Abstract
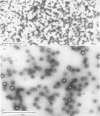
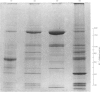
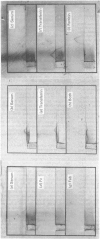
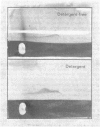
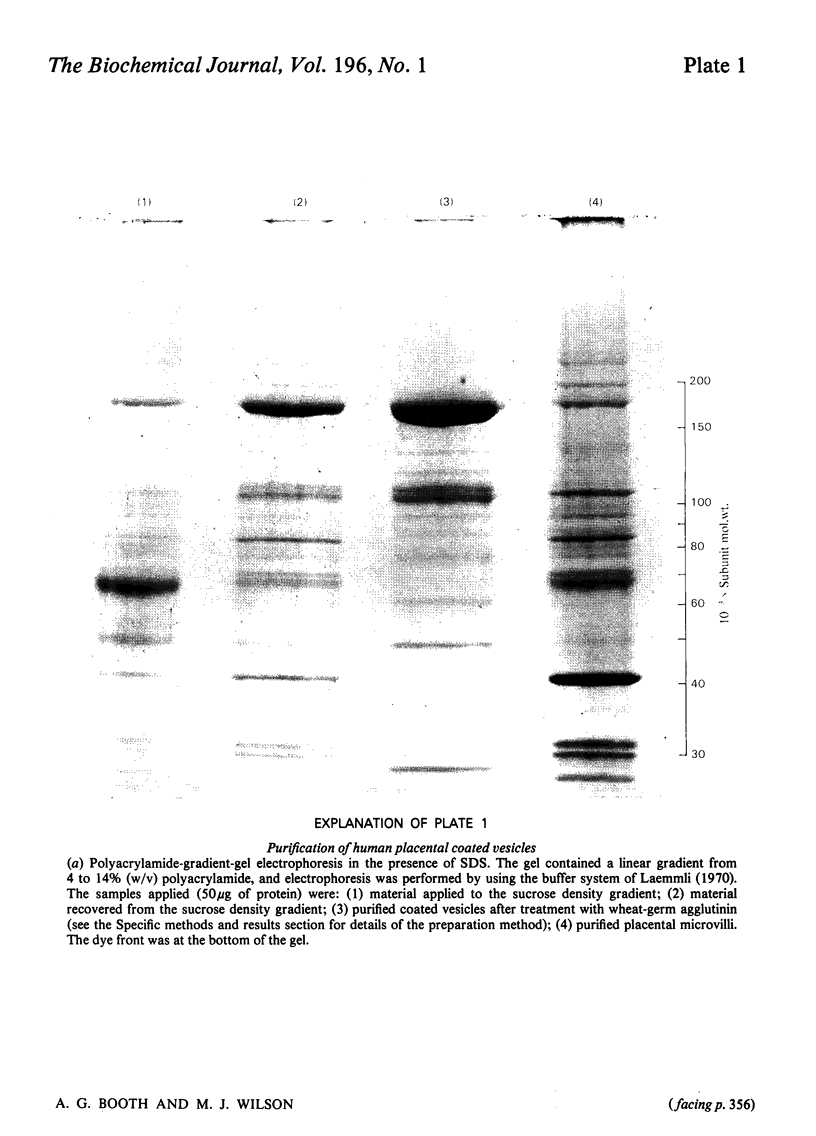
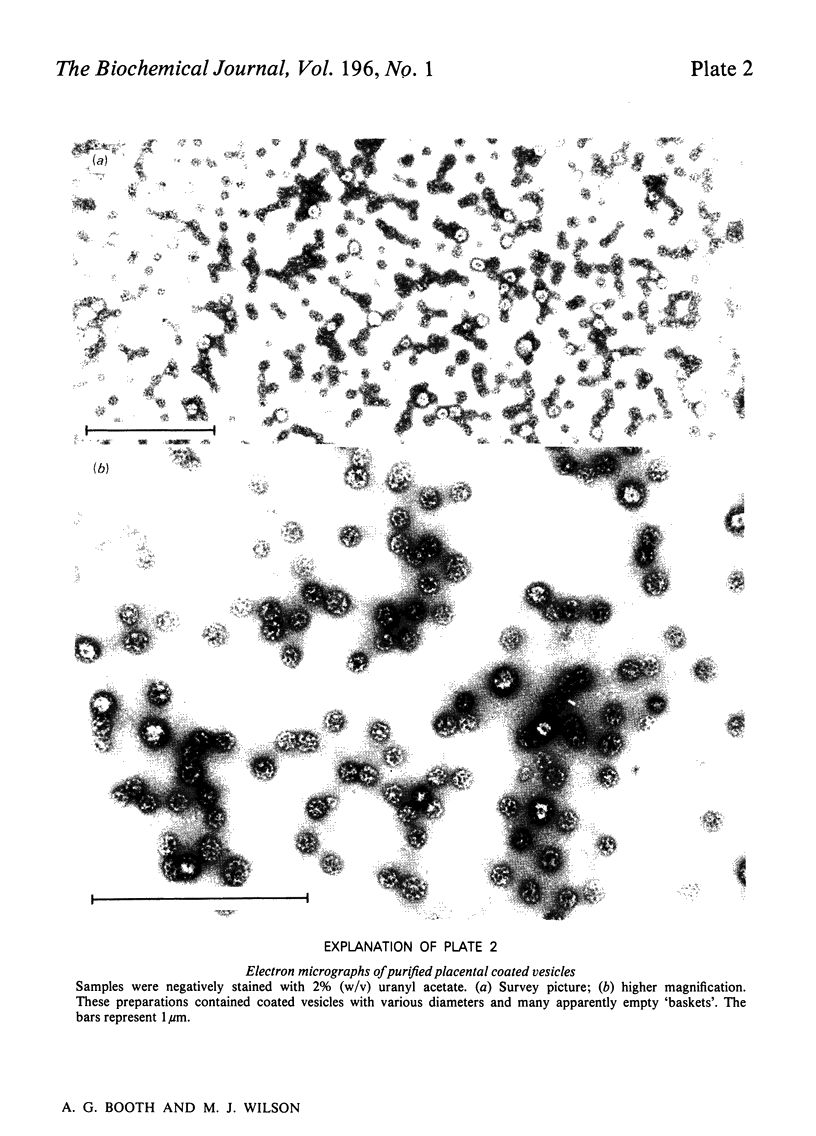
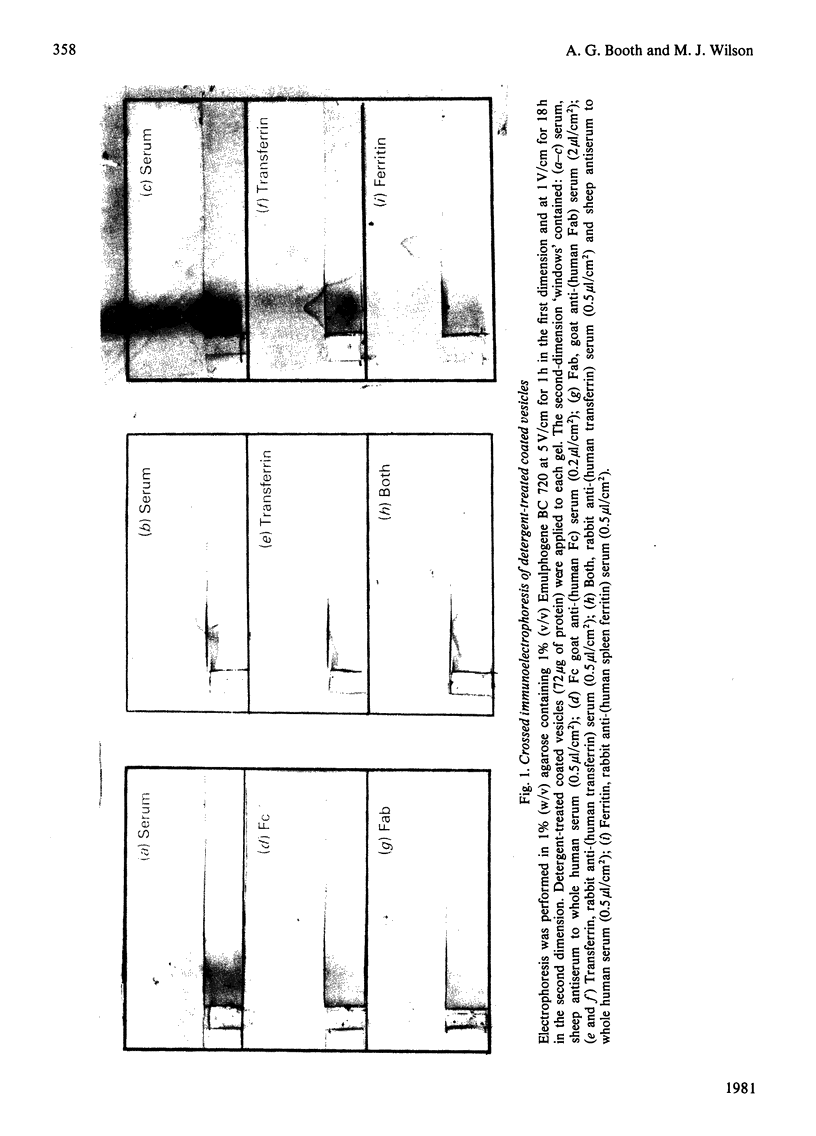
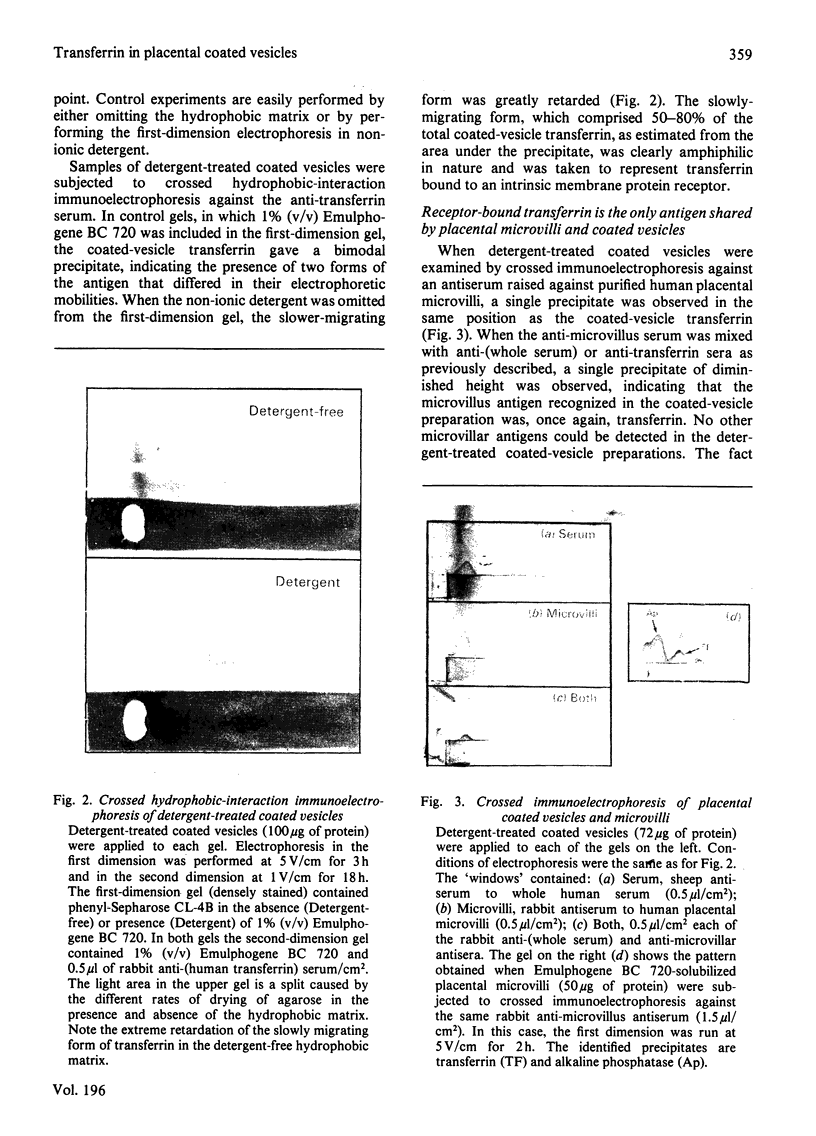
Human placental coated vesicles have been purified by a method involving sucrose-density-gradient centrifugation and treatment with wheat-germ agglutinin. These preparations were free of contamination by placental microvillus fragments. Crossed immunoelectrophoresis demonstrated that the coated vesicles contained a single serum protein, which was identified as transferrin. This transferrin was only observed after the vesicles were treated with a non-ionic detergent, and its behaviour during crossed hydrophobic-interaction immunoelectrophoresis suggested that a large proportion of it was receptor-bound. No other serum proteins, including immunoglobulin G, could be detected in these preparations. Receptor-bound transferrin was the only antigen common to placental coated vesicles and microvilli, implying that other plasma-membrane proteins are excluded from the region of membrane involved in coated-vesicle formation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P. Citrate-mediated exchange of FE3+ among tranferrin molecules. Biochem Biophys Res Commun. 1968 Jul 26;32(2):220–226. doi: 10.1016/0006-291x(68)90372-0. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J. Crossed hydrophobic interaction immunoelectrophoresis: an analytical method for detection of amphiphilic proteins in crude mixtures and for prediction of the result of hydrophobic interaction chromatography. Anal Biochem. 1978 Oct 1;90(1):331–348. doi: 10.1016/0003-2697(78)90036-2. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Olaniyan R. O., Vanderpuye O. A. An improved method for the preparation of human placental syncytiotrophoblast microvilli. Placenta. 1980 Oct-Dec;1(4):327–336. doi: 10.1016/s0143-4004(80)80034-8. [DOI] [PubMed] [Google Scholar]
- Bouchard P., Moroux Y., Tixier R., Privat J. P., Monsigny M. An improved method for purification of wheat germ agglutinin (lectin) by affinity chromatography. Biochimie. 1976;58(10):1247–1253. doi: 10.1016/s0300-9084(76)80124-1. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Temple A., Lovins R. E., Smith N. Antigens of human trophoblasts: a working hypothesis for their role in normal and abnormal pregnancies. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1947–1951. doi: 10.1073/pnas.75.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman P. A., Shia M. A., Wallace J. K. A saturable high affinity binding site for transcobalamin II-vitamin B12 complexes in human placental membrane preparations. J Clin Invest. 1977 Jan;59(1):51–58. doi: 10.1172/JCI108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITLIN D., KUMATE J., URRUSTI J., MORALES C. THE SELECTIVITY OF THE HUMAN PLACENTA IN THE TRANSFER OF PLASMA PROTEINS FROM MOTHER TO FETUS. J Clin Invest. 1964 Oct;43:1938–1951. doi: 10.1172/JCI105068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Immunological studies of transferrin and transferrin receptors of human placental trophoblast. Placenta. 1980 Jan-Mar;1(1):33–46. doi: 10.1016/s0143-4004(80)80014-2. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., George S. G., Ingram J., Kershaw D., Wood E. J., Young A. R. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J. 1976 Jul 1;157(1):169–182. doi: 10.1042/bj1570169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. T. Immunoelectron microscopy localization of immunoglobulin G in human placenta. J Histochem Cytochem. 1980 Apr;28(4):339–346. doi: 10.1177/28.4.6768794. [DOI] [PubMed] [Google Scholar]
- Loh T. T., Higuchi D. A., van Bockxmeer F. M., Smith C. H., Brown E. B. Transferrin receptors on the human placental microvillous membrane. J Clin Invest. 1980 May;65(5):1182–1191. doi: 10.1172/JCI109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb T., Koh T. Y., Dorrington K. J., Painter R. H. Structure and function of immunoglobulin domains. V. Binding, University of immunoglobulin G and fragments to placental membrane preparations. J Immunol. 1976 Sep;117(3):882–888. [PubMed] [Google Scholar]
- Ockleford C. D., Whyte A. Differeniated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: coated vesicles. J Cell Sci. 1977 Jun;25:293–312. doi: 10.1242/jcs.25.1.293. [DOI] [PubMed] [Google Scholar]
- Ogbimi A. O., Johnson P. M., Brown P. J., Fox H. Characterisation of the soluble fraction of human syncytiotrophoblast microvillous plasma membrane-associated proteins. J Reprod Immunol. 1979 Jul;1(2):127–140. doi: 10.1016/0165-0378(79)90013-5. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. On the structural and functional components of coated vesicles. J Mol Biol. 1978 Dec 25;126(4):803–812. doi: 10.1016/0022-2836(78)90021-9. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Smith C. H., Nelson D. M., King B. F., Donohue T. M., Ruzycki S., Kelley L. K. Characterization of a microvillous membrane preparation from human placental syncytiotrophoblast: a morphologic, biochemical, and physiologic, study. Am J Obstet Gynecol. 1977 May 15;128(2):190–196. doi: 10.1016/0002-9378(77)90686-x. [DOI] [PubMed] [Google Scholar]
- Surgenor D. M., Koechlin B. A., Strong L. E. CHEMICAL, CLINICAL, AND IMMUNOLOGICAL STUDIES ON THE PRODUCTS OF HUMAN PLASMA FRACTIONATION. XXXVII. THE METAL-COMBINING GLOBULIN OF HUMAN PLASMA. J Clin Invest. 1949 Jan;28(1):73–78. doi: 10.1172/JCI102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Whitsett J. A., Lessard J. L. Characteristics of the microvillus brush border of human placenta: insulin receptor localization in brush border membranes. Endocrinology. 1978 Oct;103(4):1458–1468. doi: 10.1210/endo-103-4-1458. [DOI] [PubMed] [Google Scholar]