Summary
Repetitive transcranial magnetic stimulation (rTMS) represents a non-invasive therapeutic modality acknowledged for augmenting neurological function recovery following stroke. Nonetheless, uncertainties remain regarding its efficacy in promoting cognitive function recovery in patients diagnosed with vascular dementia (VD). In this study, VD was experimentally induced in a rat model utilizing the bilateral common carotid artery occlusion method. Following a recuperation period of seven days, rats were subjected to high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) at a frequency of 10 Hz. Cognitive function was assessed utilizing the Morris water maze test, and the levels of IL-6, TNF-α, SOD, GSH, MDA, and Fe2+ in cerebral tissue were quantitatively analyzed through enzyme-linked immunosorbent assay. Moreover, the gene and protein expressions of nuclear factor erythroid 2-related factor 2 (Nrf2) and glutathione peroxidase 4 (GPx4) were meticulously investigated via quantitative polymerase chain reaction (qPCR) and Western blotting techniques. The use of HF-rTMS notably augmented cognitive function in rats with VD, concomitantly reducing neuroinflammation, oxidative stress, and ferroptosis within the brain. The group subjected to HF-rTMS demonstrated an increase in the levels of both proteins and genes associated with Nrf2 and GPx4, in comparison to the VD group. These results highlight the potential of HF-rTMS treatment in enhancing cognitive function in rats diagnosed with VD through the modulation of the Nrf2/GPx4 signaling pathway. This modulation, in turn, mitigates processes linked with neuroinflammation, oxidative stress, and ferroptosis. Nevertheless, additional studies are essential to comprehensively elucidate the underlying mechanisms and clinical implications of HF-rTMS treatment in the treatment of VD.
Keywords: Cognitive function, GPx4, High-frequency repetitive transcranial magnetic stimulation, Neural function, Nrf2, Rats, Vascular dementia
Introduction
Vascular dementia (VD) is widely recognized as a result of cerebrovascular disease, especially ischemic stroke, which precipitates cognitive and memory impairments [1,2]. Epidemiological evidence suggests a greater prevalence of VD relative to Alzheimer's disease, particularly among elderly populations, and it is more pronounced in developing countries as opposed to developed nations [3]. Despite the implementation of rehabilitation and pharmacological interventions, dementia continues to pose a significant challenge, highlighting the urgent need for novel therapeutic approaches to improve treatment outcomes and prognoses [4]. Repetitive transcranial magnetic stimulation (rTMS), a potential therapy for neurological recovery after a stroke, is gaining popularity [5]. The significant contributions of the prefrontal cortex, temporal lobe, and hippocampus to cognitive impairment have been well-documented [6]. Notably, the dorsolateral prefrontal cortex (DLPFC) plays a crucial role in higher-order cognitive processes and executive functions, such as problem-solving, decision-making, planning, and working memory [7]. Employing high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) to target the DLPFC has demonstrated potential in improving working memory and executive function in patients with stroke [8,9]. Research on animal models suggests that rTMS has the potential to reverse ultrastructural alterations in ischemic areas of the brain. This effect is linked to an increased expression of endogenous brain-derived neurotrophic factor (BDNF), which supports neuronal preservation and regeneration [10]. Nonetheless, the specific mechanisms through which cognitive improvements occur following transcranial magnetic stimulation are still predominantly undefined.
The critical function of nuclear factor E2-related factor 2 (Nrf2) in mitigating oxidative stress and diminishing neuroinflammation via its signaling pathway is indispensable [11]. In vitro studies have demonstrated that antioxidant signaling regulated by Nrf2 effectively counteracts neuronal damage induced by Amyloid-beta (Aβ) [12]. Experimental findings have shown that Nrf2 improves cognitive function, decreases infarct volume, and ameliorates brain edema in rats experiencing stroke [13]. Glutathione peroxidase 4 (GPx4) maintains antioxidative activity by diminishing glutathione levels and converting lipid peroxides into lipocalciferol, thus obstructing cellular ferroptosis and safeguarding neurological functions [14]. The initiation of ferroptosis mechanisms in ischemic stroke is characterized by elevated extracellular glutamate levels, which in turn activate the N-methyl-D-aspartate receptor (NMDAR). This activation leads to an increase in cytoplasmic Fe2+ concentrations or hinders intracellular cystine transport, consequently impairing GPx4 functionality [15]. Recent research corroborates that the activation of the Nrf2/GPx4 signaling pathway attenuates ferroptosis, thus beneficially affecting cognitive deficits post-ischemic events and laying a theoretical groundwork for our study [16,17].
The aim of this study was to evaluate the potential effects and underlying mechanisms of HF-rTMS on cognitive dysfunction in a rat model of VD. The results offer substantial evidence endorsing HF-rTMS as a viable non-pharmacological intervention for VD.
Material and Methods
Animals
The Animal Ethics Committee of Jiaxing College, authorized under license number SYXK (Zhejiang) 2020-0007, ensured compliance with the guidelines and principles set forth in the NIH Guide for the Care and Use of Laboratory Animals for all animal experiments. The study was structured into three equally sized groups, each consisting of 30 male Sprague-Dawley rats: (1) the Sham group, which underwent sham surgery and received sham stimulation; (2) the VD group, consisting of rats with induced VD models who were subjected to sham stimulation; and (3) the HF-rTMS group, comprising rats with VD models that were treated with HF-rTMS. The ambient temperature was maintained at approximately 20 °C, with ad libitum access to food and water and a 12-hour light-dark cycle (lights activated at 7:00 am). To ensure the integrity of the study results, rats displaying any abnormal behavioral effects, such as seizures, were excluded. Rigorous measures were implemented to minimize both the suffering of the animals and the total number of animals used in the study.
Establishment of VD model
To create a rat model of VD, the two-vessel occlusion (2-VO) technique, which entails the permanent blockage of both common carotid arteries, was utilized following an approved protocol [18]. In summary, each rat was anesthetized with isoflurane (4 % for induction, 2 % for maintenance), followed by a midline incision in the neck to reveal the common carotid arteries. These arteries were then carefully dissected away from the vagus nerve. Subsequently, the carotid arteries were ligated using polyglycolic acid sutures. The ligation procedures were conducted one week apart, with the right carotid artery being ligated first, followed by the left carotid artery one week later. Conversely, rats subjected to sham surgery underwent identical procedures, with the exception of the actual ligation of the carotid arteries. After the surgery, a one-week recovery period was allotted for the rats.
Morris water maze test
Cognitive function was evaluated using the Morris water maze (MWM) test 24 h following the completion of the last session of HF-rTMS treatment, in accordance with a protocol previously described [19]. The MWM pool was partitioned into four sections (1, 2, 3, and 4), with a platform of 12 cm diameter and 20 cm height positioned 2 cm beneath the surface of the water in section 3. Navigation trials were conducted over 5 consecutive days. The escape latency, representing the time taken for rats to locate the platform, was measured. Rats reaching the platform within 120 s were allowed a 20-second rest. In cases where rats failed to locate the platform within 120 s, they were gently guided toward it and given a 20-second opportunity to rest, with the escape time recorded as 120 s. Following the navigation trials, the platform was removed for a probe trial. Using a computer-based image analyzer, the time spent in the target quadrant and the frequency of rats traversing the platform site within 120 s were automatically recorded during the probe trial.
Repetitive transcranial magnetic stimulation
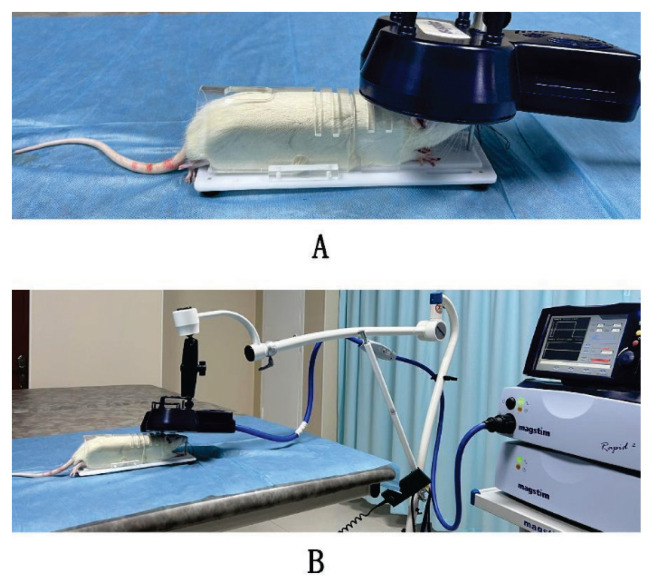
The HF-rTMS procedure involved the use of a high-focusing figure-of-eight coil (D70 P/N9925-00, with an outer wing diameter of 70 mm) in conjunction with a Magstim Rapid2 magnetic stimulator (Magstim, UK). During the HF-rTMS sessions, a fixator was used to gently immobilize rats in the HF-rTMS group. The HF-rTMS sessions were administered daily from 8:00 to 10:00 in the morning for 5 consecutive days. Following a 2-day interval, stimulation was conducted for an additional 5 days, resulting in a total of 10 days of rTMS therapy. Each HF-rTMS session involved a high-frequency stimulation protocol (10 Hz), comprising 60 standard trains. Each train comprised 20 pulses, with an interval of 8 s between each train. The stimulation intensity was set at 80 % of the maximum output of the coil. Rats in the Sham and VD groups underwent treatments analogous to those in the HF-rTMS group. The coil was positioned perpendicular to the head, allowing the rats to perceive the clicking noise without experiencing actual stimulation. Figure 1 illustrates the spatial relationship between the rat head and the coil during the rTMS procedure.
Fig. 1.
A representation of the relationship between the coil and the head of a rat during stimulation.
Enzyme-Linked Immunosorbent Assay (ELISA)
Rat brain tissue was precisely weighed, and a volume of homogenizing medium, nine times that of the tissue weight (with a weight proportion of 1 mg tissue to 9 μl medium), was added. The brain tissue and homogenizing medium were mixed under ice-water bath conditions. Mechanical homogenization of the brain tissue and homogenizing medium was conducted at 2500–3000 RPM, resulting in a 10 % homogenizing solution. Subsequently, the homogenate was subjected to centrifugation for 10 min, after which, the supernatant was collected for further analysis. To assess neuroinflammation markers, the concentrations of interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) in the rat brain tissue were determined. The levels of superoxide dismutase (SOD), malonic dialdehyde (MDA), and glutathione (GSH) were measured as oxidative stress markers. Additionally, the concentration of Fe2+ was quantified as a marker for ferroptosis. All necessary commercial kits for these analyses were provided by Nanjing Liboda Biotechnology Co., Ltd.
Quantitative Real-time PCR analyses for Nrf2 and GPx4
The total RNA was extracted using RNA extraction solution from Wuhan Servicebio Technology Co., Ltd., Wuhan, China, and its concentration was measured using a Nanodrop 2000 ultraviolet spectrophotometer from Thermo Fisher Scientific Co., Ltd., Waltham, MA, USA. Subsequently, reverse transcription (RT) was carried out using SweScript All-in-One First-Strand cDNA Synthesis SuperMix for quantitative polymerase chain reaction (qPCR) (One-Step gDNA Remover) with random primers as RT primers. The resulting cDNA was employed for real-time PCR analysis to assess the mRNA expression of Nrf2 and GPx4, with GAPDH serving as the internal reference. Real-time PCR was conducted using 2× SYBR Green qPCR Master Mix (None ROX) from Wuhan Servicebio Technology Co., Ltd., Wuhan, China. The PCR protocol involved an initial denaturation phase at 94 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 15 s, primer annealing, and extension at 60 °C for 30 s. Fluorescence signals were collected at each 0.5 °C temperature increment within the range of 65–95 °C. The relative gene expression levels were determined using the 2−ΔΔCT technique method for data analysis. The primer sequences employed in the PCR were synthesized by Wuhan ServiceBio Technology Co., Ltd., located in Wuhan, China, and are detailed in Table 1.
Table 1.
The sequence information of PCR primers.
| Name | Sequence | Tm | Length of Amplification | Gene ID |
|---|---|---|---|---|
| Nrf2 forward | 5’-GACATCCTTTGGAGGCAAGACAT-3’ | 60 | 268 bp | NM_031789.2 |
| Nrf2 reverse | 5’-TGGGAATGTGGGCAACCTG-3’ | |||
| GPx4 forward | 5’-GACATCCTTTGGAGGCAAGACAT-3’ | 60 | 212 bp | NM_001039849.3 |
| GPx4 reverse | 5’-TGGGAATGTGGGCAACCTG-3’ | |||
| NADPH forward | 5’-CTGGAGAAACCTGCCAAGTATG-3’ | 60 | 138 bp | NM_017008.4 |
| NADPH reverse | 5’-GGTGGAAGAATGGGAGTTGCT-3’ |
Western blotting analysis for Nrf2 and GPx4
To remove blood, rat brain tissue blocks were subjected to 2 to 3 cycles of rinsing with pre-chilled PBS. The tissue was then sectioned into small pieces and lysate was added for homogenization. The resulting mixture was centrifuged at 12000 RPM for 10 min at 4 °C, resulting in the separation of the supernatant. Protein concentration in the supernatant was determined using a BCA assay kit to ensure consistent protein loading for subsequent analyses. Equal amounts of proteins were loaded onto a 10 % SDS-PAGE gel for electrophoresis. Following electrophoresis, proteins were transferred onto a 0.45 μm PVDF membrane. The PVDF membranes were incubated overnight at 4 °C with primary antibodies: rabbit anti-Gpx4 (AB125066, diluted 1:1000, Abcam, Cambridge, UK), rabbit anti-Nrf2 (16396-1-AP, diluted 1:2000, Sanyin, Wuhan, China), and mouse anti-ACTIN (GB12001, diluted 1:2000, Servicebio, Wuhan, China). After three washes with TBST, membranes were incubated with secondary antibodies (GB23301, goat anti-mouse IgG HRP conjugated, diluted 1:5000; GB23303, goat anti-rabbit IgG HRP conjugated, diluted 1:5000; GB23303, goat anti-rabbit IgG HRP conjugated, diluted 1:5000; Servicebio, Wuhan, China). The strips were treated using a mixture of ECL luminescent solution, with actin serving as an internal reference for normalization. Raw data in TIFF format were analyzed using AIWBwellTM analysis software (Servicebio, Wuhan, China) to determine the relative expression of the target proteins.
Statistical analyses
Statistical analyses were conducted using SPSS 22.0 software, and GraphPad Prism (version 9.5.0, GraphPad Software, Inc.) was used for generating graphs. Multiple comparisons were assessed through one-way ANOVA, followed by post hoc Bonferroni testing. The data are presented as the mean ± standard deviation (SD), and statistical significance was considered at p<0.05.
Ethics approval
The study was approved by the Ethics Committee of Jiaxing College (approval No. SYXK (Zhejiang) 2020-0007).
Results
Effect of HF-rTMS on the learning memory ability of rats with VD
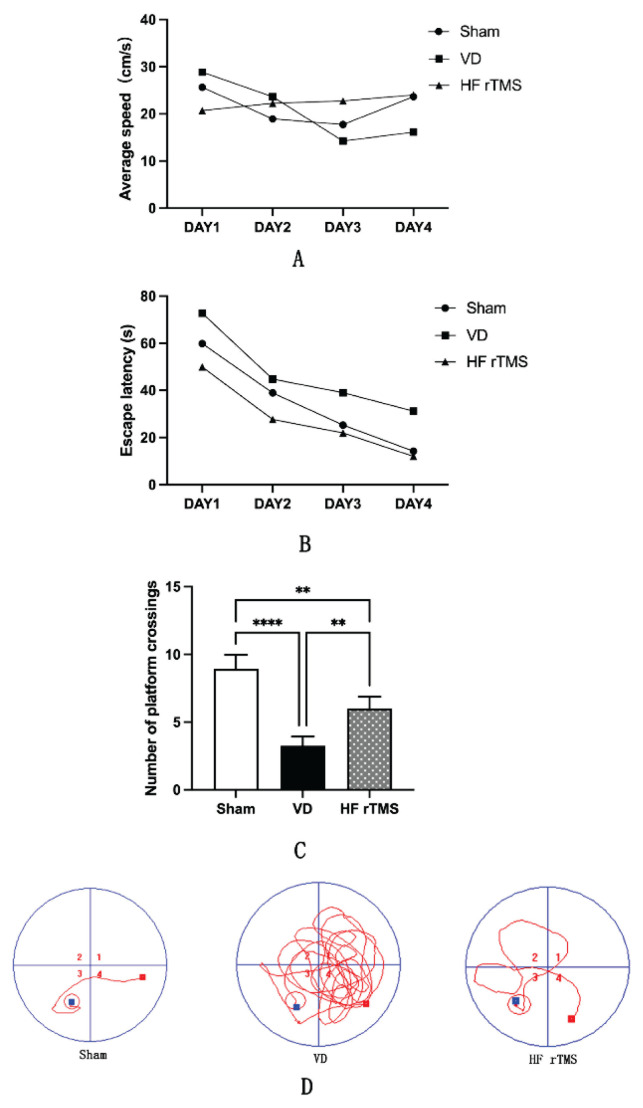
The spatial learning and memory abilities of rats were evaluated. There were no significant differences in mean velocity observed among the Sham group, VD group, and HF-rTMS group (Fig. 2A). Noteworthy distinctions were observed in the MWM test: the VD group exhibited prolonged escape latencies and fewer platform crossings from day 1 to day 4 compared to the sham group (p<0.05) (Fig. 2B, C). Conversely, over the initial four days, rats in the HF-rTMS group displayed reduced escape latencies and increased platform crossings compared to the VD group (Fig. 2B, C). Moreover, the swimming paths of the VD group demonstrated a higher level of disarray compared to the sham group, while the HF-rTMS group exhibited more organized swimming paths (Fig. 2D).
Fig. 2.
Impact of HF-rTMS on the learning memory ability of rats with VD. (A) Mean velocity; (B) Escape latency; (C) Number of platform traversals; (D) Representative swimming trajectories for all groups in the MWM test (* p<0.05, ** p<0.01, **** p<0.0001). HF-rTMS, High-Frequency Repetitive Transcranial Magnetic Stimulation; VD, Vascular Dementia; MWM, Morris water maze.
Effect of HF-rTMS on neuroinflammation in brain tissue of rats with VD
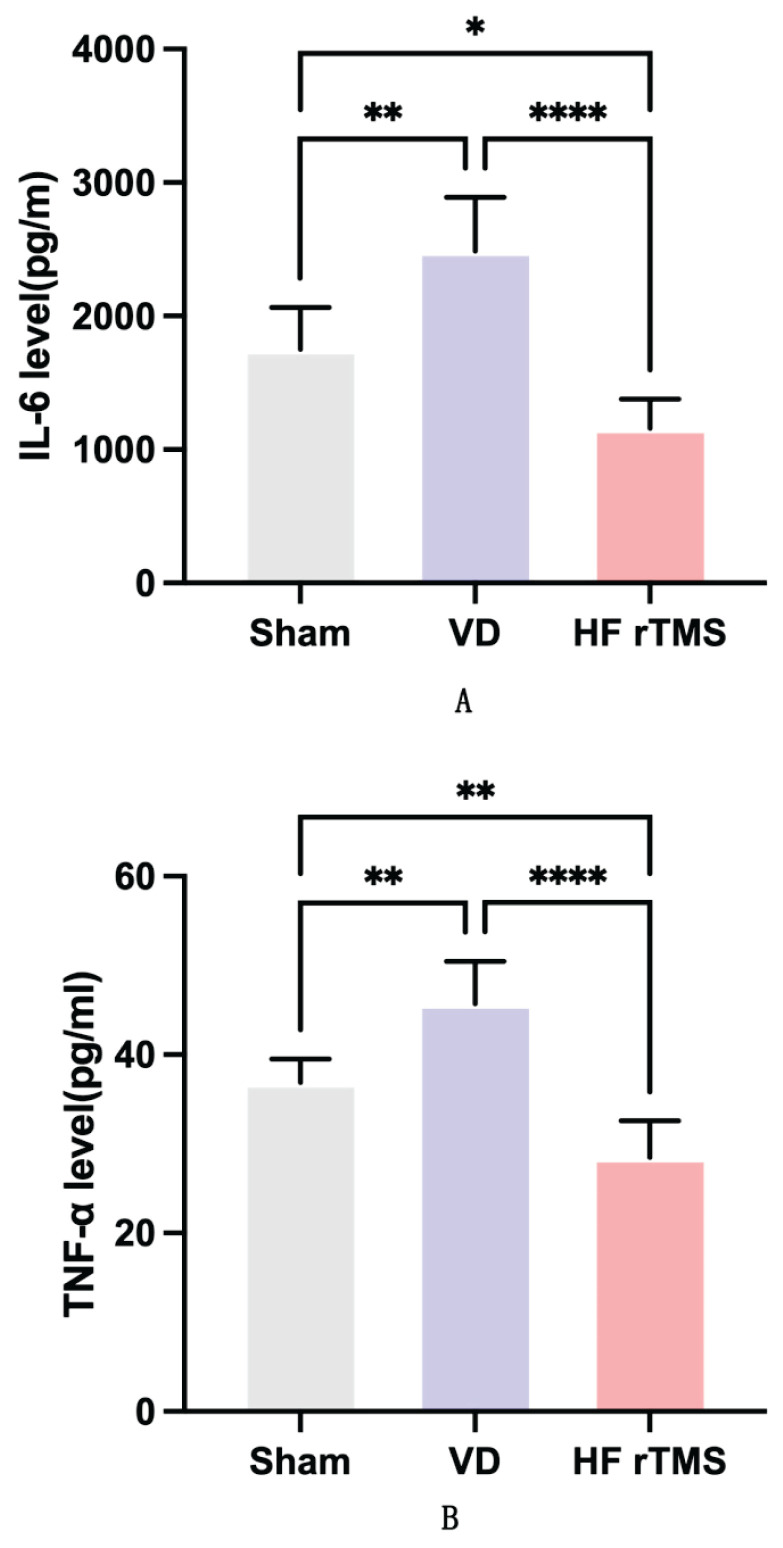
Compared to the sham group, rats in the VD group demonstrated significantly elevated levels of IL-6 and TNF-α inflammatory cytokines, as indicated by quantitative ELISA results (p<0.05). Following HF-rTMS treatment, the HF-rTMS group exhibited a significant reduction in IL-6 and TNF-α levels in brain tissue compared to the VD group (p<0.05). Notably, there were significant reductions in IL-6 and TNF-α concentrations in the brain tissue of the HF-rTMS group when compared to the sham group (p<0.05), as illustrated in Figure 3.
Fig. 3.
Impact of HF-rTMS on neuroinflammation in brain tissue of rats with VD. (A) IL-6 and (B) TNF-α levels (*p<0.05, ** p<0.01, **** p<0.0001).
Effect of HF-rTMS on oxidative stress in brain tissue of rats with VD
In the brain tissues of rats with VD, significant decreases were observed in the levels of superoxide dismutase (SOD) and glutathione (GSH) (p<0.05), whereas levels of malonic dialdehyde (MDA) experienced a significant increase (p<0.05), as determined using commercial kits. Following HF-rTMS therapy, there was a notable increase in the levels of SOD and GSH (p<0.05), and a decrease in the levels of MDA (p<0.05) compared to the VD group. However, there were no significant differences in SOD, GSH, and MDA levels between the sham group and the HF-rTMS group (p>0.05), as illustrated in Figure 4.
Fig. 4.
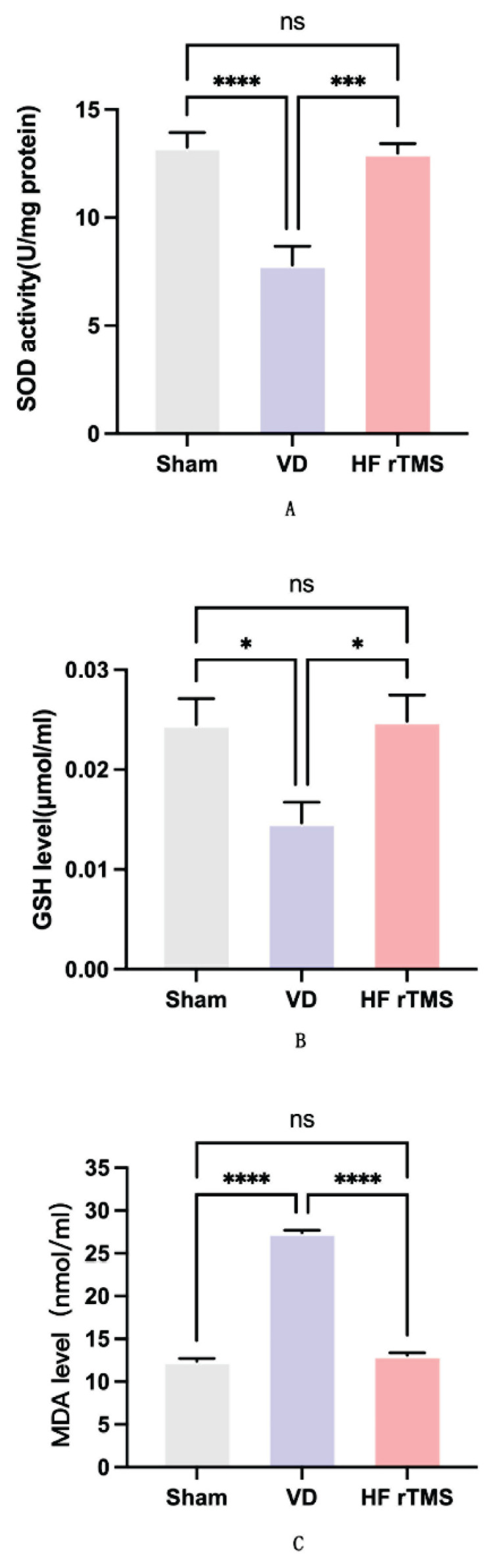
Impact of HF-rTMS on oxidative stress in the brain tissue of rats with VD. (A) SOD activity; (B) GSH level; and (C) MDA level (*p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
Effect of HF-rTMS on ferroptosis in brain tissue of rats with VD
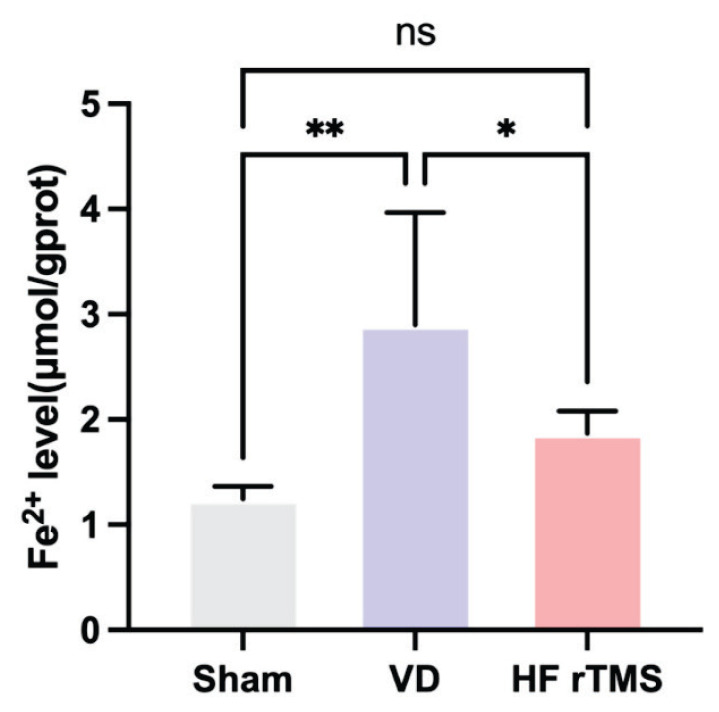
The quantitative ELISA analysis revealed a significant increase in Fe2+ levels in the rat brain tissue of the VD group compared to the sham group (p<0.05). However, the administration of HF-rTMS led to a significant decrease in Fe2+ concentrations in the brain tissue of rats with VD belonging to the HF-rTMS group (p<0.05) compared to the VD group. No notable disparities in Fe2+ concentrations were observed between the sham group and the HF-rTMS group (p>0.05), as illustrated in Figure 5.
Fig. 5.
Impact of HF-rTMS on ferroptosis in brain tissue of rats with VD. Fe2+ level (*p<0.05, ** p<0.01, ns p>0.05).
Effect of HF-rTMS on Nrf2 and GPx4 gene expression in brain tissue of rats with VD
Fluorescence PCR analysis demonstrated a significant decrease in the expression levels of both Nrf2 and GPx4 genes in the brain tissue of rats in the VD group compared to the sham group (p<0.05). Conversely, the application of HF-rTMS resulted in an increase in the levels of Nrf2 and GPx4 gene expression in the brain tissue of rats with VD (p<0.05). Importantly, there were no notable disparities in the expressions of Nrf2 and GPx4 genes between the sham group and the HF-rTMS group (p>0.05) (Fig. 6).
Fig. 6.
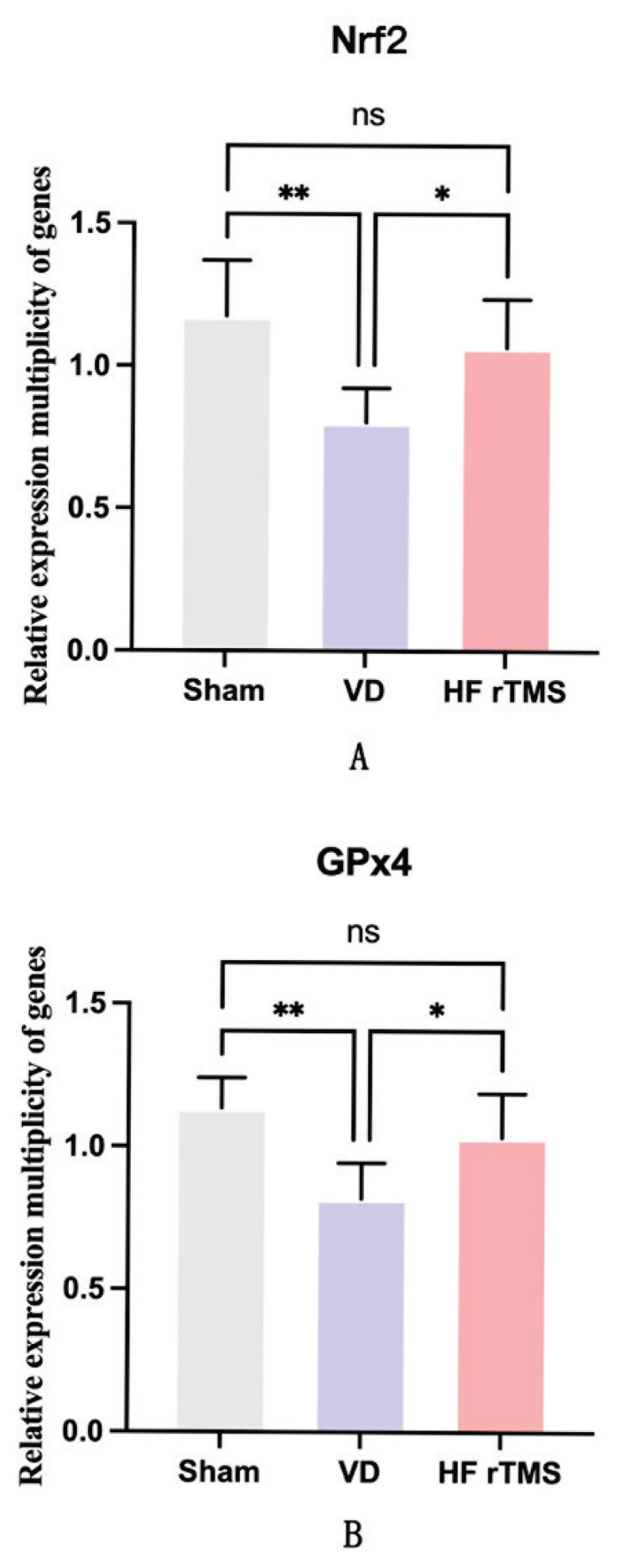
Impact of HF-rTMS on the expression of Nrf2 and GPx4 genes in the brain tissue of rats with VD. (A) Relative expression multiplicity of the Nrf2 gene; (B) Relative expression multiplicity of the GPx4 gene (*p<0.05, ** p<0.01, ns p>0.05).
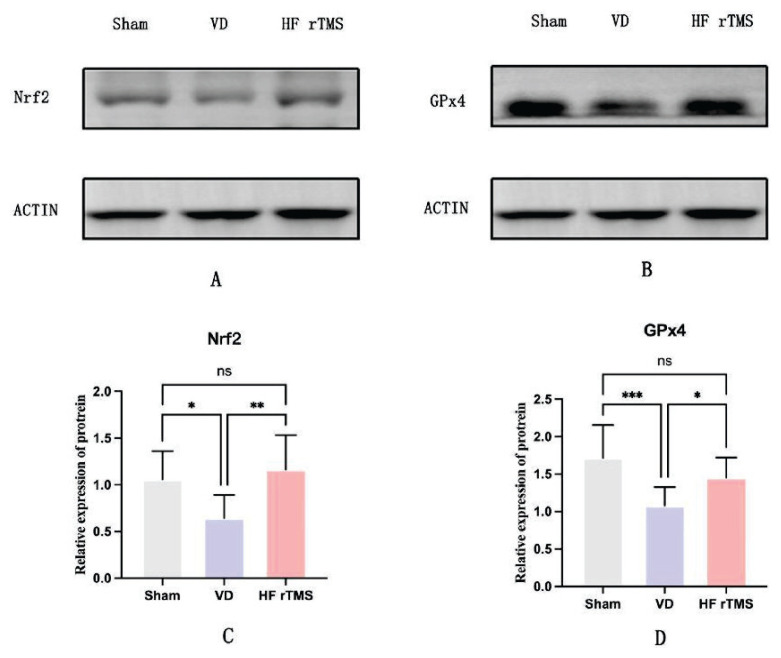
Effects of HF-rTMS on Nrf2 and GPx4 protein expression in brain tissue of rats with VD
Western blot analysis was used to assess the protein expressions of Nrf2 and GPx4 in rat brain tissues from the sham, VD, and HF-rTMS groups. In the brain tissues of rats from the VD group, the expressions of both Nrf2 and GPx4 proteins were significantly lower compared to the sham group (p<0.05). Conversely, after receiving HF-rTMS therapy, there was a notable increase in the levels of Nrf2 and GPx4 proteins compared to the VD group (p<0.05). No significant differences were observed between the sham group and the HF-rTMS group (p>0.05), as depicted in Figure 7
Fig. 7.
Effects of HF-rTMS on Nrf2 and GPx4 protein expression in brain tissue of rats with VD. (A) Western blot analysis of protein expression for Nrf2 in rat brain tissue; (B) Western blot analysis of protein expression for GPx4 in rat brain tissue; (C) Relative expression of protein Nrf2; (D) Relative expression of protein GPx4 (*p<0.05, ** p<0.01, *** p<0.001, ns p>0.05).
Discussion
Evaluating cognitive enhancement strategies for conditions like VD is considered essential due to its rising prevalence and significant impact on quality of life. The aim of this study was to explore the efficacy of HF-rTMS in modulating the Nrf2/GPx4 signaling pathway to alleviate cognitive impairments in VD rat models. Our results reveal a marked improvement in spatial learning and memory capabilities following HF-rTMS treatment in rats with VD. Moreover, in comparison to both the Sham stimulation and VD control groups, treatment with HF-rTMS resulted in a significant decrease in the levels of IL-6 and TNF-α in the brain tissues of rats. Subsequent evaluations post-treatment demonstrated notable elevations in the levels of SOD and GSH, along with a significant reduction in MDA levels, in rats with VD that underwent HF-rTMS treatment. Additionally, treatment with HF-rTMS led to a reduction in Fe2+ levels within the brain tissues of rats. Notably, both protein and gene expressions of Nrf2 and GPx4 were significantly upregulated in the HF-rTMS group in comparison to the VD group. This provides compelling evidence for the potential of HF-rTMS as an effective therapeutic strategy for VD.
Nonetheless, to fully ascertain the effectiveness and applicability of this intervention, further studies are required to uncover its underlying mechanisms. Over time, rTMS has demonstrated potential in improving cognitive functions in neurodegenerative diseases [20,21]. A recent meta-analysis underscored the significance of targeting the parietal and prefrontal cortex with TMS, revealing its neuro modulatory effects on cognitive processes such as number processing, magnitude processing, and arithmetic [22]. The DLPFC has emerged as a significant focus of therapeutic interventions, particularly for the elderly and individuals with mild cognitive impairment [23]. However, mechanistic studies elucidating the impact of TMS on VD are limited. Existing reports indicate that rTMS can modulate neurotransmitter levels such as acetylcholine, dopamine, and synaptophysin, leading to enhanced cognitive function [24,25]. In this study, we provide evidence of the positive impact of HF-rTMS on cognitive deficits in a VD rat model. Furthermore, we uncovered the anti-neuroinflammatory, antioxidant, and ferroptosis-inhibitory effects of HF-rTMS in the context of VD.
Given the pivotal roles of Nrf2 and GPx4 expression, along with established contributions of neuroinflammation, oxidative stress, and ferroptosis to cognition, a comprehensive assessment of their interplay becomes imperative [26–28]. Since the identification of Nrf2, exhaustive investigations have sought to elucidate its biological functions. A thorough understanding of its structural features, molecular mechanisms, functional attributes, activity regulation, downstream pathways, and potential therapeutic applications for diverse ailments is essential [29,30]. Recent research affirms the engagement of Nrf2 in anti-neuroinflammatory processes [31]. In the context of cerebral ischemia/reperfusion injury, activation of the NF-κB pathway triggers the expression of inflammatory factors (IL-6, TNF-α, and IL-1β), thereby exacerbating brain damage and post-stroke dysfunction. Consistent with this, elevated levels of IL-6 and TNF-α, along with reduced Nrf2 expression, are observed in the brain tissue of rats with VD in our study. Furthermore, the HF-rTMS group demonstrates a significant increase in Nrf2 expression, accompanied by notable enhancements in spatial memory and cognitive abilities when compared to the VD group. While the precise mechanisms underlying Nrf2 upregulation by rTMS remain elusive, it offers a plausible explanation for cognitive enhancement post-transcranial magnetic stimulation, aligning with findings in an Alzheimer's disease (AD) rat model where lentiviral-transfected Nrf2 injection into hippocampal tissue significantly enhanced learning and memory capabilities [32]. However, further elucidation is required regarding the specific mechanism by which HF-rTMS modulates neuroinflammatory pathways.
The burgeoning evidence underscores the pivotal role of Nrf2 in orchestrating cellular defense mechanisms, particularly in governing antioxidant systems. The results of our study validate decreased SOD and GSH activity in rats with VD, effectively restored by HF-rTMS-induced Nrf2/GPx4 activation. HF-rTMS also reduces MDA concentrations, showcasing its ability to attenuate oxidative stress from chronic cerebral hypoperfusion, providing neuronal protection. Yet, a comprehensive understanding of HF-rTMS-induced changes in the oxidation state warrants further assessment [33]. Dixon introduced ferroptosis, a distinct programmed cell death pattern induced by erastin disrupting cysteine transport, reducing glutathione, and inactivating GPx4. Ferroptosis, distinct from apoptosis and necrosis, is iron-dependent, marked by increased intracellular iron ions and lipid peroxide accumulation [34]. Multifaceted inducers of ferroptosis encompass the inhibition of system Xc-, inactivation of GPx4, elevation of ROS and iron levels, and the accumulation of lipid peroxides, constituting the participants of extensive investigation [35–37]. The investigation into neurological conditions is focused on the pivotal role of ferritin in iron storage [38,39]. GPx4, a selenoprotein glutathione peroxidase, decreases hydrogen peroxide, crucial in preventing ferroptosis by reducing phospholipid hydrogen peroxide and curbing lipoxygenase-mediated lipid peroxidation [40]. Our findings in VD-affected rat brains show significant reductions in Nrf2 and GPx4 expressions and increased Fe2+ levels. Notably, HF-rTMS activates Nrf2 and GPx4, decreases Fe2+ levels, and enhances cognitive function. Ferroptosis observed in the ischemic rat model corresponds with a decrease in GPx4 expression [41]. Heightened brain iron triggers pro-inflammatory factor release, contributing to iron accumulation. Despite increased research on ferroptosis and VD, further clarification on the connections between neuroinflammation, oxidative stress, and ferroptosis is essential [42,43].
Several limitations exist in our study. The challenge lies in the dimensions of the stimulation coil; despite opting for a 70 mm figure-of-eight coil to reduce overheating during high-frequency stimulation, even the most compact coil on the market remains excessively big for the head of a rat. This constraint prevents accurate single-brain region targeting using rTMS in rats. Thus, whole-brain stimulation was used to assess rTMS effects. Additionally, the small sample size may limit the depth of our results and the study of rTMS mechanisms. Furthermore, the complexity of cognition extends beyond neuroinflammation, oxidative stress, and iron-induced cell death; cognitive enhancement may result from a synergistic effect of multiple factors. Therefore, future research should consider the complexity of cognition and assess the interplay of these multiple factors.
Conclusions
In conclusion, our findings undeniably establish that the use of high-frequency transcranial magnetic stimulation significantly enhances cognitive capabilities in a rat model of VD. This enhancement appears to be intricately linked to the modulation of oxidative stress, neuroinflammation, and ferroptosis through the Nrf2/GPx4 signaling pathway. Overall, this study provides a conceptual framework supporting the potential use of HF-rTMS as a viable strategy to alleviate cognitive deficits associated with VD.
Acknowledgements
We express our gratitude to our colleagues at the Rehabilitation Medicine Center, The First Affiliated Hospital of Nanjing Medical University, and the Rehabilitation Medicine Center, Jiaxing Hospital of Traditional Chinese Medicine, for their support and valuable input in our research. This research was supported by the Health Science and Technology Project of Zhejiang Province (No. 2021KY1126) and the Affiliated Hospital of the University-level Scientific Research Project of Zhejiang Chinese Medicine University (Natural Science) (No. 2022FSYYZQ20) and the Jiaxing Key Laboratory of Integrated Chinese and Western Medicine for Cerebrovascular Disease Rehabilitation (Class A).
Abbreviations
- ARE
Antioxidant Response Elements
- GSH
glutathione
- GSR
Glutathione
- GSSH
Oxidized Glutathione; Reductase
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- γ-GCS
Gamma-Glutamylcysteine Synthetase
- NF-κB
Nuclear Factor-κB
- PUFAS
Polyunsaturated Fatty Acids
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Hobert MA, Hofmann W, Bartsch T, Peters S, Maetzler W. Diagnosis and treatment of vascular dementia. (Article in German) Z Gerontol Geriatr. 2020;53:687–698. doi: 10.1007/s00391-020-01786-3. [DOI] [PubMed] [Google Scholar]
- 2.Román GC. Vascular dementia revisited: diagnosis, pathogenesis, treatment, and prevention. Med Clin North Am. 2002;86:477–499. doi: 10.1016/S0025-7125(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 3.Wolters FJ, Ikram MA. Epidemiology of Vascular Dementia. Arterioscler Thromb Vasc Biol. 2019;39:1542–1549. doi: 10.1161/ATVBAHA.119.311908. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev PS, Brodaty H, Looi JC. Vascular dementia: diagnosis, management and possible prevention. Med J Aust. 1999;170:81–85. doi: 10.5694/j.1326-5377.1999.tb126889.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim WJ, Rosselin C, Amatya B, Hafezi P, Khan F. Repetitive transcranial magnetic stimulation for management of post-stroke impairments: An overview of systematic reviews. J Rehabil Med. 2020;52:jrm00015. doi: 10.2340/16501977-2637. [DOI] [PubMed] [Google Scholar]
- 6.Gomes-Osman J, Indahlastari A, Fried PJ, Cabral DLF, Rice J, Nissim NR, Aksu S, et al. Non-invasive Brain Stimulation: Probing Intracortical Circuits and Improving Cognition in the Aging Brain. Front Aging Neurosci. 2018;10:177. doi: 10.3389/fnagi.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Cha B, Lee D, Kim JM, Kim M. Effect of Cognition Recovery by Repetitive Transcranial Magnetic Stimulation on Ipsilesional Dorsolateral Prefrontal Cortex in Subacute Stroke Patients. Front Neurol. 2022;13:823108. doi: 10.3389/fneur.2022.823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilek E, Schäfer A, Ochs E, Esslinger C, Zangl M, Plichta MM, Braun U, et al. Application of alters human prefrontal-hippocampal functional interaction. J Neurosci. 2013;33:7050–7056. doi: 10.1523/JNEUROSCI.3081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeurissen D, Sack AT, Roebroeck A, Russ BE, Pascual-Leone A. TMS affects moral judgment, showing the role of DLPFC and TPJ in cognitive and emotional processing. Front Neurosci. 2014;8:18. doi: 10.3389/fnins.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, Xing M, Wang Y, Tao H, Cheng Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity a the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. 2015;311:284–291. doi: 10.1016/j.neuroscience.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. Nrf2-a Promising Therapeutic Target for Defensing Against Oxidative Stress in Stroke. Mol Neurobiol. 2017;54:6006–6017. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Zou L, Jiang X, Cheng S, Zhang J, Qin X, Qin Z, et al. Stabilization of Nrf2 leading to HO-1 activation protects against zinc oxide nanoparticles-induced endothelial cell death. Nanotoxicology. 2021;15:779–797. doi: 10.1080/17435390.2021.1919330. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wu J, Yu S, Zhu J, Zhou Y, Wang P, Li L, Zhao Y. Sestrin2 promotes angiogenesis to alleviate brain injury by activating Nrf2 through regulating the interaction between p62 and Keap1 following photothrombotic stroke in rats. Brain Res. 2020;1745:146948. doi: 10.1016/j.brainres.2020.146948. [DOI] [PubMed] [Google Scholar]
- 14.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, Wei Y, et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol. 2021;22:1127–1139. doi: 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Chen S, Guo H, Jiang H, Liu H, Fu H, Wang D. Forsythoside A Mitigates Alzheimer's-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int J Biol Sci. 2022;18:2075–2090. doi: 10.7150/ijbs.69714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation Fu C, Wu Y, Liu S, et al. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol. 2022;289:115021. doi: 10.1016/j.jep.2022.115021. [DOI] [PubMed] [Google Scholar]
- 18.Guo T, Fang J, Tong ZY, He S, Luo Y. Transcranial Direct Current Stimulation Ameliorates Cognitive Impairment via Modulating Oxidative Stress, Inflammation, and Autophagy in a Rat Model of Vascular Dementia. Front Neurosci. 2020;14:28. doi: 10.3389/fnins.2020.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo F, Lou J, Han X, Deng Y, Huang X. Repetitive Transcranial Magnetic Stimulation Ameliorates Cognitive Impairment by Enhancing Neurogenesis and Suppressing Apoptosis in the Hippocampus in Rats with Ischemic Stroke. Front Physiol. 2017;8:559. doi: 10.3389/fphys.2017.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Ma J, Zhang J, Shi WY, Mei HN, Xing Y. Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Thyroid Hormones Level and Cognition in the Recovery Stage of Stroke Patients with Cognitive Dysfunction. Med Sci Monit. 2021;27:e931914. doi: 10.12659/MSM.931914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan TF, Li WG, Zhang C, Wei H, Sun S, Xu N-J, Liu J, Xu T-L. Targeting neuroplasticity in patients with neurodegenerative diseases using brain stimulation techniques. Transl Neurodegener. 2020;9:44. doi: 10.1186/s40035-020-00224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Sanz S, Ghotme KA, Hedmont D, Arévalo-Jaimes MY, Kadosh RC, Serra-Grabulosa JM, Redolar-Ripoll D. Use of transcranial magnetic stimulation for studying the neural basis of numerical cognition: A systematic review. J Neurosci Methods. 2022;369:109485. doi: 10.1016/j.jneumeth.2022.109485. [DOI] [PubMed] [Google Scholar]
- 23.Chou YH, Ton That V, Sundman M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2020;86:1–10. doi: 10.1016/j.neurobiolaging.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Qi G, Yu C, Lian G, Zheng H, Wu S, Yuan T-F, Zhou D. Cortical plasticity is correlated with cognitive improvement in Alzheimer's disease patients after rTMS treatment. Brain Stimul. 2021;14:503–510. doi: 10.1016/j.brs.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Farina M, Vieira LE, Buttari B, Profumo E, Saso L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules. 2021;26:5001. doi: 10.3390/molecules26165001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Yuan M, Yang S, Chen X, Wu J, Wen M, Yan K, Bi X. Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway. Int J Neurosci. 2021;131:641–649. doi: 10.1080/00207454.2020.1797722. [DOI] [PubMed] [Google Scholar]
- 28.Zhu K, Zhu X, Liu S, Yu J, Wu S, Hei M. Glycyrrhizin Attenuates Hypoxic-Ischemic Brain Damage by Inhibiting Ferroptosis and Neuroinflammation in Neonatal Rats via the HMGB1/GPX4 Pathway. Oxid Med Cell Longev. 2022;2022:8438528. doi: 10.1155/2022/8438528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazur A, Fangman M, Ashouri R, Arcenas A, Doré S. Nrf2 as a therapeutic target in ischemic stroke. Expert Opin Ther Targets. 2021;25:163–166. doi: 10.1080/14728222.2021.1890716. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, Ghezzi P, et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 33.Kerins MJ, Ooi A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gou Z, Su X, Hu X, Zjou Y, Huang L, Fan Y, Li J, Lu L. Melatonin improves hypoxic-ischemic brain damage through the Akt/Nrf2/Gpx4 signaling pathway. Brain Res Bull. 2020;163:40–48. doi: 10.1016/j.brainresbull.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Fang Y, Zhang Z, Luo Y, Zhang A, Lenahan C, Chen S. Ferroptosis: An emerging therapeutic target in stroke. J Neurochem. 2022;160:64–73. doi: 10.1111/jnc.15351. [DOI] [PubMed] [Google Scholar]
- 37.Tuo QZ, Lei P, Jackman KA, Li X-L, Xiong H, Li X-L, Liuyang Z-Y, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 38.Ji Y, Zheng K, Li S, Ren C, Shen Y, Tian L, Zhu H, et al. Insight into the potential role of ferroptosis in neurodegenerative diseases. Front Cell Neurosci. 2022;16:1005182. doi: 10.3389/fncel.2022.1005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Cao F, Yin H-L, Huang Z-J, Lin Z-T, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo SE, Chen L, Na R, Liu Y, Rios C, Van Remmen H, Richardson A, Ran Q. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic Biol Med. 2012;52:1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Xu G, Fu L, Li Y, Fu R, Zhao D, Ding C. The effects of repetitive transcranial magnetic stimulation on the cognition and neuronal excitability of mice. Electromagn Biol Med. 2020;39:9–19. doi: 10.1080/15368378.2019.1696358. [DOI] [PubMed] [Google Scholar]
- 42.Ayton S, Portbury S, Kalinowski P, Agarwal P, Diouf I, Schneider JA, Morris AM, Bush AI. Regional brain iron associated with deterioration in Alzheimer's disease: A large cohort study and theoretical significance. Alzheimers Dement. 2021;17:1244–1256. doi: 10.1002/alz.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Zhang E, Yang H, Chen Y, Tao L, Xu Y, Chen T, Shen X. Gastrodin Ameliorates Cognitive Dysfunction in Vascular Dementia Rats by Suppressing Ferroptosis via the Regulation of the Nrf2/Keap1-GPx4 Signaling Pathway. Molecules. 2022;27:6311. doi: 10.3390/molecules27196311. [DOI] [PMC free article] [PubMed] [Google Scholar]