Abstract
The structural and functional integrity of conduits used for coronary artery bypass grafting is critical for graft patency. Disruption of endothelial integrity and endothelial dysfunction are incurred during conduit harvesting subsequent to mechanical or thermal injury and during conduit storage prior to grafting, leading to acute thrombosis and early graft failure. Late graft failure, in particular that of vein grafts, is precipitated by progressive atherogenesis. Intra-operative management includes appropriate selection of conduit-specific harvesting techniques and storage solutions. Arterial grafts are prone to vasospasm subsequent to surgical manipulation, and application of intra-operative vasodilatory protocols is critical. Post-operative management includes continuation of oral vasodilator therapy and selection of antithrombotic and lipid-lowering agents to attenuate atherosclerotic disease progression in conduits. In this review, the scientific evidence underlying the key aspects of intra- and post-operative management of conduits for coronary artery bypass grafting is examined. Clinical consensus statements for best clinical practice are provided, and areas requiring further research are highlighted.
Keywords: Coronary artery bypass grafting, Internal thoracic artery, Radial artery, Saphenous vein, Harvesting technique, Vasospasm, Graft failure
Graft patency is the mechanism for the sustained clinical benefits of coronary artery bypass grafting (CABG).
Graphical Abstract
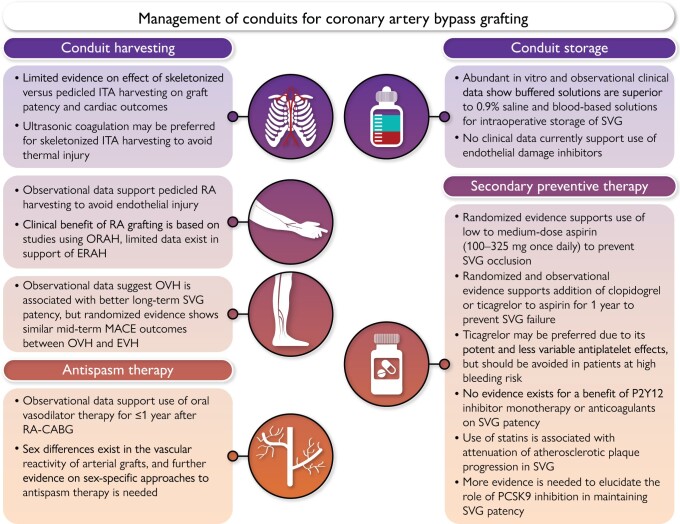
Intra-operative and post-operative management of conduits for coronary artery bypass grafting. CABG, coronary artery bypass grafting; ERAH, endoscopic radial artery harvesting; EVH, endoscopic vein harvesting; ITA, internal thoracic artery; MACE, major adverse cardiac events; ORAH, open radial artery harvesting; OVH, open vein harvesting; PCSK9, proprotein convertase subtilisin/kexin type 9; RA, radial artery; SVG, saphenous vein graft.
Introduction
Graft patency is the mechanism for the sustained clinical benefits of coronary artery bypass grafting (CABG). Continued patency of bypass grafts protects against spontaneous myocardial infarction (MI) and re- duces the need for repeat revascularization.1 In the largest individual participant data pooled analysis on graft failure to date [seven randomized clinical trials (RCTs) involving 4413 patients and 13 163 grafts], graft failure was strongly associated with non-fatal cardiac events, as well as mortality after CABG.2 Graft failure is a multifactorial process that involves acute thrombosis, intimal hyperplasia, inflammation, spasm and atherosclerosis.3 Conduit harvesting techniques, intra- operative storage prior to reimplantation into the coronary circulation, and targeted pharmacotherapy therefore represent the key determinants to preserve the structural and functional integrity and, ultimately, the efficacy of CABG conduits. In this clinical consensus statement by the European Society of Cardiology (ESC) Working Group on Cardiovascular Surgery and the European Association for Cardio-Thoracic Surgery Coronary Task Force, we review the scientific evidence and provide best practice statements for the intra- and post-operative management of CABG conduits. We also highlight gaps in knowledge and future research directions.
Mechanisms and consequences of impaired endothelial function
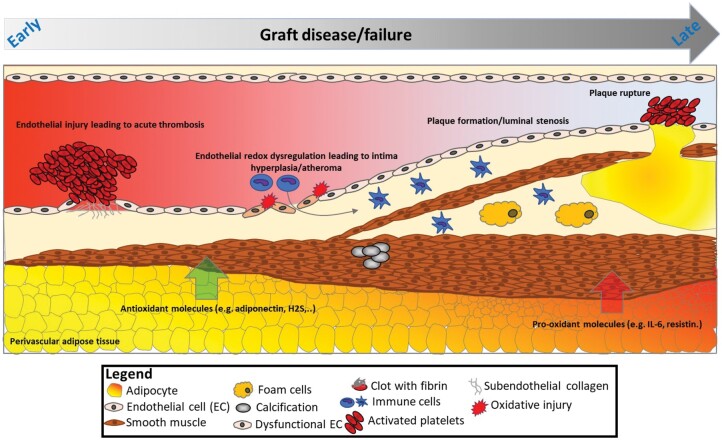
The long-term patency of vein or arterial grafts is highly dependent on the anatomical integrity of the graft in situ and the anatomical and haemodynamic characteristics of the target vessel, but also the biology of the graft. The integrity and the biological ‘health’ of the endothelial layer of the graft are critical factors that determine its early patency given that trauma to the graft during harvesting and storage may lead to disruption of the endothelial layer exposing the subendothelial collagen to the circulating platelets, leading to acute graft thrombosis and failure early after CABG.3 This mechanism, together with technical anastomotic issues, lead to slow blood flow through the graft and largely explain the early thrombosis and graft failure observed in ∼11% of saphenous vein grafts (SVGs) within the first few weeks post-surgery.4,5
Beyond these mechanical factors, endothelial dysfunction [related to reduced endothelial nitric oxide (NO) bioavailability] leads to redox dysregulation in the graft wall and triggers pro-inflammatory and pro- thrombotic mechanisms that may result in graft occlusion.3,6 Indeed, endothelial dysfunction related to clinical risk factors, such as smoking, diabetes or insulin resistance, obesity, and hypercholesterolaemia, is driven by activation of pro-oxidant enzymatic systems in the endothelial cell, such as nicotinamide adenine dinucleotide phosphate oxidases, which generate free radicals like superoxide (O2-), damaging endothelial cell structures.7–9 The same redox dysregulation results in oxidative degradation of endothelial NO synthase (eNOS) co-factor tetrahydro- biopterin, which then induces eNOS uncoupling in the graft’s endothelial cell, further increasing superoxide generation and endothelial dysfunction.8,10 On the other hand, late (>1 year) graft failure is often associated with intimal hyperplasia as part of atherogenesis. Clinical risk factors and the graft biology (e.g. redox dysregulation and endothelial dysfunction)11 lead to proliferation and migration of smooth muscle cells and may also trigger the classic mechanisms of plaque formation, plaque rupture, and late graft failure. Size mismatch, particularly when larger SVGs are grafted to small coronary targets, may predispose to non-laminar flow patterns, which may lead to intimal hyperplasia or graft occlusion.12 Grafts are also prone to spasm, driven by the imbalance between vasoconstrictors (e.g. thromboxane A2 and endothelin) and vasodilators [e.g. NO, endothelial derived relaxation factor, and prostacyclin (PGI2)] subsequent to endothelial dysfunction. Finally, evidence suggests that maintaining perivascular adipose tissue around the graft [internal thoracic artery (ITA) or SVG] could have a beneficial effect on graft patency,13,14 given that perivascular adipose tissue secretes a range of vasodilatory agents [e.g. adiponectin and hydrogen sulfide (H2S)] that could improve endothelial function and the graft’s overall redox state.15,16 An overview of the role of endothelial dysfunction and vascular redox dysregulation in graft failure is shown in Fig. 1.
Figure 1:
Graft failure: from early endothelial injury to late atherosclerotic plaque formation and rupture.
Conduit harvesting
Skeletonized vs. pedicled harvesting of arterial grafts
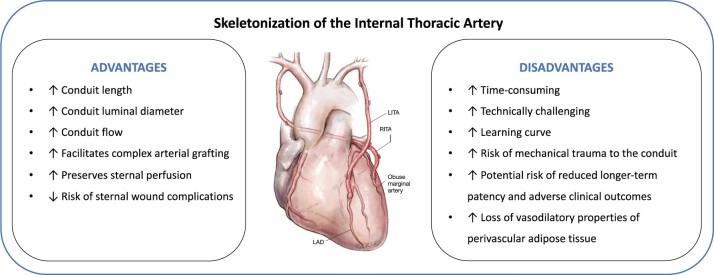
The ITA can be harvested as a pedicled graft (including perivascular fat, veins, and the endothoracic fascia) or as a skeletonized graft (without surrounding tissue). The skeletonized method is technically more challenging, but results in a longer and more versatile conduit that facilitates sequential and composite grafting, and has been shown to improve conduit flow (Fig. 2).17–19 Skeletonizing the ITA reduces sternal devascularization25 and has been associated with lower risk of deep sternal wound infection in non-randomized studies and meta-analyses.20,21,26 This benefit is especially pronounced in diabetic patients and when harvesting bilateral ITAs.20,27 Patients with deep sternal wound infection have an increased risk of adverse short- and long-term clinical out- comes, including an increased mortality risk.28,29 However, recent re- ports have suggested that the skeletonized technique may result in lower patency rates and worse long-term clinical outcomes than the pedicled technique, probably as a result of mechanical trauma to the ITA during harvesting.21–23 Limited evidence suggests that semi- skeletonized harvesting30 may be associated with better results when compared with pedicled harvesting with respect to graft length and flow without increasing operative time, although there are insufficient data to compare the incidence of sternal wound complications or long- term clinical outcomes.31
Figure 2:
Advantages and disadvantages of the skeletonization technique for internal thoracic artery harvesting. The supporting evidence is based on data from meta-analyses of non-randomized studies and small randomized clinical trials and non-randomized studies.19–23 LAD, left anterior descending artery; LITA, left internal thoracic artery; RITA, right internal thoracic artery. Image reproduced with permission from Taggart DP et al.24
While skeletonization of the radial artery (RA) theoretically attenuates potential sympathetic responses and vasoconstriction due to denervation,32 it does not result in significant added conduit length and is more frequently associated with endothelial damage.33 When using non-skeletonized RA grafts, incising the RA fascia after harvesting for the entire length of the RA to allow for maximal dilatation and to protect against local constrictive fibrous bands may combine the advantages of both techniques.32,34 Limited skeletonization for 2-3 cm at the distal and proximal ends of the RA allows maximal dilatation at the anastomotic points and protects against accidental incorporation of any fibrous bands that may distort the anastomosis.34 The gastroepiploic artery (GEA) is mainly used to revascularize the distal branches of the right coronary artery and has shown excellent early and long-term patency rates when harvested as a pedicle including omental tissue.35 As reported for other arterial grafts, skeletonization of the GEA results in larger diameter conduits and may prevent spasm due to arterial denervation and facilitate visual inspection and sequential anastomosis.36 In observational studies, graft patency of skeletonized GEA conduits up to 4 years after surgery was either similar or superior to that of pedicled GEA conduits.37
Electrocautery vs. harmonic scalpel harvesting
Conventional electrocautery enables easy and rapid harvest of the ITA. However, the heat that is transmitted to the artery can injure the endothelium leading to segmental vasospasm.38,39 Yoshida et al.40 using scanning electron microscopy found nearly complete loss of endothelium on the flow surface of the ITA in the branch orifice area following monopolar cauterization vs. partial loss with bipolar cauterization. Bipolar electrocautery enables precise control of current and avoids random spraying of heat in contrast to monopolar electrocautery.41
The harmonic scalpel is an alternative to electrocautery and may be preferred when harvesting the ITA using a skeletonized technique. Ultrasonic coagulation generates lower temperature compared with electrocautery, which reduces thermal-related injuries and tissue charring42 as well as vasospasm.43 Isomura et al.43 found that the tissue temperature is <80°C when ultrasonic coagulation is used, while it is >300°C when electrocautery is used. In addition to generating less heat, ultrasonic coagulation produces less surgical smoke and requires fewer surgical clips.Urso et al.44 in a randomized comparison of electrocautery vs. harmonic scalpel harvesting found that the intra-operative mean graft flow was similar with both techniques. Kieser et al.45 in the largest observational series of harmonic ITA skeletonization found no significant differences in the risk of reoperation for bleeding [0.80, 95% confidence interval (CI) -3.20-4.80], ITA damage (0.25, 95% CI -1.10-1.60), sternal wound complications (-0.40, 95% CI -2.80-2.00), or peri-operative MI (0.70, 95% CI -2.60-4.00) compared with electrocautery.
In observational analyses of RA harvesting, harmonic scalpel induced less spasm and intimal injury compared with electrocautery46,47 and was associated with larger conduit luminal diameter. Nonetheless, no differences in intra-operative graft flow or post-operative graft patency were found.47
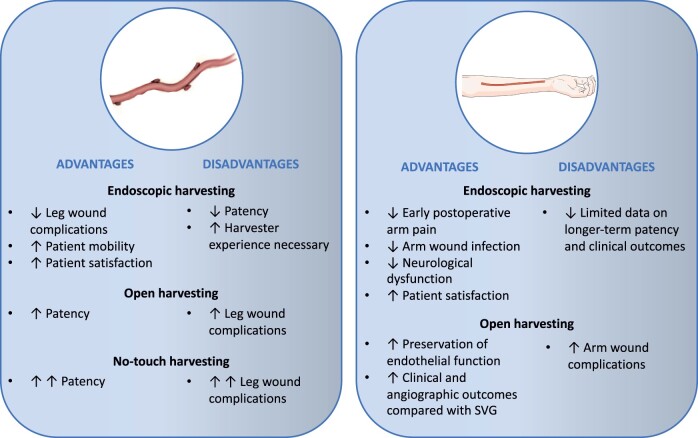
Open vs. endoscopic harvesting techniques
The effectiveness of the endoscopic technique for SVG harvesting [endoscopic vein harvesting (EVH)] in reducing the incidence of harvesting site complications and post-operative pain, as well as increasing patient satisfaction and mobility relative to the open technique [open vein harvesting (OVH)], is well established48 and supports the use of EVH as standard of care in patients who are at risk of leg wound complications (Fig. 3). A pooled analysis of 29 studies (11 919 patients) showed that the odds of wound complications (including abscess, necrosis, dehiscence, drainage, seroma, oedema, and haematoma) were significantly reduced by 71% with EVH compared with OVH [odds ratio (OR) 0.29, 95% CI 0.22-0.37, P < .00001].48 However, patency data for EVH compared with OVH are mixed. Whereas two small RCTs with angiographic follow-up of 3 and 6 months, respectively, did not find a difference in the rate of SVG failure between EVH and OVH,49,50 observational evidence with longer angiographic follow-up, in particular the non-randomized post hoc analyses of the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT)-IV51 and Randomized On/Off Bypass (ROOBY)52 trials, has suggested that EVH is associated with reduced long-term SVG patency.53 A meta-analysis of 11 studies (18 131 patients) reported lower SVG failure rates with OVH at a mean follow-up of 2.6 years (OVH 17.7% vs. EVH 24.9%, OR 0.61, 95% CI 0.43-0.87, P = .01).54 A meta-analysis that included only studies with angiographic follow-up > 1 year (5 studies, 5235 patients) also reported lower SVG patency with EVH (OR 0.80, 95% CI 0.70-0.91).55 The Randomized Endovein Graft Prospective (REGROUP) trial56 did not find a significant difference between OVH and EVH in the risk of the composite of death, MI, or repeat revascularization at a median follow-up of 2.78 years [15.5% vs. 13.9%, hazard ratio (HR) 1.12, 95% CI 0.83-1.51, P = .47] that was confirmed at median follow-up of 4.7 years (OVH 23.5% vs. EVH 21.9%, HR 0.92, 95% CI 0.72-1.18, P = .52).57 Importantly, the REGROUP trial did not include angiographic follow-up and specified minimum harvester experience for both techniques, which has been shown to affect SVG quality.58 In a comparison of OVH vs. EVH performed by experienced (>30/month, >900 total cases) vs. less experienced (<3/month, <100 total cases) harvesters, the incidence of SVG endothelial injury was significantly lower when grafts were procured by experienced harvesters and when using OVH.58
Figure 3:
Advantages and disadvantages of harvesting techniques for the saphenous vein (left) and radial artery (right). SVG, saphenous vein graft. Parts of the figure were drawn using Servier Medical Art (smart.servier.com) licensed under a Creative Commons Attribution 4.0 International Licence (https://creativecommons.org/licenses/by/4.0/).
The effectiveness of the endoscopic RA harvesting (ERAH) technique compared with open RA harvesting (ORAH) in reducing the incidence of arm wound complications, including infection,48 haematoma,48 and incisional pain,59 is consistent with the benefits of endoscopic harvesting of the SVG (Fig. 3). However, similarly, there are concerns that ERAH may adversely affect RA patency and cardiac outcomes due to potential mechanical injury to the endothelium.
This consideration is particularly important for a predominantly muscular and highly spastic conduit such as the RA. Whereas older studies have reported no difference between ORAH and ERAH,60,61 a more contemporary organ bath study showed that ORAH was associated with better preservation of endothelial function compared with ERAH.62 Meta-expertise bias, short follow-up, and low statistical power, reported that ERAH was associated with similar 30-day and longer-term mortality and graft patency rates compared with ORAH.63,64 No adequately powered RCT exists evaluating a strategy of ORAH vs. ERAH on cardiac outcomes. The vast majority of the evidence in support of the efficacy and safety of RA grafting is based on studies that used ORAH. Open radial artery harvesting should therefore currently be considered standard of care.
High-pressure distension
During preparation, the SVG is frequently distended using a handheld syringe to overcome graft spasm and check for leaks. Manual distension leads to intraluminal pressures in excess of 600 mmHg65 that results in endothelial and medial damage66 that has been associated with reduced patency rates.67 Galea et al.68 found that apoptosis was increased in SVGs after distension with 350 mmHg for 2 min. Levels of eNOS remained unchanged in SVGs distended with 100 and 200 mmHg but were significantly lower in SVGs distended with 300 mmHg.69 Stigler et al.70 showed that distension pressures above 50 mmHg were associated with incrementally increased endothelial cell loss and neointimal proliferation. At 50, 100, and 300 mmHg pressures, endothelial loss levels assessed by CD31 immunostaining were 29%, 54%, and 91%, respectively. Although only limited data exist, a pressure-controlling syringe may be helpful in preventing excess graft dilatation and subsequent endothelial damage.
No-touch SVG
Mechanism of benefit
Ahmed et al.71 performed multiple studies assessing changes associated with no-touch (NT) compared with conventional (CON) SVG harvesting using discarded segments of human SVGs from the operating room. On light microscopy, endothelial cushions were present in the NT-SVGs while the endothelial surface was flattened with loss of endothelial integrity in the CON-SVGs. In addition, after dilatation, the total wall thickness was typically greater after NT than CON-SVG harvesting.72 On transmission electron microscopy, the medial smooth muscle cells had a normal appearance in the NT-SVGs but were non-uniform in the CON-SVGs.71 Others have documented the appearance of markers of smooth muscle cell activation, potential precursors of intimal hyperplasia, in the CON compared with the NT-SVGs.73 Furthermore, the adventitial layer consisting of connective tissue, fat, vasa vasorum, and perivascular nerves is preserved in the NT-SVGs, while it is removed with CON-SVG harvesting. Studies with and without retention of the surrounding tissue showed partial reduction of distension-induced endothelial injury in the NT-SVGs.72 The basis for this protective effect may include partial buttressing of the SVG which then limits conduit overdistension. Other possible mechanisms are preservation of eNOS activity, which is highly expressed in the adventitia tissue,72 and certain adipose specific markers such as leptin and adiponectin which are expressed in the perivascular fat.74 More recent studies have shown that the pheno- type of peri-saphenous and peri-ITA fat has similarities with the perivascular fat of atherosclerosis-prone vessels such coronary arteries or the aorta.75 Finally, some data suggest that the vasa vasorum of NT-SVGs remain patent unlike with CON-SVGs.76
Angiographic and clinical outcomes
A recently published network meta-analysis of 18 graft patency RCTs (6543 patients and 8272 grafts) concluded that graft occlusion was substantially reduced [risk ratio (RR) 0.56; 95% CI, 0.44-0.70] at a mean follow-up time of 3.5 years compared with the CON-SVG and that the NT-SVG and RA were ranked as the best conduits.77 Tian et al.78 in an RCT that included 2655 patients showed that SVG occlusion on computed tomography angiography (CTA) was significantly reduced for NT-SVG grafts compared with CON-SVG both at 3 months (2.8% vs. 4.8%, OR 0.57, 95% CI 0.41-0.80, P<.001) and at 12 months (3.7% vs. 6.5%, OR 0.56, 95% CI 0.41-0.76, P < .001). The SWEDEGRAFT registry-based RCT (NCT03501303) compares NT-SVG vs. CON-SVG in 900 patients with a primary endpoint of graft failure on protocol-specified CTA imaging or death at 2 years.79 To date, there is no convincing evidence for better cardiac outcomes when using the NT-SVG compared with the CON-SVG.53 No-touch saphenous vein graft harvesting is however associated with a significantly higher risk of leg wound complications (Fig. 3).53 Tian et al.78 reported that the NT technique was associated with higher rates of leg wound surgical interventions at 3 months (10.3% vs. 4.3%; OR, 2.55; 95% CI, 1.85-3.52; P < .001). Minimally invasive NT-SVG harvesting techniques have recently been described,80,81 including one approach whereby the NT-SVG is harvested endoscopically with the perivascular tissue intact,82 thus combining the advantage of endoscopic harvesting with respect to harvest site complications and the improved patency of NT-SVG.
| Best practice clinical consensus statements: conduit harvesting | Strength of evidence |
|---|---|
|
Meta-analyses of non-randomized studies and small RCTs20,21,26 |
|
Single large RCT,56 meta-analyses of non-randomized studies and small RCTs48 |
|
Large RCT,78 meta-analyses of non-randomized studies and RCTs54,55 |
| Avoid high-pressure distension of SVGs, using a pressure-controlling syringe when possible | Multiple in vitro studies65,70 |
Conduit storage
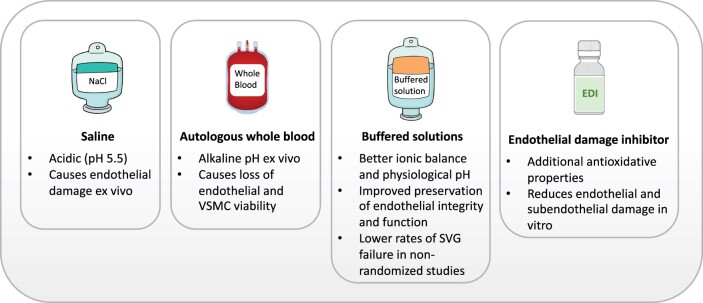
The evidence on the effect of conduit storage solutions is mixed, and data are derived mainly from in vitro studies (Fig. 4). Traditionally, he- parinized 0.9% saline or autologous whole blood (AWB) has been used in clinical practice.83 However, at a pH of 5.5, saline is acidic and has been shown to cause endothelial damage when used as an ex vivo storage solution.84,85 Unlike circulating blood which is under arterial and venous pressure, extracorporeal blood is under atmospheric pressure, which results in loss of partial pressure of CO2 and causes the pH of blood to rapidly become alkaline. Loss of endothelial and smooth muscle cell viability has been shown to occur even after short-term exposure to slightly alkaline solutions at a pH of 8.0.86 Whereas some studies have shown less endothelial injury, inflammatory changes, and tissue necrosis with the use of AWB compared with saline,84 other studies did not find a difference between the two solutions.87 In functional tests, AWB was superior to saline with regard to contraction and relaxation rates, likely due to improved preservation of vascular contractile and endothelial function.88–91
Figure 4:
Comparison of solutions for intra-operative conduit storage. SVG, saphenous vein graft.
Buffered solutions (such as University of Wisconsin preservation solution, histidine-tryptophan-ketoglutarate, TiProtec, and He solutions) provide better ionic balance and physiological pH, and in vitro studies have shown improved preservation of endothelial structural integrity and function compared with both AWB and saline.92 A post hoc analysis of the PREVENT-IV trial showed that use of buffered solutions was associated with lower rates of SVG failure and possibly better clinical outcomes.93 There is evidence to suggest that AWB may increase the susceptibility of the RA to spasm94 and a buffered asanguineous solution may be preferred for intra-operative storage of the RA.32,34
An endothelial damage inhibitor (EDI) is a buffered solution with antioxidative, radical-scavenging, and eNOS-supporting properties that were developed based on the GALA formulation (reduced gluta- thione, L-ascorbic acid, and L-arginine).95 In a small RCT using multide- tector CTA, lower mean SVG wall thickness at 12 months was found for SVGs treated with EDI compared with saline.96 Recent ex vivo studies using EDI on SVGs97,98 as well as RA grafts99 have suggested significant reduction of endothelial and subendothelial damage and reduced levels of reactive oxygen species that correlated with a reduction of hypoxic damage (eNOS and caveolin-1) and significant increase of oxidation-reduction potential when compared with standard buffered solutions98,99 and saline or AWB.97 No comparative studies of EDI vs. other buffered storage solutions have been performed with regard to graft patency or clinical outcomes. Use of EDI increases the cost of the CABG procedure.100
The temperature of the storage solution is probably important in endothelial protection, but evidence is limited. Bush et al.67 reported that the best protection is achieved at room temperature and 37°C, whereas temperature at 4°C causes separation at the basal membrane and spherical changes in cells.
| Best practice clinical consensus statements: conduit storage | Strength of evidence |
|---|---|
|
Multiple in vitro studies89 |
|
Large non-randomized study93 |
|
In vitro study and expert opinion32,94 |
Prevention and treatment of arterial graft spasm
Pathophysiology of arterial graft spasm
The mechanisms of vasospasm in arterial CABG grafts have been described by He and Taggart.101 Vasospasm may be precipitated by vasoconstrictor substances (spasmogens), which in arterial grafts include endothelium-derived contracting factors (e.g. endothelin-1), prostaglandins (e.g. thromboxane A2), alpha-adrenoceptor agonists (e.g. norepinephrine), and platelet-derived substances (e.g. serotonin), among others.101 Arterial grafts such as the ITA and RA are predominantly alpha-adrenoceptor vessels with a high constriction responsiveness to norepinephrine. In comparison with the ITA, the RA has higher receptor-mediated contractility to endothelin, angiotensin II, vasopressin, serotonin, and thromboxane A2. Vasospasm in arterial grafts may also be related to endothelial dysfunction.101 The intact endothelium prevents spasm of the graft by releasing endothelium- derived relaxing factors (e.g. NO and PGI2) which balance vasoconstriction and relaxation in arterial grafts. Whereas the ITA has better endothelial function and releases more NO and other vasorelaxing factors, the RA and GEA have less eNOS expression and require more active pharmacologic interventions.101 The diameter of the ITA is inversely correlated with its tendency for spasm, suggesting that the distal end of the ITA should not be harvested.102 Aspirin exhibits vasoconstrictive properties and inhibits arachidonic acid-dependent vasodilator pathways even at low doses (75-300 mg).103
Sex-related differences in arterial graft physiology
Radial artery size and flow are smaller in women,104,105 whereas ITA segments have been noted to be comparable in size between women and men,106 and both are more likely to be related to body size rather than sex.
Internal thoracic artery endothelial cells in post-menopausal women show impaired expression of messenger RNA for eNOS and reduced eNOS protein levels compared with men,107 suggesting NO-mediated endothelial dysfunction. This is consistent with the findings of lower levels of vasodilators (including NO) and higher levels of vasoconstrictors in the circulating blood of women vs. men.108 It is unclear if these differences contribute to function or the propensity for graft spasm in either sex. Sex differences in vascular reactivity, plasma levels of mediators of microvascular tone, and pharmacologic responses have been described and postulated to be related to higher levels of NO or eNOS in younger women due to higher levels of oestrogen.108
The presence of oestrogen is postulated to delay cellular senescence by a NO-dependent mechanism, and menopause would thus lead to less NO bioavailability and impaired endothelial metabolism.107
Endothelial cyclooxygenase pathway-mediated ITA hypersensitivity to serotonin and to alpha1-adrenergic stimuli in women may be a biological mechanism contributing to post-operative ITA graft spasm in women,109 and excessive ITA graft constriction in women administered catecholamines.110 Table 1 summarizes the sex differences in vascular reactivity responses of ex vivo ITA segments to mediators of vascular tone.
Table 1:
Summary of published internal thoracic artery and saphenous vein segment vascular reactivity testing in women and men
| Author, year | Conduit evaluated | Vascular reactivity results | Patient age | Endothelium dependent | Cyclooxygenase dependent | NO dependent |
|---|---|---|---|---|---|---|
| Dignan,1992106 | IMA segments |
|
50–76 years | |||
| Akar, 2007111 | IMA segments |
|
|
|||
| Muir, 2010112 | IMA and SV segments |
|
|
|||
| Mannacio, 2012107 | IMA segments |
|
|
|||
| Lamin, 2018109 | IMA segments |
|
|
Yes—serotonin response | Yes—serotonin response | No—serotonin response |
| Riedel, 2019113 | IMA segments |
|
Men and Women 48–55 years | Yes—norepinephrine, isoprenaline | Yes—norepinephrine, isoprenaline | |
| Jaghoori, 2020110 | IMA and SV segments |
|
|
Yes—phenylephrine | Yes—phenylephrine | No—phenylephrine |
KATP, adenosine triphosphate-sensitive potassium channel; KCa, calcium-activated potassium channel; ITA, internal thoracic artery; SV, saphenous vein; NO, nitric oxide.
Vasodilatory protocols
Intra-operative protocols
Papaverine is the most widely used agent for vasodilation of the ITA. Papaverine can either be injected into endothoracic fascia before harvesting or topically applied after harvesting and the ITA covered with a papaverine-soaked gauze.114 Several studies have shown the beneficial effect on ITA graft flow after periarterial or intraluminal administration of papaverine.115 Intraluminal papaverine administration may increase vasodilation over topical administration, but it is associated with the risk of intimal injury.116,117 Sodium nitroprusside has also been shown to be a potent vasodilator when used topically on the ITA, but is less frequently used.118
Several RA bath options have been described.32,119 The most commonly used topical vasodilating agents for the RA in clinical practice are calcium channel blockers (CCBs) and nitrates. In particular, the combined use of verapamil and nitroglycerine is favoured and is more effective at RA vasospasm prevention than when each agent is used individually. A verapamil/nitroglycerine solution better preserves RA endothelial function than does papaverine.120 For an RA bath including papaverine, a buffered solution such as Ringer's lactate, or heparinized blood at 37° may be used.34 The phosphodiesterase inhibitor milrinone has a potent vasodilatory effect on the RA and may be used topically in heparinized arterial blood.32,34
Post-operative protocols
Patients with RA grafts commonly receive oral antispasm therapy postoperatively, and the CCBs amlodipine and diltiazem are used most frequently121 (Table 2). However, the evidence on the effect of CCB on the RA is inconsistent. A small RCT that assigned 100 patients to either receive or not receive diltiazem for 1 year starting in the early postoperative period showed no difference in clinical or angiographic outcomes at 1 year.132 Similarly, a post hoc analysis of the Radial Artery Patency Study found that among 440 patients with RA grafts, the incidence of string sign (the highest degree of RA spasm) was not associated with patients' compliance with the prescribed post-operative CCB therapy.133 In a post hoc analysis of the RADIAL database that included 732 patients with RA grafts, CCB therapy was associated with a significantly lower risk of major adverse cardiac events (MACE) (HR, 0.52; 95% CI, 0.31-0.89; P = .02) and RA occlusion (HR, 0.20; 95% CI, 0.08-0.49; P < .001).134 Calcium channel blocker therapy for 1 year was associated with a greater reduction in the risk of MACE (P < .001) and RA occlusion (P = .006) than a shorter duration of CCB therapy. A benefit of a longer duration of CCB therapy was not demonstrated (P = .08), although the numbers of patients on prolonged CCB therapy was small. After implantation in the coronary circulation, RA grafts undergo remodelling of the vessel wall with a progressive reduction in the muscular component of the media and thus a reduction in the propensity for spasm.135 This process is completed 1 year post-operatively,135 suggesting that in clinical practice, the duration of CCB therapy may be limited to the first post-operative year.
Table 2:
Therapeutic strategies for preventing arterial graft spasm and graft atherogenesis after coronary artery bypass grafting
| Agent | Mechanisms of action | Time of initiation after CABG and treatment duration | Main study findings | Strength of evidence |
|---|---|---|---|---|
| Vasospasm prevention | ||||
| Amlodipin, diltiazem | Calcium channel antagonist | Treatment duration 1 year | ↓ incidence of RA occlusion | Observational32 |
| Inhibition of platelet aggregation | ||||
| Aspirin | Cyclooxygenase inhibition | Within 24 h (ideally with 6 h) after CABG and continued indefinitely | ↓ incidence of SVG occlusion | Multiple RCTs and MAs of RCTs122–124 |
| Clopidogrel | Irreversible P2Y12 receptor inhibitor | Variable timing of post-operative initiation; treatment duration 3–12 months | ↓ incidence of SVG failure or occlusion Conflicting findings with regard to incidence of major bleeding | Several study-level MA of small RCTs and observational studies125–127 |
| Ticagrelor | Reversible P2Y12 receptor inhibitor Pleiotropic effects including attenuation of ischaemia–reperfusion injury, inflammation, and atherosclerosis128 | Within 48 h after CABG and continued for 1 year |
|
Single RCT of 500 patients,129 study-level MA of RCTs,125 IPD-MA of RCTs130 |
| LDL-C-lowering | ||||
| Statins | HMG-CoA reductase inhibition Pleiotropic effect on inflammation | Continued peri-operative treatment to LDL-C target level | ↓ progression of graft atherosclerosis | One large RCT131 |
BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass grafting; HMG-CoA, hydroxy-methylglutaryl coenzyme A; MA, meta-analysis; RA, radial artery; RCT, randomized clinical trial; SVG, saphenous vein graft.
It is unclear whether there is a difference in the antispasm efficacy on the RA between amlodipine and diltiazem. In the post hoc analysis of RADIAL, use of amlodipine (HR, 0.30; 95% CI, 0.12-0.74; P = .009) and diltiazem (HR, 0.20; 95% CI, 0.07-0.51; P < .001) was associated with a similar protective effect on the risk of RA occlusion when compared with non-use of CCBs.134
It should be noted that chronic CCB use has side effects including headache, tachycardia, flushing, and peripheral oedema. In addition, use of CCB therapy due to its hypotensive effect may preclude the use of secondary preventive medications such as beta-blockers or renin-angiotensin-aldosterone system inhibitors.
An RCT that compared a strategy of 24 h i.v. infusion of nitroglycerine with diltiazem, followed by 6-month treatment with a daily oral dose of isosorbide mononitrate or diltiazem, found no differences in clinical outcomes.136 Tachyphylaxis may render oral nitrates less effective for continued prevention of RA graft vasospasm, and no evidence exists evaluating their post-operative use with regard to graft patency.
| Best practice clinical consensus statements: antispasm prophylaxis | Strength of evidence |
|---|---|
|
Non-randomized study134 |
Secondary prevention of graft failure
The mechanism of graft failure is distinctly different between arterial grafts and SVGs. Acute thrombosis and late atherosclerosis are observed predominantly in SVGs, and pharmacological therapy is thus mainly aimed at preventing SVG failure (Table 2). The role of pharmacological therapies in optimizing the late patency of arterial grafts is not well characterized.
Antithrombotic therapy Aspirin
The routine use of aspirin is based on decades-old RCTs demonstrating the benefit of aspirin compared with placebo to prevent SVG occlusion.122–124 Goldman et al.122 in the largest RCT with angiographic follow-up including 772 patients (Veterans Administration Cooperative Study) found that aspirin significantly decreased SVG occlusion vs. placebo early and at 1 year after CABG (15.8% vs. 22.6%, P = .029).137 A meta-analysis of 17 RCTs that included 1443 patients showed that a low (100 mg) to medium (325 mg) daily aspirin dose initiated within 6 h post-CABG is most effective, without an increase in post-operative bleeding.124,138 Randomized clinical trials of delayed (>24 h post-operatively) initiation of aspirin did not find a benefit on138,139 SVG patency. Low-dose aspirin (75-100 mg daily) appears sufficient as maintenance therapy as it exceeds the minimal effective dose required for platelet thromboxane A2 suppression and overcomes interindividual variability in drug response.140 More than once-daily dosing may be considered in the immediate post-operative phase after on-pump CABG. Use of cardiopulmonary bypass promotes postoperative platelet turnover leading to increased synthesis of thromboxane and may reduce early post-operative aspirin efficacy. However, more frequent aspirin dosing must be balanced with an increased risk of bleeding. Based on an association between aspirin dosing and outcomes in a post hoc analysis of the Platelet Inhibition and Patient Outcomes Study, low-dose aspirin should be used in patients treated with ticagrelor.141
Current clinical practice is to continue antiplatelet therapy life-long after CABG, but evidence in support of a clinical benefit is limited. There is in fact little evidence to support a clinical benefit, as opposed to a graft patency benefit, from aspirin use after CABG.142,143 Most studies showed no effect on mortality, or even a trend to excess mortality,124,144 but they were underpowered for small to moderate differences in clinical outcomes and, in particular, in mortal- ity.125,137,145 However, a pooled analysis of 7 contemporary RCTs with systematic graft imaging (4413 patients, 13 163 grafts) showed that graft failure is strongly associated with adverse cardiac events (adjusted OR 3.98, 95% CI 3.54-4.47, P < .001) and mortality after CABG (adjusted OR 2.79, 95% CI 2.01-3.89, P < .001),2 indirectly supporting a potential clinical benefit of aspirin. On the other hand, 1 trial randomized 213 patients 1 year after CABG to continue aspirin 325 mg/day or switch to placebo for the following 2 years and found no difference in the rate of graft occlusion, MI, or death (although the trial was not formally powered and the described power limitations apply).145
Dual antiplatelet therapy
Evidence from RCTs and observational studies supports a strategy of dual antiplatelet therapy (DAPT) after CABG to reduce SVG failure. An early meta-analysis of 11 studies (5 RCTs, 6 observational studies) and 25 728 patients showed that aspirin + clopidogrel compared with aspirin was associated with a significantly lower risk of SVG occlusion (RR 0.59, 95% CI 0.43-0.82, P = .02), but also with a higher risk of major bleeding events (RR 1.17, 95% CI 1.00-1.37, P = .05).126 A sub-analysis that included 2 RCT (560 patients) showed that aspirin + clopidogrel was associated with a lower risk of SVG occlusion after off-pump CABG. Dual antiplatelet therapy compared with aspirin was associated with a lower risk of 30-day/in-hospital mortality (RR 0.38, 95% CI 0.26-0.57, P < .001); there was no difference between the treatment strategies in the risk of angina or MI (RR 0.60, 95% CI 0.31-1.14, P = .12).126 Another meta-analysis of 5 RCTs and 958 patients that compared aspirin + clopidogrel with aspirin also showed an association between aspirin and the risk of SVG occlusion (OR 1.70, 95% CI 1.20-2.40) but not arterial graft occlusion (OR 1.17, 95% CI 0.54-2.56).146 A network meta-analysis that included 20 RCTs (4803 patients) investigating 9 different antithrombotic strategies showed that the use of either aspirin + ticagrelor (2 RCTs, OR 0.50, 95% CI 0.31-0.79) or aspirin + clopidogrel (7 RCTs, OR 0.60, 95% CI 0.420.86) was associated with a lower risk of SVG failure compared with aspirin alone, without significant differences in major bleeding, MI, and death.125 However, the analyses were likely underpowered to detect small to moderate differences in clinical outcomes. All these study- level meta-analyses were limited by heterogeneity with regard to type and duration of P2Y12 inhibitor treatment, duration of follow-up, and definitions of SVG failure and bleeding.
In an individual patient data meta-analysis of 4 RCTs (1316 patients) that used rigorous re-adjudication of outcomes, aspirin + ticagrelor was associated with a significantly lower incidence of SVG failure compared with aspirin (11.2% vs. 20%; OR 0.51, 95% CI 0.35-0.74; P < .001).130
This finding was consistent for patients undergoing on- or off-pump CABG (Pint = .15) and for SVG and arterial grafts (including ITA and RA grafts, Pint = .93). However, patients receiving aspirin + ticagrelor had a significantly increased risk of clinically important bleeding events [Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding: 22.1% vs. 8.7%, OR 2.98, 95% CI 1.99-4.47; P < .001]. Of note, the median treatment duration with aspirin + ticagrelor was 1 year. The specific impact of DAPT on arterial graft patency, particularly in relation to different arterial graft types, has not yet been studied in detail.
These findings highlight the importance of a DAPT strategy after CABG that reduces bleeding risk while retaining its efficacy in reducing SVG failure. Platelet-driven thrombosis is the predominant mechanism of early SVG failure and typically occurs during the first month after surgery,147 providing a biological rationale for intensified antiplatelet therapy in the first month after CABG. The 1-month DAPT with ticagrelor in coronary artery bypass graft patients (ODIN) trial (NCT05997693) is an investigator-initiated prospective, randomized, international, multicentre trial that is designed to compare the effect of treatment with ticagrelor in addition to low-dose aspirin for 1 month vs. aspirin alone on the 1-year incidence of ischaemic events and graft failure among patients with chronic coronary syndromes undergoing CABG.148 ODIN will also inform whether short-term DAPT provides a net clinical benefit in this patient population. The Ticagrelor-based De-escalation of Dual Antiplatelet Therapy after Coronary Artery Bypass Grafting (TOP-CABG) trial (NCT05380063) will investigate whether de-escalation of DAPT (ticagrelor + aspirin) to aspirin monotherapy after 3 months is non-inferior to DAPT for 12 months in reducing SVG occlusion and superior in reducing bleeding events.
No randomized head-to-head comparison of ticagrelor vs. clopidogrel (on a background of aspirin) for SVG patency exists. Ticagrelor has a rapid onset and offset of action and provides faster, more powerful, and predictable platelet inhibition than clopidogrel.149 Clopidogrel has a variable interindividual response, with approximately one-third of patients having inadequate platelet inhibitory effects. Importantly, such patients who continue to have high platelet reactivity despite use of clopidogrel are at increased risk of thrombotic events.150 Clopidogrel may be preferred when ticagrelor is not available, not tolerated or contraindicated, and in patients at high bleeding risk.
P2Y12 inhibitor monotherapy and anticoagulant therapy
Current evidence does not support P2Y12 inhibitor monotherapy as analternative to aspirin after CABG.129,151,152 When pooling individual patient data from the two RCTs investigating the effect of ticagrelor monotherapy, ticagrelor was not associated with a significant difference in the risk of SVG failure compared with aspirin, although the point estimate favoured ticagrelor monotherapy (OR 0.86, 95% CI 0.58-1.27).130 In a sub- study of the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial, the factor Xa inhibitor rivaroxaban either alone or in combination with aspirin did not reduce the 1-year incidence of graft failure compared with aspirin alone (rivaroxaban vs. aspirin: 7.8% vs. 8.0%; OR: 0.95, 95% CI: 0.67-1.33; P = .75; rivaroxaban + aspirin vs. aspirin: 9.1% vs. 8.0%; OR: 1.13, 95% CI 0.82-1.57; P = .45).153
Lipid-lowering therapy
In patients with clinical atherosclerotic cardiovascular disease (ASCVD), including those with a history of CABG, high-intensity statin therapy is guidelines recommended with the aim of achieving a >50% reduction in LDL cholesterol (LDL-C) to reduce the risk of cardiovascular events.154 The magnitude of the benefit of high-intensity statins is similar among women and men,155,156 although high-intensity statins remain underused in women. Elevated LDL-C levels are associated with atherosclerotic plaque progression in SVGs.157 In addition to lowering LDL-C, statins are also known to have pleiotropic effects, improving endothelial function, NO levels, and antioxidant function, as well as inhibiting inflammatory response, vasoconstriction, thrombosis, and platelet aggregation.158 Several studies have examined the effect of statins on graft patency.131,159,160 The Post Coronary Artery Bypass Graft (Post CABG) trial showed that aggressive (target LDL-C < 85 mg/dL) compared with moderate (target LDL-C < 140 mg/dL) lowering of LDL-C using lovastatin decreased obstructive changes in CABG grafts by 31% at >4 years of follow-up.131 In a non-randomized post hoc comparison of participants on statin therapy in the Clopidogrel after Surgery for Coronary Artery Disease trial, 12-month graft patency as assessed by coronary angiography was higher in those with LDL-C levels < 100 mg/dL than in those with LDL-C levels > 100 mg/dL (96.5% vs. 83.3%, P = .03).161 The ACTIVE trial, comparing a strategy of 10 mg (moderate-intensity) vs. 80 mg (high-intensity) atorvastatin, did not find a difference in the incidence of SVG occlusion at 1 year; however, the trial was limited by small sample size and high rate of protocol violations, with approximately one-third of patients in each arm discontinuing the assigned treatment over the course of the study.160
More recently, circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) has been shown to induce macrophage activation and vein graft lesion development via LDL receptor-independent mechanisms,162 representing a potential target for pharmacologic intervention. A small cross-sectional study from China (231 patients) showed a significant association between circulating PCSK9 levels and the presence of SVG disease at >1 year after CABG. The Effect of Evolocumab on Saphenous Vein Graft Patency Following Coronary Artery Bypass Surgery (NEWTON-CABG) trial (NCT03900026) will examine the effect of the PCSK9 inhibitor evolocumab vs. placebo in addition to statin therapy for 24 months on SVG disease (defined as significant stenosis > 50% or total occlusion) on protocol-specified CTA or earlier clinically indicated coronary angiography.
| Best practice clinical consensus statements: secondary prevention of graft failure | Strength of evidence |
|---|---|
|
Meta-analysis of RCTs124 |
|
Study-level and individual participant data meta-analyses of RCTs125,130 |
|
Large RCT131 |
Future research directions
Several gaps in our knowledge remain with respect to intra-operative and post-operative management of conduits, and further research is urgently needed to address these.
Randomized studies are needed comparing skeletonized and pedicled ITA harvesting to determine how these techniques affect ITA graft patency and post-operative cardiac outcomes.
Further studies are needed to evaluate the effect of ERAH on cardiovascular and patient-reported outcomes, given that the majority of randomized trials demonstrating superiority of the RA over the SVG have used ORAH.
Further research is needed to investigate potential relative clinical benefits of available storage solutions and address cost-effectiveness.
The role of oestrogen in arterial graft physiology remains unclear. Outcomes following CABG are worse in women (pre- and postmenopausal) compared with men and have been noted to be significantly worse in younger women.164 Clarification of pathways that are influenced by the levels of oestrogen will be important for future therapeutics. Sex-specific management of arterial conduits should be a focus of future research.
Randomized studies are needed to evaluate the efficacy of continued oral antispasm therapy in patients with RA grafts.
Low-grade systemic inflammation is a more powerful determinant of recurrent cardiovascular events and death than LDL-C in patients with stable ASCVD.165 The clinical benefit of targeted inflammation inhibition has been shown in particular for low-dose colchicine.166 Given the pro-inflammatory mechanisms implicated in graft failure subsequent to endothelial injury, further studies are needed to determine whether patients after CABG would benefit from the addition of these pharmacotherapies to reduce the risk of graft failure.
Summary
Preserving the structural and functional integrity of the conduit during graft harvesting and storage, prevention and treatment of vasospasm, and attenuating atherogenesis are integral to graft patency and the clinical benefits of CABG. The best practice clinical consensus statements outlined in this document provide a comprehensive, evidence-based approach to the intra-operative and post-operative management of conduits for CABG surgery. These strategies can serve as a valuable resource for multidisciplinary heart teams, facilitating more informed and effective treatment planning tailored to individual patient needs and local practices.
Contributor Information
Sigrid Sandner, Department of Cardiac Surgery, Medical University of Vienna, Spitalgasse 23, 1090 Vienna, Austria; Department of Cardiothoracic Surgery, Weill Cornell Medicine, 525 E 68th St, New York, NY 10065, USA.
Charalambos Antoniades, Division of Cardiovascular Medicine, Radcliffe Department Medicine, University of Oxford, Oxford, UK.
Etem Caliskan, Department of Cardiothoracic and Vascular Surgery, Deutsches Herzzentrum der Charité (DHZC), Berlin, Germany.
Martin Czerny, Department of Cardiovascular Surgery, University Heart Center Freiburg—Bad Krozingen, Germany; Faculty of Medicine, Albert Ludwigs University Freiburg, Freiburg, Germany.
Victor Dayan, University Cardiovascular Center, National Institute of Cardiac Surgery, Montevideo, Uruguay.
Stephen E Fremes, Schulich Heart Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada.
David Glineur, Division of Cardiac Surgery, Memorial University, St John’s, Newfoundland, Canada; Department of Cardiac Surgery, University of Ottawa Heart Institute, Ottawa, Ontario, Canada.
Jennifer S Lawton, Department of Surgery, Division of Cardiac Surgery, Johns Hopkins University, Baltimore, MD, USA.
Matthias Thielmann, Department of Thoracic and Cardiovascular Surgery, West-German Heart and Vascular Center, University of Duisburg-Essen, Essen, Germany.
Mario Gaudino, Department of Cardiothoracic Surgery, Weill Cornell Medicine, 525 E 68th St, New York, NY 10065, USA.
Declarations
Disclosure of Interest
This document is submitted following review and approval by the ESC Scientific Documents Committee (SDoC). The declarations of interests review is handled by the respective oversight body according to the section 6.3.3 ‘DOI review’ of the ESC Scientific Documents policy.
Data Availability
No data were generated or analysed for this manuscript.
Funding
All authors declare no funding for this contribution.
REFERENCES
- 1. Gaudino M, Di Franco A, Bhatt DL, Alexander JH, Abbate A, Azzalini L, et al. The association between coronary graft patency and clinical status in patients with coronary artery disease. Eur Heart J 2021;42:1433–41. https://doi.org/10.1093/eurheartj/ehab096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaudino M, Sandner S, An KR, Dimagli A, Di Franco A, Audisio K, et al. Graft failure after coronary artery bypass grafting and its association with patient characteristics and clinical events: a pooled individual patient data analysis of clinical trials with imaging follow-up. Circulation 2023;148:1305–15. https://doi.org/10.1161/CIRCULATIONAHA.123.064090 [DOI] [PubMed] [Google Scholar]
- 3. Gaudino M, Antoniades C, Benedetto U, Deb S, Di Franco A, Di Giammarco G, et al. Mechanisms, consequences, and prevention of coronary graft failure. Circulation 2017; 136:1749–64. https://doi.org/10.1161/CIRCULATIONAHA.117.027597 [DOI] [PubMed] [Google Scholar]
- 4. Antonopoulos AS, Odutayo A, Oikonomou EK, Trivella M, Petrou M, Collins GS, et al. Development of a risk score for early saphenous vein graft failure: an individual patient data meta-analysis. J Thorac Cardiovasc Surg 2020;160:116–27.e4. https://doi.org/10.1016/j.jtcvs.2019.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wood A, Antonopoulos A, Chuaiphichai S, Kyriakou T, Diaz R, Al Hussaini A, et al. PHACTR1 modulates vascular compliance but not endothelial function: a translational study. Cardiovasc Res 2023;119:599–610. https://doi.org/10.1093/cvr/cvac092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spadaccio C, Antoniades C, Nenna A, Chung C, Will R, Chello M, et al. Preventing treatment failures in coronary artery disease: what can we learn from the biology of in-stent restenosis, vein graft failure, and internal thoracic arteries? Cardiovasc Res 2020;116:505–19. https://doi.org/10.1093/cvr/cvz214 [DOI] [PubMed] [Google Scholar]
- 7. Oikonomou EK, Antoniades C.. Immunometabolic regulation of vascular redox state: the role of adipose tissue. Antioxid Redox Signal 2018;29:313–36. https://doi.org/10.1089/ars.2017.7017 [DOI] [PubMed] [Google Scholar]
- 8. Akoumianakis I, Badi I, Douglas G, Chuaiphichai S, Herdman L, Akawi N, et al. Insulin-induced vascular redox dysregulation in human atherosclerosis is ameliorated by dipeptidyl peptidase 4 inhibition. Sci Transl Med 2020;12:eaav8824. https://doi.org/10.1126/scitranslmed.aav8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, et al. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft re- dox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation 2010;122: S66–73. https://doi.org/10.1161/CIRCULATIONAHA.109.927376 [DOI] [PubMed] [Google Scholar]
- 10. Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation 2007;116:2851–9. https://doi.org/10.1161/CIRCULATIONAHA.107.704155 [DOI] [PubMed] [Google Scholar]
- 11. Akoumianakis I, Polkinghorne M, Antoniades C.. Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications. Nat Rev Cardiol 2022;19: 783–97. https://doi.org/10.1038/s41569-022-00718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desai ND, Naylor CD, Kiss A, Cohen EA, Feder-Elituv R, Miwa S, et al. Impact of patient and target-vessel characteristics on arterial and venous bypass graft patency: insight from a randomized trial. Circulation 2007;115:684–91. https://doi.org/10.1161/CIRCULATIONAHA.105.567495 [DOI] [PubMed] [Google Scholar]
- 13. Saito T, Kurazumi H, Suzuki R, Matsunaga K, Tsubone S, Lv B, et al. Perivascular adipose tissue is a major source of nitric oxide in saphenous vein grafts harvested via the no- touch technique. J Am Heart Assoc 2022;11:e020637. https://doi.org/10.1161/JAHA.120.020637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 2005;130:1130–6. https://doi.org/10.1016/jjtcvs.2005.05.028 [DOI] [PubMed] [Google Scholar]
- 15. Akoumianakis I, Sanna F, Margaritis M, Badi I, Akawi N, Herdman L, et al. Adipose tissue-derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci Transl Med 2019;11:eaav5055. https://doi.org/10.1126/scitranslmed.aav5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akawi N, Checa A, Antonopoulos AS, Akoumianakis I, Daskalaki E, Kotanidis CP, et al. Fat-secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. J Am Coll Cardiol 2021;77:2494–513. https://doi.org/10.1016/j.jacc.2021.03.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deja MA, Wos S, Golba KS, Zurek P, Domaradzki W, Bachowski R, et al. Intraoperative and laboratory evaluation of skeletonized versus pedicled internal thoracic artery. Ann Thorac Surg 1999;68:2164–8. https://doi.org/10.1016/S0003-4975(99)00820-6 [DOI] [PubMed] [Google Scholar]
- 18. Wendler O, Tscholl D, Huang Q, Schafers HJ.. Free flow capacity of skeletonized versus pedicled internal thoracic artery grafts in coronary artery bypass grafts. Eur J Cardiothorac Surg 1999;15:247–50. https://doi.org/10.1016/S1010-7940(99)00012-3 [DOI] [PubMed] [Google Scholar]
- 19. Kusu-Orkar TE, Kermali M, Masharani K, Noshirwani A, MacCarthy-Ofosu B, Oguamanam N, et al. Skeletonized or pedicled harvesting of left internal mammary artery: a systematic review and meta-analysis. Semin Thorac Cardiovasc Surg 2021;33: 10–8. https://doi.org/10.1053Zj.semtcvs.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Saso S, James D, Vecht JA, Kidher E, Kokotsakis J, Malinovski V, et al. Effect of skeleto- nization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg 2010;89:661–70. https://doi.org/10.1016/j.athoracsur.2009.08.018 [DOI] [PubMed] [Google Scholar]
- 21. Dimagli A, Gemelli M, Kumar N, Mitra M, Sinha S, Fudulu D, et al. A systematic review and meta-analysis of internal thoracic artery harvesting techniques: skeletonized vs pedicled. IntJ Cardiol 2024;395:131577. https://doi.org/10.1016/jjjcard.2023.131577 [DOI] [PubMed] [Google Scholar]
- 22. Lamy A, Browne A, Sheth T, Zheng Z, Dagenais F, Noiseux N, et al. Skeletonized vs pedicled internal mammary artery graft harvesting in coronary artery bypass surgery: a post hoc analysis from the COMPASS trial. JAMA Cardiol 2021;6:1042–9. https://doi.org/10.1001/jamacardio.2021.1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaudino M, Audisio K, Rahouma M, Chadow D, Cancelli G, Soletti GJ, et al. Comparison of long-term clinical outcomes of skeletonized vs pedicled internal thoracic artery harvesting techniques in the arterial revascularization trial. JAMA Cardiol 2021;6:1380–6. https://doi.org/10.1001/jamacardio.2021.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taggart DP. How I deploy arterial grafts. Ann Cardiothorac Surg 2018;7:690–7. https://doi.org/10.21037/acs.2018.09.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boodhwani M, Lam BK, Nathan HJ, Mesana TG, Ruel M, Zeng W, et al. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation 2006;114:766–73. https://doi.org/10.1161/CIRCULATIONAHA.106.615427 [DOI] [PubMed] [Google Scholar]
- 26. Sa MP, Ferraz PE, Escobar RR, Vasconcelos FP, Ferraz AA, Braile DM, et al. Skeletonized versus pedicled internal thoracic artery and risk of sternal wound infection after coronary bypass surgery: meta-analysis and meta-regression of 4817 patients. Interact Cardiovasc Thorac Surg 2013;16:849–57. https://doi.org/10.1093/icvts/ivt012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benedetto U, Altman DG, Gerry S, Gray A, Lees B, Pawlaczyk R, et al. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: insights from the arterial revascularization trial. J Thorac Cardiovasc Surg 2016;152:270–6. https://doi.org/10.1016/jjtcvs.2016.03.056 [DOI] [PubMed] [Google Scholar]
- 28. Gaudino M, Audisio K, Rahouma M, Robinson NB, Soletti GJ, Cancelli G, et al. Association between sternal wound complications and 10-year mortality following coronary artery bypass grafting. J Thorac Cardiovasc Surg 2023;166:532–9.e4. https://doi.org/10.1016/j.jtcvs.2021.10.067 [DOI] [PubMed] [Google Scholar]
- 29. Perezgrovas-Olaria R, Audisio K, Cancelli G, Rahouma M, Ibrahim M, Soletti GJ, et al. Deep sternal wound infection and mortality in cardiac surgery: a meta-analysis. Ann Thorac Surg 2023;115:272–80. https://doi.org/10.1016/j.athoracsur.2022.04.054 [DOI] [PubMed] [Google Scholar]
- 30. Horii T, Suma H.. Semiskeletonization of internal thoracic artery: alternative harvest technique. Ann Thorac Surg 1997;63:867–8. https://doi.org/10.1016/S0003-4975(96)01123-X [DOI] [PubMed] [Google Scholar]
- 31. Maskell P, Berks M, Vibhishanan J, Harky A.. In patients undergoing coronary artery bypass grafting is semi-skeletonization superior to pedicled harvesting of the left internal mammary artery? Interact Cardiovasc Thorac Surg 2021;33:362–6. https://doi.org/10.1093/icvts/ivab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaudino M, Fremes S, Schwann TA, Tatoulis J, Wingo M, Tranbaugh RF.. Technical aspects of the use of the radial artery in coronary artery bypass surgery. Ann Thorac Surg 2019;108:613–22. https://doi.org/10.1016/j.athoracsur.2018.10.066 [DOI] [PubMed] [Google Scholar]
- 33. Rukosujew A, Reichelt R, Fabricius AM, Drees G, Tjan TD, Rothenburger M, et al. Skeletonization versus pedicle preparation of the radial artery with and without the ultrasonic scalpel. Ann Thorac Surg 2004;77:120–5. https://doi.org/10.1016/S0003-4975(03)01488-7 [DOI] [PubMed] [Google Scholar]
- 34. Tatoulis J. The radial artery: an important component of multiarterial coronary surgery and considerations for its optimal harvest.JTCVS Tech 2021;5:46–55. https://doi.org/10.1016/j.xjtc.2020.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suma H, Tanabe H, Takahashi A, Horii T, Isomura T, Hirose H, et al. Twenty years experience with the gastroepiploic artery graft for CABG. Circulation 2007;116:I188–91. https://doi.org/10.1161/CIRCULATIONAHA.106.678813 [DOI] [PubMed] [Google Scholar]
- 36. Gagliardotto P, Coste P, Lazreg M, Dor V.. Skeletonized right gastroepiploic artery used for coronary artery bypass grafting. Ann Thorac Surg 1998;66:240–2. https://doi.org/10.1016/S0003-4975(98)00403-2 [DOI] [PubMed] [Google Scholar]
- 37. Ali E, Saso S, Ashrafian H, Athanasiou T.. Does a skeletonized or pedicled right gastroepiploic artery improve patency when used as a conduit in coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg 2010;10:293–8. https://doi.org/10.1510/icvts.2009.221226 [DOI] [PubMed] [Google Scholar]
- 38. Blake KL, Watt PA, Smith JM, De Souza AC, Spyt TJ, Thurston H.. Randomized comparison of ultrasonic aspiration versus conventional electrocautery for dissection of the human internal thoracic artery. J Thorac Cardiovasc Surg 1996;111:1194–9. https://doi.org/10.1016/S0022-5223(96)70221-0 [DOI] [PubMed] [Google Scholar]
- 39. Lehtola A, Verkkala K, Jarvinen A.. Is electrocautery safe for internal mammary artery (IMA) mobilization? A study using scanning electron microscopy (SEM). Thorac Cardiovasc Surg 1989;37:55–7. https://doi.org/10.1055/s-2007-1013906 [DOI] [PubMed] [Google Scholar]
- 40. Yoshida H, Wu MH, Kouchi Y, Onuki Y, Shi Q, Sauvage LR.. Comparison of the effect of monopolar and bipolar cauterization on skeletonized, dissected internal thoracic arteries. J Thorac Cardiovasc Surg 1995;110:504–10. https://doi.org/10.1016/S0022-5223(95)70247-4 [DOI] [PubMed] [Google Scholar]
- 41. Keeley SB. The skeletonized internal mammary artery. Ann Thorac Surg 1987;44:324–5. https://doi.org/10.1016/S0003-4975(10)62088-7 [DOI] [PubMed] [Google Scholar]
- 42. Lamm P, Juchem G, Weyrich P, Schutz A, Reichart B.. The harmonic scalpel: optimizing the quality of mammary artery bypass grafts. Ann Thorac Surg 2000;69:1833–5. https://doi.org/10.1016/S0003-4975(00)01288-1 [DOI] [PubMed] [Google Scholar]
- 43. Isomura T, Suma H, Sato T, Horii T.. Use of the harmonic scalpel for harvesting arterial conduits in coronary artery bypass. Eur J Cardiothorac Surg 1998;14:101–3. https://doi.org/10.1016/S1010-7940(98)00146-8 [DOI] [PubMed] [Google Scholar]
- 44. Urso S, Alvarez L, Sadaba R, Greco E.. Skeletonization of the internal thoracic artery: a randomized comparison of harvesting methods. Interact Cardiovasc Thorac Surg 2008;7: 23–6. https://doi.org/10.1510/icvts.2007.168831 [DOI] [PubMed] [Google Scholar]
- 45. Kieser TM, Rose MS, Aluthman U, Narine K.. Quicker yet safe: skeletonization of 1640 internal mammary arteries with harmonic technology in 965 patients. Eur J Cardiothorac Surg 2014;45:e142–50. https://doi.org/10.1093/ejcts/ezu024 [DOI] [PubMed] [Google Scholar]
- 46. Oz BS, Mataraci I, Iyem H, Kuralay E, Doganci S, Demirkilic U, et al. Comparison of ultrasonically activated scalpel and traditional technique in radial artery harvesting: clinical research. Thorac Cardiovasc Surg 2007;55:104–7. https://doi.org/10.1055/s-2006-924504 [DOI] [PubMed] [Google Scholar]
- 47. Brazio PS, Laird PC, Xu C, Gu J, Burris NS, Brown EN, et al. Harmonic scalpel versus electrocautery for harvest of radial artery conduits: reduced risk of spasm and intimal injury on optical coherence tomography. J Thorac Cardiovasc Surg 2008;136:1302–8. https://doi.org/10.1016/jjtcvs.2008.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferdinand FD, MacDonald JK, Balkhy HH, Bisleri G, Hwang HY, Northrup P, et al. Endoscopic conduit harvest in coronary artery bypass grafting surgery: an ISMICS systematic review and consensus conference statements. Innovations (Phi/a) 2017;12: 301–19. https://doi.org/10.1097/imi.0000000000000410 [DOI] [PubMed] [Google Scholar]
- 49. Perrault LP, Jeanmart H, Bilodeau L, Lesperance J, Tanguay JF, Bouchard D, et al. Early quantitative coronary angiography of saphenous vein grafts for coronary artery bypass grafting harvested by means of open versus endoscopic saphenectomy: a prospective randomized trial. J Thorac Cardiovasc Surg 2004;127:1402–7. https://doi.org/10.1016/j. jtcvs.2003.10.040 [DOI] [PubMed] [Google Scholar]
- 50. Yun KL, Wu Y, Aharonian V, Mansukhani P, Pfeffer TA, Sintek CF, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: six- month patency rates. J Thorac Cardiovasc Surg 2005;129:496–503. https://doi.org/10. 1016/j.jtcvs.2004.08.054 [DOI] [PubMed] [Google Scholar]
- 51. Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA, et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N EnglJ Med 2009;361:235–44. https://doi.org/10.1056/NEJMoa0900708 [DOI] [PubMed] [Google Scholar]
- 52. Zenati MA, Shroyer AL, Collins JF, Hattler B, Ota T, Almassi GH, et al. Impact of endo- scopic versus open saphenous vein harvest technique on late coronary artery bypass grafting patient outcomes in the ROOBY (Randomized On/Off Bypass) trial. J Thorac Cardiovasc Surg 2011;141:338–44. https://doi.org/10.1016/jjtcvs.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 53. Gaudino M, Bakaeen FG, Sandner S, Aldea GS, Arai H, Chikwe J, et al. Expert systematic review on the choice of conduits for coronary artery bypass grafting: endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and The Society of Thoracic Surgeons (STS). Eur J Cardiothorac Surg 2023;64:ezad163. https://doi.org/10. 1093/ejcts/ezad163 [DOI] [PubMed] [Google Scholar]
- 54. Kodia K, Patel S, Weber MP, Luc JGY, Choi JH, Maynes EJ, et al. Graft patency after open versus endoscopic saphenous vein harvest in coronary artery bypass grafting surgery: a systematic review and meta-analysis. Ann Cardiothorac Surg 2018;7:586–97. https://doi.org/10.21037/acs.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li G, Zhang Y, Wu Z, Liu Z, Zheng J.. Mid-term and long-term outcomes of endoscopic versus open vein harvesting for coronary artery bypass: a systematic review and meta-analysis. IntJ Surg 2019;72:167–73. https://doi.org/10.1016Zj.ijsu.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 56. Zenati MA, Bhatt DL, Bakaeen FG, Stock EM, Biswas K, Gaziano JM, et al. Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med 2019;380:132–41. https://doi.org/10.1056/NEJMoa1812390 [DOI] [PubMed] [Google Scholar]
- 57. Zenati MA, Bhatt DL, Stock EM, Hattler B, Wagner TH, Bakaeen FG, et al. Intermediate-term outcomes of endoscopic or open vein harvesting for coronary artery bypass grafting: the REGROUP randomized clinical trial. JAMA Netw Open 2021;4: e211439. https://doi.org/10.1001/jamanetworkopen.2021.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Desai P, Kiani S, Thiruvanthan N, Henkin S, Kurian D, Ziu P, et al. Impact of the learning curve for endoscopic vein harvest on conduit quality and early graft patency. Ann Thorac Surg 2011;91:1385–91. discussion 91-2. https://doi.org/10.1016/j.athoracsur.2011.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kiaii BB, Swinamer SA, Fox SA, Stitt L, Quantz MA, Novick RJ.. A prospective randomized study of endoscopic versus conventional harvesting of the radial artery. Innovations (Phi/a) 2017;12:231–8. https://doi.org/10.1097/imi.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 60. Shapira OM, Eskenazi BR, Anter E, Joseph L, Christensen TG, Hunter CT, et al. Endoscopic versus conventional radial artery harvest for coronary artery bypass grafting: functional and histologic assessment of the conduit. J Thorac Cardiovasc Surg 2006; 131:388–94. https://doi.org/10.1016/jjtcvs.2005.07.036 [DOI] [PubMed] [Google Scholar]
- 61. Medalion B, Tobar A, Yosibash Z, Stamler A, Sharoni E, Snir E, et al. Vasoreactivity and histology of the radial artery: comparison of open versus endoscopic approaches. Eur J Cardiothorac Surg 2008;34:845–9. https://doi.org/10.1016/j.ejcts.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 62. Gaudino MF, Lorusso R, Ohmes LB, Narula N, McIntire P, Gargiulo A, et al. Open radial artery harvesting better preserves endothelial function compared to the endoscopic approach. Interact Cardiovasc Thorac Surg 2019;29:561–7. https://doi.org/10.1093/icvts/ivz129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cao C, Tian DH, Ang SC, Peeceeyen S, Allan J, Fu B, et al. A meta-analysis of endoscopic versus conventional open radial artery harvesting for coronary artery bypass graft surgery. Innovations (Phiia) 2014;9:269–75. https://doi.org/10.1097/imi.0000000000000087 [DOI] [PubMed] [Google Scholar]
- 64. Rahouma M, Kamel M, Benedetto U, Ohmes LB, Di Franco A, Lau C, et al. Endoscopic versus open radial artery harvesting: a meta-analysis of randomized controlled and propensity matched studies. J Card Surg 2017;32:334–41. https://doi.org/10.1111/jocs.13148 [DOI] [PubMed] [Google Scholar]
- 65. Li FD, Eagle S, Brophy C, Hocking KM, Osgood M, Komalavilas P, et al. Pressure control during preparation of saphenous veins. JAMA Surg 2014;149:655–62. https://doi.org/10.1001/jamasurg.2013.5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Angelini GD, Passani SL, Breckenridge IM, Newby AC.. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res 1987;21:902–7. https://doi.org/10.1093/cvr/21.12.902 [DOI] [PubMed] [Google Scholar]
- 67. Bush HL Jr., McCabe ME, Nabseth DC.. Functional injury of vein graft endothelium. Role of hypothermia and distention. Arch Surg 1984;119:770–4. https://doi.org/10. 1001/archsurg.1984.01390190014003 [DOI] [PubMed] [Google Scholar]
- 68. Galea J, Armstrong J, Francis SE, Cooper G, Crossman DC, Holt CM.. Alterations in c-fos expression, cell proliferation and apoptosis in pressure distended human saphenous vein. Cardiovasc Res 1999;44:436–48. https://doi.org/10.1016/S0008-6363(99)00220-5 [DOI] [PubMed] [Google Scholar]
- 69. Viaro F, Capellini VK, Celotto AC, Carlotti CG Jr., Rodrigues AJ, Reis GS, et al. Immunohistochemical evaluation of three nitric oxide synthase isoforms in human sa- phenous vein exposed to different degrees of distension pressures. Cardiovasc Patho /2010;19:e211–20. https://doi.org/10.1016/j.carpath.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 70. Stigler R, Steger C, Schachner T, Holfeld J, Edlinger M, Grimm M, et al. The impact of distension pressure on acute endothelial cell loss and neointimal proliferation in saphenous vein grafts. Eur J Cardiothorac Surg 2012;42:e74–9. https://doi.org/10.1093/ejcts/ezs402 [DOI] [PubMed] [Google Scholar]
- 71. Ahmed SR, Johansson BL, Karlsson MG, Souza DS, Dashwood MR, Loesch A.. Human saphenous vein and coronary bypass surgery: ultrastructural aspects of conventional and “no-touch” vein graft preparations. Histo/Histopatho /2004;19:421–33. https://doi.org/10.14670/HH-19.421 [DOI] [PubMed] [Google Scholar]
- 72. Dashwood MR, Savage K, Tsui JC, Dooley A, Shaw SG, Fernandez Alfonso MS, et al. Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension-induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg 2009;138:334–40. https://doi.org/10.1016/j.jtcvs.2008.11.060 [DOI] [PubMed] [Google Scholar]
- 73. Verma S, Lovren F, Pan Y, Yanagawa B, Deb S, Karkhanis R, et al. Pedicled no-touch saphenous vein graft harvest limits vascular smooth muscle cell activation: the PATENT saphenous vein graft study. Eur J Cardiothorac Surg 2014;45:717–25. https://doi.org/10.1093/ejcts/ezt560 [DOI] [PubMed] [Google Scholar]
- 74. Fernandez-Alfonso MS, Gil-Ortega M, Aranguez I, Souza D, Dreifaldt M, Somoza B, et al. Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol 2017;174:3561–72. https://doi.org/10.1111/bph.13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mikami T, Furuhashi M, Sakai A, Numaguchi R, Harada R, Naraoka S, et al. Antiatherosclerotic phenotype of perivascular adipose tissue surrounding the saphe- nous vein in coronary artery bypass grafting. J Am Heart Assoc 2021;10:e018905. https://doi.org/10.1161/JAHA.120.018905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dreifaldt M, Souza DS, Loesch A, Muddle JR, Karlsson MG, Filbey D, et al. The “no- touch” harvesting technique for vein grafts in coronary artery bypass surgery preserves an intact vasa vasorum. J Thorac Cardiovasc Surg 2011;141:145–50. https://doi.org/10. 1016/j.jtcvs.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 77. Deng MX, Lia H, Lee G, Rahouma M, Di Franco A, Demetres M, et al. Angiographic patency of coronary artery bypass conduits: an updated network meta-analysis of randomized trials. Braz J Cardiovasc Surg 2022;37:7–31. https://doi.org/10.21470/1678- 9741-2022-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tian M, Wang X, Sun H, Feng W, Song Y, Lu F, et al. No-touch versus conventional vein harvesting techniques at 12 months after coronary artery bypass grafting surgery: mul- ticenter randomized, controlled trial. Circu/ation 2021;144:1120–9. https://doi.org/10.1161/CIRCULATIONAHA.121.055525 [DOI] [PubMed] [Google Scholar]
- 79. Ragnarsson S, Janiec M, Modrau IS, Dreifaldt M, Ericsson A, Holmgren A, et al. No-touch saphenous vein grafts in coronary artery surgery (SWEDEGRAFT): rationale and design of a multicenter, prospective, registry-based randomized clinical trial. Am Heart J 2020;224:17–24. https://doi.org/10.1016/j.ahj.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 80. Hayashi I, Kashima I, Yoshikawa E.. The endoscopic no-touch saphenous vein harvesting technique. Mu/timed Man Cardiothorac Surg 2020;2020. https://doi.org/10.1510/mmcts.2020.049 [DOI] [PubMed] [Google Scholar]
- 81. Hayashi I, Kashima I, Yoshikawa E.. Use of the no-touch saphenous vein harvesting technique via small incisions. Innovations (Phi/a) 2020;15:81–4. https://doi.org/10.1177/1556984519886549 [DOI] [PubMed] [Google Scholar]
- 82. Yoshino K, Abe K, Suzuki K, Tamaki R, Mituishi A, Yamasaki M, et al. A novel technique of endoscopic vein harvesting with preserved perivascular tissue. Innovations (Phi/a) 2020;15:475–7. https://doi.org/10.1177/1556984520948139 [DOI] [PubMed] [Google Scholar]
- 83. Williams JB, Harskamp RE, Bose S, Lawson JH, Alexander JH, Smith PK, et al. The preservation and handling of vein grafts in current surgical practice: findings of a survey among cardiovascular surgeons of top-ranked US hospitals. JAMA Surg 2015;150: 681–3. https://doi.org/10.1001/jamasurg.2015.0404 [DOI] [PubMed] [Google Scholar]
- 84. Gundry SR, Jones M, Ishihara T, Ferrans VJ.. Optimal preparation techniques for human saphenous vein grafts. Surgery 1980;88:785–94. [PubMed] [Google Scholar]
- 85. Wilbring M, Tugtekin SM, Zatschler B, Ebner A, Reichenspurner H, Matschke K, et al. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur J Cardiothorac Surg 2011;40:811–5. https://doi. org/10.1016/j.ejcts.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 86. Biswas KS, Thatte HS, Najjar SF, Rhee JH, Birjiniuk V, Crittenden MD, et al. Multi-photon microscopy in the evaluation of human saphenous vein. J Surg Res 2001;95:37–43. https://doi.org/10.1006/jsre.2000.6019 [DOI] [PubMed] [Google Scholar]
- 87. Catinella FP, Cunningham JN Jr., Srungaram RK, Baumann FG, Nathan IM, Glassman EA, et al. The factors influencing early patency of coronary artery bypass vein grafts: correlation of angiographic and ultrastructural findings. J Thorac Cardiovasc Surg 1982;83:686–700. https://doi.org/10.1016/S0022-5223(19)37208-3 [PubMed] [Google Scholar]
- 88. Lawrie GM, Weilbacher DE, Henry PD.. Endothelium-dependent relaxation in human saphenous vein grafts. Effects of preparation and clinicopathologic correlations. J Thorac Cardiovasc Surg 1990;100:612–20. https://doi.org/10.1016/S0022-5223(19)35507-2 [PubMed] [Google Scholar]
- 89. Wilbring M, Ebner A, Schoenemann K, Knaut M, Tugtekin SM, Zatschler B, et al. Heparinized blood better preserves cellular energy charge and vascular functions of in- traoperatively stored saphenous vein grafts in comparison to isotonic sodium-chloride- solution. Clin Hemorheol Microcirc 2013;55:445–55. https://doi.org/10.3233/CH-131781 [DOI] [PubMed] [Google Scholar]
- 90. Zerkowski HR, Knocks M, Konerding MA, Doetsch N, Roth G, Hakim K, et al. Endothelial damage of the venous graft in CABG. Influence of solutions used for storage and rinsing on endothelial function. Eur J Cardiothorac Surg 1993;7:376–82. https://doi.org/10.1016/1010-7940(93)90070-R [DOI] [PubMed] [Google Scholar]
- 91. Chong WC, Ong PJ, Hayward C, Moat N, Collins P.. Effects of storage solutions on in vitro vasoreactivity of radial artery conduits. J Thorac Cardiovasc Surg 2001;122:470–5. https://doi.org/10.1067/mtc.2001.115921 [DOI] [PubMed] [Google Scholar]
- 92. Ben Ali W, Bouhout I, Perrault LP.. The effect of storage solutions, gene therapy, and antiproliferative agents on endothelial function and saphenous vein graft patency. J Card Surg 2018;33:235^2. https://doi.org/10.1111/jocs.13608 [DOI] [PubMed] [Google Scholar]
- 93. Harskamp RE, Alexander JH, Schulte PJ, Brophy CM, Mack MJ, Peterson ED, et al. Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA Surg 2014; 149:798–805. https://doi.org/10.1001/jamasurg.2014.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tatoulis J, Jiang GC, Moffatt JD, Cocks TM.. Storage of radial artery grafts in blood increases vessel reactivity to vasoconstrictors in vitro. Ann Thorac Surg 1999;68:2191–5. https://doi.org/10.1016/S0003-4975(99)01065-6 [DOI] [PubMed] [Google Scholar]
- 95. Thatte HS, Biswas KS, Najjar SF, Birjiniuk V, Crittenden MD, Michel T, et al. Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann Thorac Surg 2003;75:1145–52; discussion 52. https://doi.org/10. 1016/S0003-4975(02)04705-7 [DOI] [PubMed] [Google Scholar]
- 96. Perrault LP, Carrier M, Voisine P, Olsen PS, Noiseux N, Jeanmart H, et al. Sequential multidetector computed tomography assessments after venous graft treatment solution in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2021;161:96–106.e2. https://doi.org/10.1016/jjtcvs.2019.10.115 [DOI] [PubMed] [Google Scholar]
- 97. Tekin I, Demir M, Ozdem S.. Effect of different storage solutions on oxidative stress in human saphenous vein grafts. J Cardiothorac Surg 2022;17:7. https://doi.org/10.1186/s13019-022-01752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nazari-Shafti TZ, Thau H, Zacharova E, Beez CM, Exarchos V, Neuber S, et al. Endothelial damage inhibitor preserves the integrity of venous endothelial cells from patients undergoing coronary bypass surgery. Eur J Cardiothorac Surg 2023;64: ezad327. https://doi.org/10.1093/ejcts/ezad327 [DOI] [PubMed] [Google Scholar]
- 99. Aschacher T, Baranyi U, Aschacher O, Eichmair E, Messner B, Zimpfer D, et al. A novel endothelial damage inhibitor reduces oxidative stress and improves cellular integrity in radial artery grafts for coronary artery bypass. Front Cardiovasc Med 2021;8:736503. https://doi.org/10.3389/fcvm.2021.736503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.https://www.nice.org.uk/advice/mib184/chapter/The-technology.
- 101. He GW, Taggart DP.. Spasm in arterial grafts in coronary artery bypass grafting surgery. Ann Thorac Surg 2016;101:1222–9. https://doi.org/10.1016Zj.athoracsur.2015.09.071 [DOI] [PubMed] [Google Scholar]
- 102. He GW. Contractility of the human internal mammary artery at the distal section increases toward the end. Emphasis on not using the end of the internal mammary artery for grafting. J Thorac Cardiovasc Surg 1993;106:406–11. https://doi.org/10.1016/S0022- 5223(19)34072-3 [PubMed] [Google Scholar]
- 103. Davie AP, Love MP, McMurray JJ.. Even low-dose aspirin inhibits arachidonic acid- induced vasodilation in heart failure. Clin Pharmacol Ther 2000;67:530–7. https://doi. org/10.1067/mcp.2000.106290 [DOI] [PubMed] [Google Scholar]
- 104. Lawton JS, Barner HB, Bailey MS, Guthrie TJ, Moazami N, Pasque MK, et al. Radial artery grafts in women: utilization and results. Ann Thorac Surg 2005;80:559–63. https://doi.org/10.1016/j.athoracsur.2005.02.055 [DOI] [PubMed] [Google Scholar]
- 105. Joannides R, Costentin A, Iacob M, Compagnon P, Lahary A, Thuillez C.. Influence of vascular dimension on gender difference in flow-dependent dilatation of peripheral conduit arteries. Am J Physiol Heart Circ Physiol 2002;282:H1262–9. https://doi.org/10.1152/ajpheart.00209.2001 [DOI] [PubMed] [Google Scholar]
- 106. Dignan RJ, Yeh T Jr., Dyke CM, Lutz HA Jr., Wechsler AS.. The influence of age and sex on human internal mammary artery size and reactivity. Ann Thorac Surg 1992;53: 792–7. https://doi.org/10.1016/0003-4975(92)91438-F [DOI] [PubMed] [Google Scholar]
- 107. Mannacio V, Di Tommaso L, Antignano A, De Amicis V, Stassano P, Pinna GB, et al. Endothelial nitric oxide synthase expression in postmenopausal women: a sex-specific risk factor in coronary surgery. Ann Thorac Surg 2012;94:1934–9. https://doi.org/10. 1016/j.athoracsur.2012.06.040 [DOI] [PubMed] [Google Scholar]
- 108. Huxley VH, Kemp SS.. Sex-specific characteristics of the microcirculation. Adv Exp Med Biol 2018;1065:307–28. https://doi.org/10.1007/978-3-319-77932-4_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lamin V, Jaghoori A, Jakobczak R, Stafford I, Heresztyn T, Worthington M, et al. Mechanisms responsible for serotonin vascular reactivity sex differences in the internal mammary artery. J Am Heart Assoc 2018;7:e007126. https://doi.org/10.1161/JAHA.117. 007126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jaghoori A, Lamin V, Jacobczak R, Worthington M, Edwards J, Viana F, et al. Sex differences in vascular reactivity of coronary artery bypass graft conduits. Heart Vessels 2020;35:422–31. https://doi.org/10.1007/s00380-019-01508-9 [DOI] [PubMed] [Google Scholar]
- 111. Akar F, Manavbasi Y, Parlar AI, Ulus AT, Katircioglu SF.. The gender differences in the relaxation to levosimendan of human internal mammary artery. Cardiovasc Drugs Ther 2007;21:331–8. https://doi.org/10.1007/s10557-007-6047-x [DOI] [PubMed] [Google Scholar]
- 112. Muir AD, McKeown PP, Bayraktutan U.. Role of gender, smoking profile, hypertension, and diabetes on saphenous vein and internal mammary artery endothelial relaxation in patients with coronary artery bypass grafting. Oxid Med Cell Longev 2010;3:199–205. https://doi.org/10.4161/oxim.3.3.11757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Riedel K, Deussen AJ, Tolkmitt J, Weber S, Schlinkert P, Zatschler B, et al. Estrogen determines sex differences in adrenergic vessel tone by regulation of endothelial beta-adrenoceptor expression. Am J Physiol Heart Circ Physiol 2019;317:H243–54. https://doi.org/10.1152/ajpheart.00456.2018 [DOI] [PubMed] [Google Scholar]
- 114. Masroor M, Zhou K, Chen C, Fu X, Zhao Y.. All we need to know about internal thoracic artery harvesting and preparation for myocardial revascularization: a systematic review. J Cardiothorac Surg 2021;16:354. https://doi.org/10.1186/s13019-021-01733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Formica F, Ferro O, Brustia M, Corti F, Colagrande L, Bosisio E, et al. Effects of papaverine and glycerylnitrate-verapamil solution as topical and intraluminal vasodilators for internal thoracic artery. Ann Thorac Surg 2006;81:120–4. https://doi.org/10.1016/j.athoracsur.2005.06.037 [DOI] [PubMed] [Google Scholar]
- 116. Dregelid E, Heldal K, Resch F, Stangeland L, Breivik K, Svendsen E.. Dilation of the internal mammary artery by external and intraluminal papaverine application. J Thorac Cardiovasc Surg 1995;110:697–703. https://doi.org/10.1016/S0022-5223(95)70101-X [DOI] [PubMed] [Google Scholar]
- 117. Mayranpaa M, Simpanen J, Hess MW, Werkkala K, Kovanen PT.. Arterial endothelial denudation by intraluminal use of papaverine-NaCl solution in coronary bypass surgery. Eur J Cardiothorac Surg 2004;25:560–6. https://doi.org/10.1016/j.ejcts.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 118. Cooper GJ, Wilkinson GA, Angelini GD.. Overcoming perioperative spasm of the internal mammary artery: which is the best vasodilator? J Thorac Cardiovasc Surg 1992;104:465–8. https://doi.org/10.1016/S0022-5223(19)34805-6 [PubMed] [Google Scholar]
- 119. He GW, Taggart DP.. Antispastic management in arterial grafts in coronary artery bypass grafting surgery. Ann Thorac Surg 2016;102:659–68. https://doi.org/10.1016/j. athoracsur.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 120. He GW. Verapamil plus nitroglycerin solution maximally preserves endothelial function of the radial artery: comparison with papaverine solution. J Thorac Cardiovasc Surg 1998;115:1321–7. https://doi.org/10.1016/S0022-5223(98)70215-6 [DOI] [PubMed] [Google Scholar]
- 121. Robinson NB, Audisio K, Cancelli G, Masterson Creber RM, Fremes SE, Gelijns AC, et al. Current practice patterns for use of the radial artery for coronary surgery in dedicated centers. J Thorac Cardiovasc Surg 2022;163:e251–2. https://doi.org/10.1016/j. jtcvs.2021.09.035 [DOI] [PubMed] [Google Scholar]
- 122. Goldman S, Copeland J, Mortiz T, Henderson W, Zadina K, Ovitt T, et al. Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a veterans administration cooperative study. Circulation 1988;77:1324–32. https://doi.org/10.1161/01.CIR.77.6.1324 [DOI] [PubMed] [Google Scholar]
- 123. Lorenz RL, Schacky CV, Weber M, Meister W, Kotzur J, Reichardt B, et al. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily). Effects on platelet aggregation and thromboxane formation. Lancet 1984;1:1261–4. https://doi.org/10. 1016/S0140-6736(84)92446-2 [DOI] [PubMed] [Google Scholar]
- 124. Fremes SE, Levinton C, Naylor CD, Chen E, Christakis GT, Goldman BS.. Optimal antithrombotic therapy following aortocoronary bypass: a meta-analysis. Eur J Cardiothorac Surg 1993;7:169–80. https://doi.org/10.1016/1010-7940(93)90155-5 [DOI] [PubMed] [Google Scholar]
- 125. Solo K, Lavi S, Kabali C, Levine GN, Kulik A, John-Baptiste AA, et al. Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. BMJ 2019;367:l5476. https://doi.org/10.1136/bmj.l5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Deo SV, Dunlay SM, Shah IK, Altarabsheh SE, Enwin PJ, Boilson BA, et al. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg 2013;28:109–16. https://doi.org/10.1111/jocs.12074 [DOI] [PubMed] [Google Scholar]
- 127. Cardoso R, Knijnik L, Whelton SP, Rivera M, Gluckman TJ, Metkus TS, et al. Dual versus single antiplatelet therapy after coronary artery bypass graft surgery: an updated meta-analysis. IntJ Cardiol 2018;269:80–8. https://doi.org/10.1016/j.ijcard.2018.07.083 [DOI] [PubMed] [Google Scholar]
- 128. Triska J, Maitra N, Deshotels MR, Haddadin F, Angiolillo DJ, Vilahur G, et al. A comprehensive review of the pleiotropic effects of ticagrelor. Cardiovasc Drugs Ther 2022;38: 775–97. https://doi.org/10.1007/s10557-022-07373-5 [DOI] [PubMed] [Google Scholar]
- 129. Zhao Q, Zhu Y, Xu Z, Cheng Z, Mei J, Chen X, et al. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA 2018;319:1677–86. https://doi. org/10.1001/jama.2018.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sandner S, Redfors B, Angiolillo DJ, Audisio K, Fremes SE, Janssen PWA, et al. Association of dual antiplatelet therapy with ticagrelor with vein graft failure after coronary artery bypass graft surgery: a systematic review and meta-analysis. JAMA 2022; 328:554–62. https://doi.org/10.1001/jama.2022.11966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Post Coronary Artery Bypass Graft Trial I. The effect of aggressive lowering of low- density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med 1997;336: 153–62. https://doi.org/10.1056/NEJM199701163360301 [DOI] [PubMed] [Google Scholar]
- 132. Gaudino M, Luciani N, Nasso G, Salica A, Canosa C, Possati G.. Is postoperative calcium channel blocker therapy needed in patients with radial artery grafts? J Thorac Cardiovasc Surg 2005;129:532–5. https://doi.org/10.1016/j.jtcvs.2004.07.054 [DOI] [PubMed] [Google Scholar]
- 133. Miwa S, Desai N, Koyama T, Chan E, Cohen EA, Fremes SE, et al. Radial artery angiograph- ic string sign: clinical consequences and the role of pharmacologic therapy. Ann Thorac Surg 2006;81:112–8; discussion 9. https://doi.org/10.1016/j.athoracsur.2005.06.076 [DOI] [PubMed] [Google Scholar]
- 134. Gaudino M, Benedetto U, Fremes SE, Hare DL, Hayward P, Moat N, et al. Effect of calcium-channel blocker therapy on radial artery grafts after coronary bypass surgery. J Am Coll Cardiol 2019;73:2299–306. https://doi.org/10.1016/jjacc.2019.02.054 [DOI] [PubMed] [Google Scholar]
- 135. Gaudino M, Prati F, Caradonna E, Trani C, Burzotta F, Schiavoni G, et al. Implantation in coronary circulation induces morphofunctional transformation of radial grafts from muscular to elastomuscular. Circulation 2005; 112:I208–11. https://doi.org/10.1161/CIRCULATIONAHA.104.512889 [DOI] [PubMed] [Google Scholar]
- 136. Shapira OM, Alkon JD, Macron DS, Keaney JF Jr., Vita JA, Aldea GS, et al. Nitroglycerin is preferable to diltiazem for prevention of coronary bypass conduit spasm. Ann Thorac Surg 2000;70:883–8. https://doi.org/10.1016/S0003-4975(00)01628-3 [DOI] [PubMed] [Google Scholar]
- 137. Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, et al. Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplate- let therapy. Results of a veterans administration cooperative study. Circulation 1989; 80:1190–7. https://doi.org/10.1161/01.CIR.80.5.1190 [DOI] [PubMed] [Google Scholar]
- 138. Musleh G, Dunning J.. Does aspirin 6 h after coronary artery bypass grafting optimise graft patency? Interact Cardiovasc Thorac Surg 2003;2:413–5. https://doi.org/10.1016/S1569-9293(03)00181-6 [DOI] [PubMed] [Google Scholar]
- 139. Sharma GV, Khuri SF, Josa M, Folland ED, Parisi AF.. The effect of antiplatelet therapy on saphenous vein coronary artery bypass graft patency. Circulation 1983;68:II218—21. [PubMed] [Google Scholar]
- 140. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C.. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373–83. https://doi.org/10. 1056/NEJMra052717 [DOI] [PubMed] [Google Scholar]
- 141. Mahaffey KW, Held C, Wojdyla DM, James SK, Katus HA, Husted S, et al. Ticagrelor effects on myocardial infarction and the impact of event adjudication in the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol 2014;63:1493–9. https://doi.org/10.1016/jjacc.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 142. Mangano DT; Multicenter study of perioperative ischemia research G. Aspirin and mortality from coronary bypass surgery. N Engl J Med 2002;347:1309–17. https://doi.org/10.1056/NEJMoa020798 [DOI] [PubMed] [Google Scholar]
- 143. Cleland JGF. Aspirin for primary and secondary prevention of cardiovascular disease: time to stop? Thromb Haemost 2022;122:311^. https://doi.org/10.1055/s-0041-1740639 [DOI] [PubMed] [Google Scholar]
- 144. Hastings S, Myles P, McIlroy D.. Aspirin and coronary artery surgery: a systematic review and meta-analysis. Br J Anaesth 2015;115:376–85. https://doi.org/10.1093/bja/aev164 [DOI] [PubMed] [Google Scholar]
- 145. Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, et al. Long-term graft patency (3 years) after coronary artery surgery: effects of aspirin: results of a VA cooperative study. Circulation 1994;89:1138^3. https://doi.org/10.1161/01.CIR.89.3.1138 [DOI] [PubMed] [Google Scholar]
- 146. Nocerino AG, Achenbach S, Taylor AJ.. Meta-analysis of effect of single versus dual anti- platelet therapy on early patency of bypass conduits after coronary artery bypass grafting. Am J Cardiol 2013;112:1576–9. https://doi.org/10.1016/j.amjcard.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 147. Harik L, Perezgrovas-Olaria R, Soletti G Jr., Dimagli A, Alzghari T, An KR, et al. Graft thrombosis after coronary artery bypass surgery and current practice for prevention. Front Cardiovasc Med 2023;10:1125126. https://doi.org/10.3389/fcvm.2023.1125126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sandner S, Gaudino M, Redfors B, Angiolillo DJ, Ben-Yehuda O, Bhatt DL, et al. One-month DAPT with ticagrelor and aspirin for patients undergoing coronary artery bypass grafting: rationale and design of the randomised, multicentre, double-blind, placebo-controlled ODIN trial. EuroIntervention 2024;20:e322–8. https://doi.org/10. 4244/EIJ-D-23-00699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Calderone D, Capodanno D, Angiolillo DJ.. An updated drug profile of ticagrelor with considerations on the treatment of patients with coronary artery disease and diabetes mellitus. Expert Rev Cardiovasc Ther 2020;18:449–64. https://doi.org/10. 1080/14779072.2020.1792293 [DOI] [PubMed] [Google Scholar]
- 150. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–37. https://doi.org/10.1016/jjcin.2019.03.034 [DOI] [PubMed] [Google Scholar]
- 151. Gao C, Ren C, Li D, Li L.. Clopidogrel and aspirin versus clopidogrel alone on graft patency after coronary artery bypass grafting. Ann Thorac Surg 2009;88:59–62. https://doi. org/10.1016/j.athoracsur.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 152. Kulik A, Abreu AM, Boronat V, Kouchoukos NT, Ruel M.. Ticagrelor versus aspirin and vein graft patency after coronary bypass: a randomized trial. J Card Surg 2022;37: 563–70. https://doi.org/10.1111/jocs.16189 [DOI] [PubMed] [Google Scholar]
- 153. Lamy A, Eikelboom J, Sheth T, Connolly S, Bosch J, Fox KAA, et al. Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: the COMPASS- CABG study. J Am Coll Cardiol 2019;73:121–30. https://doi.org/10.1016/jjacc.2018. 10.048 [DOI] [PubMed] [Google Scholar]
- 154. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart j 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 155. Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB.. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012;59:572–82. https://doi.org/10.1016/j.jacc.2011.09.067 [DOI] [PubMed] [Google Scholar]
- 156. Gaudino M, Di Franco A, Cao D, Giustino G, Bairey Merz CN, Fremes SE, et al. Sex-related outcomes of medical, percutaneous, and surgical interventions for coronary artery disease: JACC focus seminar 3/7. J Am Coll Cardiol 2022;79:1407–25. https://doi.org/10.1016/j.jacc.2021.07.066 [DOI] [PubMed] [Google Scholar]
- 157. Campeau L, Enjalbert M, Lesperance J, Bourassa MG, Kwiterovich P Jr., Wacholder S, et al. The relation of risk factors to the development of atherosclerosis in saphenous- vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med 1984;311:1329–32. https://doi.org/10.1056/NEJM198411223112101 [DOI] [PubMed] [Google Scholar]
- 158. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–72. https://doi.org/10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Makuuchi H, Furuse A, Endo M, Nakamura H, Daida H, Watanabe M, et al. Effect of pravastatin on progression of coronary atherosclerosis in patients after coronary artery bypass surgery. CircJ 2005;69:636–43. https://doi.org/10.1253/circj.69.636 [DOI] [PubMed] [Google Scholar]
- 160. Kulik A, Abreu AM, Boronat V, Ruel M.. Intensive versus moderate statin therapy and early graft occlusion after coronary bypass surgery: The Aggressive Cholesterol Therapy to Inhibit Vein Graft Events randomized clinical trial. J Thorac Cardiovasc Surg 2019;157:151–61.e1. https://doi.org/10.1016/jjtcvs.2018.05.123 [DOI] [PubMed] [Google Scholar]
- 161. Kulik A, Voisine P, Mathieu P, Masters RG, Mesana TG, Le May MR, et al. Statin therapy and saphenous vein graft disease after coronary bypass surgery: analysis from the CASCADE randomized trial. Ann Thorac Surg 2011;92:1284–91. https://doi.org/10.1016/j.athoracsur.2011.04.107 [DOI] [PubMed] [Google Scholar]
- 162. Katsuki S, Jha PK, Lupieri A, Nakano T, Passos LSA, Rogers MA, et al. Proprotein convertase subtilisin/kexin 9 (PCSK9) promotes macrophage activation via LDL receptor-independent mechanisms. Circ Res 2022;131:873–89. https://doi.org/10.1161/CIRCRESAHA.121.320056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gao J, Wang HB, Xiao JY, Ren M, Reilly KH, Li YM, et al. Association between propro- tein convertase subtilisin/kexin type 9 and late saphenous vein graft disease after coronary artery bypass grafting: a cross-sectional study. BMJ Open 2018;8:e021951. https://doi.org/10.1136/bmjopen-2018-021951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Vaccarino V, Abramson JL, Veledar E, Weintraub WS.. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation 2002;105:1176–81. https://doi.org/10.1161/hc1002. 105133 [DOI] [PubMed] [Google Scholar]
- 165. Nelson K, Fuster V, Ridker PM.. Low-dose colchicine for secondary prevention of coronary artery disease: JACC review topic of the week.J Am Coll Cardiol 2023;82:648–60. https://doi.org/10.1016/j.jacc.2023.05.055 [DOI] [PubMed] [Google Scholar]
- 166. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–47. https://doi.org/10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for this manuscript.