower-extremity amputation (ie, major limb amputation), each of these studies used nonstandard methods for scoring PVC.26–29 Similarly, our study employed a distinct scoring approach, noting the number of anatomical locations within the foot of PVC and focusing on its concordant relationship with both MAFEs and CKD. It is vital to acknowledge the intricate relationship between peripheral artery disease and PVC and warrants careful consideration in the interpretation of our findings.
Another limitation of our study was the lack of documentation or exploration of vascular calcification biomarkers, either promoters (eg, sclerostin, FGF-23, etc) or inhibitors (eg, Klotho, Vitamin K, etc).30 The unexpected discovery of ectopic PVC (identified incidentally on a plain foot radiograph in our study), along with reduced bone mineral density or disrupted bone turnover presents a perplexing association found in patients with CKD (as well as the general population and individuals with osteoporosis).31 It is essential to conduct further investigations aimed at comprehending the mechanisms behind this calcification paradox to develop strategies that can treat or even prevent cardiovascular morbidity and mortality in the diabetic nephropathic patient.
CONCLUSIONS
Our study provides valuable insights into the relationships between PVC, MAFEs, and CKD stage in patients with DM and PN. The findings support the use of a single, plain film foot radiographic series as a surrogate marker for assessing the relative risk of foot complications and renal dysfunction in patients with DM and PN. Early recognition of PVCs can prompt appropriate evaluation, intervention, and referral for at risk patients with DM, with the end goal to ultimately improve overall foot health and kidney disease management. Larger prospective studies are warranted to validate these findings and further explore the clinical utility of PVC scoring in diabetic foot disease management. Nonetheless, this study significantly enriches our comprehension and potential clinical handling of foot-related complications in the diabetic neuropathic, nephropathic patient.
Acknowledgements:
The authors wish to thank Indra M. Newman, PhD, for critical manuscript feedback and suggestions. The authors thank Tyler George, who was supported by National Institute of Diabetes and Digestive and Kidney Diseases NIDDK T35 Training Grant DK007400, for his invaluable assistance with data gathering and entry.
Footnotes
Conflict of Interest: None reported.
Financial Disclosure: None reported.
Note: Portions of this study were presented as a virtual poster at the Medical Student Research Project (MSRP) in October 2021.
References
- 1.Wu M, Rementer C, Giachelli CM: Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 93: 365, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan C, Ni L, Zhang C: Vascular calcification: New insights into endothelial cells. Microvasc Res 134: 104105, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Schaper CN, Van Netten JJ, Apelqvist J, et al. : Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab Res Rev 40: e3657, 2024. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DG, Boulton AJM, Bus SA: Diabetic foot ulcers and their recurrence. N Engl J Med 376: 2367, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Rocha-Singh KJ, Zeller T, Jaff MR: Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 83: E212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David Smith C, Gavin Bilmen J, Iqbal S, et al. : Medial artery calcification as an indicator of diabetic peripheral vascular disease. Foot Ankle Int 29: 185, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Persy V, D’haese P: Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 15: 405, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Kakani E, Elyamny M, Ayach T, et al. : Pathogenesis and management of vascular calcification in CKD and dialysis patients. Semin Dial 32: 553, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Beulens JWJ, Yauw JS, Elders PJM, et al. : Prognostic models for predicting the risk of foot ulcer or amputation in people with type 2 diabetes: a systematic review and external validation study. Diabetologia 64: 1550, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Lazzarini PA, Mcphail SM, et al. : Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care 43: 964, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Eggers PW, Gohdes D, Pugh J: Non-traumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney International 56: 1524, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Bosco E, Hsueh L, Mcconeghy KW, et al. : Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol 21: 241, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Kidney Foundation: Kidney Disease: The Basics, 2022. Available at: https://www.kidney.org/news/newsroom/fsindex#fast-facts. Accessed October 15, 2023.
- 15.Jupiter DC, Thorud JC, Buckley CJ, et al. : The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J 13: 892, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazzaruso C, Gallotti P, Pujia A, et al. : Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: a 10-year retrospective cohort study. Endocrine 71: 59, 2021. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt BM, Holmes CM: Updates on Diabetic Foot and Charcot Osteopathic Arthropathy. Curr Diab Rep 18: 74, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Rathnayake A, Saboo A, Malabu UH, et al. : Lower extremity amputations and long-term outcomes in diabetic foot ulcers: A systematic review. World J Diabetes 11: 391, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everhart JE, Pettitt DJ, Knowler WC, et al. : Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia 31: 16, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Watkins PJ, Edmonds ME: Sympathetic nerve failure in diabetes. Diabetologia 1983 25: 73, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Edmonds ME: Medial arterial calcification and diabetes mellitus. Z Kardiol 89: 101, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet JB, Sultan A: Narrative review of the relationship between CKD and diabetic foot ulcer. Kidney Int Rep 7: 381, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur R, Singh R: Mechanistic insights into CKD-MBD-related vascular calcification and its clinical implications. Life Sci 311: 121148, 2022. [DOI] [PubMed] [Google Scholar]
- 24.Durham AL, Speer MY, Scatena M, et al. : Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 114: 590, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leskinen Y, Salenius JP, Lehtimäki T, et al. : The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis 40: 472, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Liu IH, Wu B, Krepkiy V, et al. : Pedal arterial calcification score is associated with the risk of major amputation in chronic limb-threatening ischemia. J Vasc Surg 75: 270, 2022. [DOI] [PubMed] [Google Scholar]
- 27.An WS, Son YK, Kim SE, et al. : Vascular calcification score on plain radiographs of the feet as a predictor of peripheral arterial disease in patients with chronic kidney disease. Int Urol Nephrol 42: 773, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Skolnik J, Weiss R, Meyr AJ, et al. : Evaluating the impact of medial arterial calcification on outcomes of infrageniculate endovascular interventions for treatment of diabetic foot ulcers. Vasc Endovascular Surg 55: 382, 2021. [DOI] [PubMed] [Google Scholar]
- 29.Ferraresi R, Ucci A, Pizzuto A, et al. : A novel scoring system for small artery disease and medial arterial calcification is strongly associated with major adverse limb events in patients with chronic limb-threatening ischemia. J Endovasc Ther 28: 194, 2021. [DOI] [PubMed] [Google Scholar]
- 30.Yamada S, Giachelli CM: Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 100: 87, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toussaint ND, Lau KK, Strauss BJ, et al. : Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23: 586, 2008. [DOI] [PubMed] [Google Scholar]


