Abstract
STUDY QUESTION
Can secondary follicles be obtained from cultured cryopreserved-thawed human ovarian cortical tissue?
SUMMARY ANSWER
We obtained high-quality secondary follicles from cultured cryopreserved-thawed human ovarian cortical tissue from cis female donors (cOVA), but not from trans masculine donors (tOVA) in the same culture conditions.
WHAT IS KNOWN ALREADY
The in vitro growth of oocytes present in unilaminar follicles into metaphase II stage (MII) oocytes has been previously achieved starting from freshly obtained ovarian cortical tissue from adult cis female donors. This involved a multi-step culture protocol and the first step included the transition from unilaminar follicles to multilayered secondary follicles. Given that the ovarian cortex (from both cis female and trans masculine donors) used for fertility preservation is cryopreserved, it is crucial to investigate the potential of unilaminar follicles from cryopreserved-thawed ovarian cortex to grow in culture.
STUDY DESIGN, SIZE, DURATION
Cryopreserved-thawed ovarian cortical tissue from adult trans masculine donors (n = 3) and adult cis female donors (n = 3) was used for in vitro culture following the first culture step described in two published culture protocols (7–8 days and 21 days) and compared to freshly isolated ovarian cortex from trans masculine donors (n = 3) and to ovarian cortex prior to culture.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Ovarian cortical tissue was obtained from adult trans masculine donors undergoing gender-affirming surgery while using testosterone, and from adult cis female donors undergoing oophorectomy for fertility preservation purposes before chemotherapy. The ovarian cortex was fixed either prior (day 0) or after the culture period. Follicular survival, growth, and morphology were assessed through histology and immunofluorescence.
MAIN RESULTS AND THE ROLE OF CHANCE
We quantified the different stages of follicular development (primordial, primary, secondary, and atretic) after culture and observed an increase in the percentage of secondary follicles as well as an increase in COLIV deposition in the stromal compartment regardless of the culture media used. The quality of the secondary follicles obtained from cOVA was comparable to those prior to culture. However, in the same culture conditions, the secondary follicles from tOVA (fresh and cryo) showed low-quality secondary follicles, containing oocytes with small diameter, granulosa cells that expressed abnormal levels of KRT19 and steroidogenic-marker STAR and lacked ACTA2+ theca cells, when compared to tOVA secondary follicles prior to culture.
LIMITATIONS, REASONS FOR CAUTION
The number of different donors used was limited.
WIDER IMPLICATIONS OF THE FINDINGS
Our study revealed that cryopreserved-thawed cOVA can be used to generate high-quality secondary follicles after culture and those can now be further tested to evaluate their potential to generate functional MII oocytes that could be used in the clinic. However, using the same culture protocol on tOVA (fresh and cryo) did not yield high-quality secondary follicles, suggesting that either the testosterone treatment affects follicular quality or adapted culture protocols are necessary to obtain high-quality secondary follicles from tOVA. Importantly, caution must be taken when using tOVA to optimize folliculogenesis in vitro.
STUDY FUNDING/COMPETING INTEREST(S)
This research was funded by the European Research Council Consolidator Grant OVOGROWTH (ERC-CoG-2016-725722 to J.S.D.V. and S.M.C.D.S.L.), the Novo Nordisk Foundation (reNEW NNF21CC0073729 to H.C., F.W., J.S.D.V., S.M.C.D.S.L.), and China Scholarship Council (CSC 202008320362 and CSC 202008450034 to H.C. and F.W.), respectively. The authors have no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: fertility preservation, follicular growth, ovarian tissue cryopreservation, secondary follicle, in vitro culture, human, oocyte, granulosa cells, ovarian cortex, trans masculine
Introduction
Fertility preservation is becoming increasingly important and beneficial not only for individuals diagnosed with certain (oncological) diseases but also for social reasons and as part of gender-affirming procedures (Coleman et al., 2022; ESHRE Guideline Group on Female Fertility Preservation et al., 2020; Practice Committee of the American Society for Reproductive Medicine, 2019, 2021). There are currently several available options for fertility preservation, including embryo cryopreservation, oocyte cryopreservation after ovarian stimulation and ovarian tissue cryopreservation (OTC), as outlined by the European Society of Human Reproduction and Embryology (ESHRE Guideline Group on Female Fertility Preservation et al., 2020) and the American Society for Reproductive Medicine (Practice Committee of the American Society for Reproductive Medicine, 2019, 2021).
OTC involves the isolation of the outer cortex of the ovary (about 1 mm), where the pool of unilaminar follicles (primordial and primary follicles) reside (Gougeon, 2010). The large majority of the follicles in the adult ovary are dormant primordial follicles formed by one oocyte surrounded by flat granulosa cells. Upon follicular activation, the flat granulosa cells become cuboidal granulosa cells, marking the formation of primary follicles. Subsequently, the oocyte increases in size, the granulosa cells start proliferating, giving rise to a multilayered secondary follicle and an ovarian-specific cell type differentiates in the ovarian stromal compartment, the theca cells. Thereafter, a growing fluid-filled antral cavity emerges in small antral follicles (1–5 mm in diameter) (Gougeon, 2010). During folliculogenesis in humans, most follicles degenerate (atresia) (Wei et al., 2023), and typically only one manages to reach dominance and the ovulatory stage per menstrual cycle (Baerwald et al., 2012).
OTC was initially developed to preserve fertility in individuals undergoing gonadotoxic treatment for malignant diseases when oocyte cryopreservation was not possible but is now offered to individuals for both medical or social indications (Yding Andersen et al., 2019). Currently, the procedure to restore fertility after OTC includes a necessary step of autologous (or heterologous) transplantation of the ovarian cortex tissue containing the unilaminar follicles. After transplantation life birth rate is about 21% using IVF and about 33% from spontaneous pregnancies (Fraison et al., 2023), however, this fertility preservation option depends on the number of follicles present in the transplanted ovarian cortex tissue. Moreover, this procedure is not indicated for several groups of patients, such as individuals with blood-related cancers, due to the risk of reintroduction of malignant cells (Bastings et al., 2013; Rosendahl et al., 2013) or trans masculine people, due to gender dysphoria and the need for female hormones, not in line with their gender identity. From a social and medical point of view, it would be tremendously beneficial to establish clinical protocols to allow for efficient follicular growth and maturation that would lead to functional oocytes in vitro, for further use in medically assisted reproduction, avoiding ovarian tissue transplantation.
Protocols for the in vitro maturation (IVM) of oocytes in cumulus-oocyte complexes (COCs), found during ovarian tissue preparation for OCT, into functionally mature metaphase II (MII) oocytes have been established from cis females and trans masculine individuals (De Roo et al., 2017; Lierman et al., 2017; ESHRE Guideline Group on Female Fertility Preservation et al., 2020). However, MII oocytes from trans masculine individuals show poor cytoplasmic maturation and consequently result in low fertilization rates and even lower rates of development to the blastocyst stage (Lierman et al., 2021; Christodoulaki et al., 2023).
The in vitro growth and maturation of oocytes in unilaminar follicles into MII oocytes remains challenging and has been reported in only two studies, both using a multi-step culture protocol (McLaughlin et al., 2018; Xu et al., 2021). Importantly, the capacity of those MII oocytes to be fertilized and give rise to pre-implantation embryos was not investigated. Moreover, these protocols were only applied to freshly collected ovarian cortex tissue from adult cis female donors. However, as the ovarian cortex tissue used for fertility preservation purposes (from both cis female and trans masculine individuals) is standardly cryopreserved, it is crucial to optimize the growth and maturation of unilaminar follicles in vitro starting from cryopreserved ovarian cortex tissue to use in future clinical applications. Therefore, we have applied the two culture protocols by McLaughlin et al. (2018) and by Xu et al. (2021) to induce the first step of in vitro growth from unilaminar follicles using cryopreserved ovarian cortical tissue from adult trans masculine and cis female donors.
We report here that cryopreserved-thawed ovarian cortex from both adult trans masculine and cis female donors yielded secondary follicles after culture. In contrast to trans masculine ovarian cortex, the quality of the secondary follicles from cis female ovarian cortex was high regarding oocyte diameter, characteristics of granulosa cells, and emergence of theca cell progenitors, suggesting that this material has great potency to be further cultured to generate MII oocytes. This is an important first step to achieve in vitro growth and maturation of oocytes from cryopreserved-thawed human ovarian cortical tissue that could be used for clinical applications.
Materials and methods
Ethical permission for the collection and use of human ovarian tissue
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and letters of no objection were issued by the Medical Ethical Committee of the Leiden University Medical Center (B16.050, B18.029, B22.3077). Signed informed consent was obtained from two groups of adult donors (Supplementary Table S1). The first group consisted of adult individuals on testosterone treatment for more than one year and undergoing gender-affirming surgery (trans masculine donors) at the Amsterdam UMC hospital (n = 3 donors). The second group consisted of adult individuals undergoing oophorectomy for fertility preservation purposes before chemotherapy (cis female donors) at the Leiden University Medical Center (n = 3 donors).
Ovarian cortex isolation, cryopreservation, and thawing
The adult ovaries were bisected and immersed in 0.9% w/v NaCl solution (B230551, Fresenius Kabi, Germany). The outer cortex of the ovary, approximately 1 mm thick, was isolated by gently scrapping the medulla and the inner cortex using scalpels (0507, Swann-Morton, UK), and subsequently, the outer cortex of the ovary was cut into strips of about 10 × 5 mm (Anderson and Baird, 2019).
For cryopreservation by slow freezing, one or two ovarian cortex strips were placed per cryovial (123263, Greiner, Germany) containing 1 ml of cryoprotectant solution [0.1 M sucrose (S0389-500G, Sigma-Aldrich, USA) and 1.5 M ethylene glycol (1.00949.1000, Merck, Germany) in phosphate-buffered saline (PBS) (14190-094, Thermo Fisher, UK)]. The tissue was allowed to equilibrate in the cryoprotectant solution for 30 min before initiating the slow freezing process using a Planer Kryosave Compact Controlled Rate Freezer System LNP 4 (ITEM-04781, GDMRV lab, UK). The freezing program used was as follows: a cooling rate of 2°C/min to −9°C, a 5 min soaking period, manual seeding for ice crystal nucleation induction using a cotton swab dipped into liquid nitrogen, a cooling rate of 0.3°C/min to −40°C, a rapid cooling rate of 10°C/min to −140°C, and finally, the cryovials were transferred to liquid nitrogen (−196°C) for long-term storage.
For thawing, the cryovials were submerged in a bead bath (74300-714, Lab Armor, USA) at 37°C until the medium surrounding the ovarian strips thawed (approximately 3–5 min). To remove the cryoprotectant solution, the ovarian strips were washed in a solution of 0.75 M ethylene glycol and 0.25 M sucrose in PBS at room temperature (RT) for 10 min with occasional shaking. This was followed by a 10-min wash in a solution of 0.35 M sucrose in PBS, and finally, a 10-min wash in PBS.
Culture of ovarian cortex
Freshly isolated (n = 3 tOVA donors) and cryopreserved-thawed (n = 3 tOVA donors and n = 3 cOVA donors) ovarian cortex strips were then manually cut into 1 mm3 cubes using scalpels in PBS. Ovarian cortex cubes were cultured in either McLaughlin’s first-step medium (Mc medium) for 8 days (McLaughlin et al., 2018) or in Xu’s first-step medium (Xu medium) for 1 or 3 weeks (Xu et al., 2021). The ovarian cortex cubes (n = 6–8 cubes/well) were cultured in 1 ml medium/well of 24-well plates (92024, TPP, Switzerland) at 37°C in 5% CO2 in air in a humidified incubator. Half of the medium was replaced every other day.
Mc medium used consisted of McCoy’s 5A medium (26600-023, Invitrogen, UK) supplemented with 1 ng/ml recombinant human FSH (rhFSH) (GONAL-F 450U/0.75ML, Merck, USA), 0.1% human serum albumin (HSA) (RVG105901, CSL Behring, UK), 2.5 μg/ml apo-transferrin (T1147, Merck, USA), 4 ng/ml selenium (209651, Merck, USA), 10 ng/ml human insulin (I9278, Merck, USA), 3 mM glutaMAX (35050-038, Thermo Fisher, UK), 20 mM HEPES (15630-049, Thermo Fisher, UK), 0.1% penicillin-streptomycin (15070063, Life Technologies, USA), and 50 μg/ml ascorbic acid (A92902, Merck, USA).
Xu medium used consisted of alpha minimum essential medium (10712124, Thermo Fisher, USA) supplemented with 15 mIU/ml rhFSH, 6% HSA, 5 μg/ml apo-transferrin, 5 ng/ml selenium, 5 μg/ml insulin, 0.1% penicillin-streptomycin and 0.5 mg/ml bovine fetuin (F3004, Merck, USA).
Histochemistry and immunofluorescence
Ovarian tissue cubes (n = 6 donors) were fixed overnight at 4°C in 4% paraformaldehyde (PFA) (1.04005.1000, Merck, Germany). Subsequently, the tissue was washed three times with PBS, transferred to 70% ethanol, and embedded in paraffin using a Shandon Excelsior ES tissue processor (A78410120, Thermo Fisher Scientific, USA). After embedding, the tissue was sectioned into 5 μm slices using a microtome (RM2065, Leica Microsystems, Germany), and the sections were placed onto StarFrost microscope slides (3057-1, Waldemar Knittel, Germany). Sections were deparaffinized and used for hematoxylin and eosin (HE) staining following standard procedures.
For immunofluorescence, deparaffinized sections were subjected to antigen retrieval using Tris-EDTA buffer [10 mM Trizma base (T6066, Merck, Germany), 1 mM EDTA (M101, VWR, USA) solution, pH 9.0] and heated for 12 min at 98°C using a TissueWave 2 Microwave (B35600002, Thermo Fisher Scientific, USA). After cooling down, the sections were washed in PBS (2 × 5 min) and in 0.05% Tween-20 (822184, Merck, Germany) in PBS (PBST) (5 min). To prevent non-specific binding, the sections were first treated with 1% bovine serum albumin (BSA) (10735086001, Merck, Germany) in PBST for 1 h at RT, followed by overnight incubation with primary antibodies at 4°C. The primary antibodies used were goat anti-FOXL2 (1:250, NB100-1277, Bio-Techne, USA), goat anti-DDX4 (1:250, AF2030, R&D, USA), mouse anti-AMH (1:30, MCA2246T, BioRad, USA), rabbit anti-KRT19 (1:150, ab76539, Abcam, UK), rabbit anti-COLIV (1:150, AB748, Merck, Germany), mouse anti-STAR (1:100, sc166821, Santa Cruz, USA), rabbit anti-ACTA2 (1:100, sc-79, Santa Cruz, USA), mouse anti-CD68 (1:100, M087629-2, DAKO, Denmark), and mouse anti-PCNA (1:100, sc-56, Santa Cruz, USA). Subsequently, the slides were washed with PBS (2 × 5 min) and PBST (5 min) and incubated for 1 h at RT with the appropriate secondary antibodies and DAPI (1:1000, D1306, Life Technologies, USA). The secondary antibodies used were Alexa Fluor 488 donkey anti-rabbit IgG (1:500, A21206, Life Technologies, USA), Alexa Fluor 594 donkey anti-mouse IgG (1:500, A21203, Life Technologies, USA), and Alexa Fluor 647 donkey anti-goat IgG (1:500, A-21447, Life Technologies, USA). The antibodies were diluted in 1% BSA in PBST. The TUNEL assay was performed using the In Situ Cell Detection Kit FITC (11684817910, Roche, Germany) following the manufacturer’s instructions. Finally, the slides were rinsed with PBS (2 × 2 min), PBST (2 min), distilled water (2 min), and mounted with Pro-Long Gold (P36930, Life Technologies, USA).
Imaging and quantification
The HE-stained slides were scanned using a Panoramic 250 digital scanner (3DHISTECH Ltd, Budapest, Hungary) and imaged using CaseViewer v2.4.0.119028 software (3DHISTECH Ltd, Budapest, Hungary). To measure the oocyte diameter, we selected healthy follicles with a diameter >30 µm containing a clear boundary between the oocyte and GCs in HE-stained sections. The average between the longest and shortest diameter was used.
Immunofluorescence images were acquired on an SP8 TC inverted confocal microscope (Leica, Germany) with LAS v3.7.4.23463 software (Leica, Germany). For the quantification of follicles, we counted the total number of follicles in eight different HE-stained sections, with a separation of 40 µm between sections to avoid double-counting, per ovarian tissue cube (5–8 ovarian cubes per condition). The number of follicles per cube per donor (n = 6 donors) were summed and the percentage of follicular stages was determined regarding the total number of follicles per donor. We classified follicular stages based on morphology as described previously (Del Valle et al., 2022). Briefly, primordial follicles had a single layer of flat granulosa cells; primary follicles were unilaminar but contained at least some cuboidal granulosa cells; secondary follicles had at least two layers of granulosa cells and no antrum. Atretic follicles showed pyknotic nuclei in the oocyte and granulosa cells, and exhibited oocyte shrinkage with red coloration in HE staining and/or detachment of granulosa cells from the follicular basement membrane. Early atretic follicles were unilaminar and secondary atretic follicles multilaminar.
Statistical analysis
Each experiment was performed in triplicate. Data were analyzed using GraphPad Prism v9.0.1 software (GraphPad Software Inc, USA) with one-way analysis of variance. Bar graphs show mean ± standard deviation and dot plots show mean ± standard deviation. Statistical significance was considered with P-value <0.05 (*), <0.01 (**), and <0.001 (***).
Results
In vitro growth of follicles from freshly isolated trans masculine ovarian cortex (fresh tOVA)
Initially, we isolated ovarian cortex from fresh tOVA (n = 3 donors) and after cutting it into different-sized cubes or strips (Supplementary Fig. S1), we performed the first of three culture steps, to obtain secondary follicles from unilaminar follicles, using the Step1 from McLaughlin et al. (2018) consisting of 8 days (D8) culture in Mc medium. We cultured different-sized ovarian cortex pieces either alone or grouped (6–8× 1 mm3 cubes) in 24-well plates and after quantification of the different follicular stages, we observed no significant differences between the different conditions tested (Supplementary Fig. S1), hence we cultured 6–8× 1 mm3 cubes/well in this work.
Next, we isolated ovarian cortex strips from fresh tOVA (n = 3 donors) and after cutting them into small 1 mm3 cubes (Fig. 1A), we performed again the first of three culture steps using either the Step1 from McLaughlin et al. (2018) consisting of 8 days (D8) culture in Mc medium; or the Step1 from Xu and colleagues (Xu et al., 2021) consisting of 3 weeks (W3) culture in Xu medium. For comparison, we also analyzed follicular development after one week (W1) of culture in Xu medium.
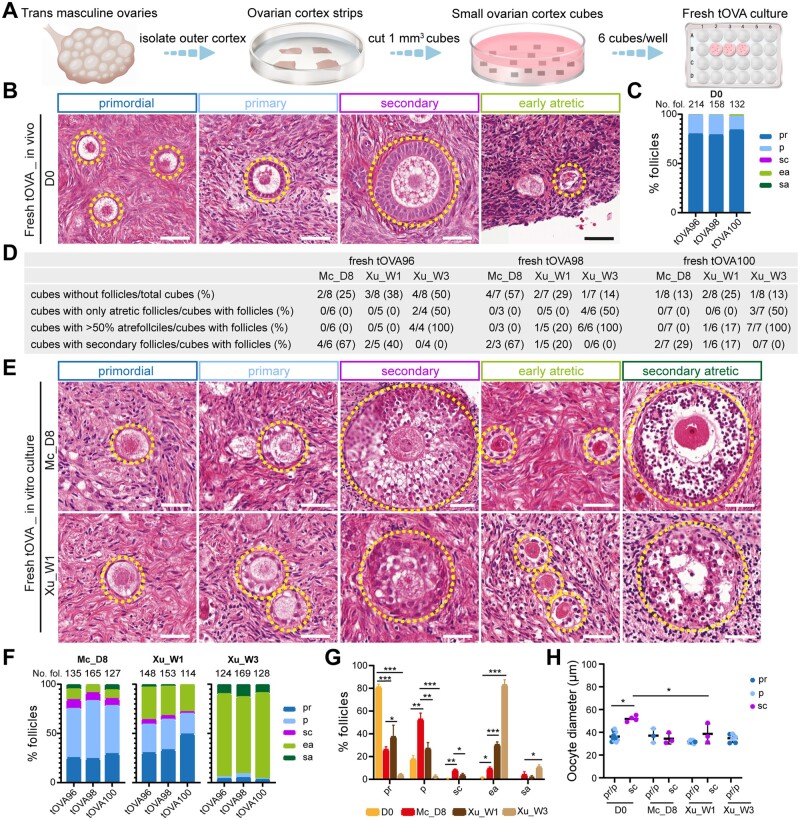
Figure 1.
Follicular distribution in fresh trans masculine ovarian cortex before and after culture. (A) Schematic workflow used to culture freshly isolated trans masculine ovarian cortex (tOVA). (B) Hematoxn and eosin (HE) staining of freshly isolated tOVA prior to culture (day 0, D0) showing different types of follicles (dashed line). Scale bars are 50 µm. (C) Percentage of follicular distribution in freshly -isolated tOVA per donor prior to culture (D0). In the top of the graph are the total number of follicles counted per donor. (D) Characteristics of the tOVA tissue cubes analyzed per donor regarding follicles. (E) HE staining of freshly isolated tOVA after culture in two different media showing different types of follicles (dashed line). Scale bars are 50 µm. (F) Percentage of follicular distribution in freshly isolated tOVA per donor after culture. In the top of the graph are the total number of follicles counted per donor. (G) Percentage of follicular distribution in freshly isolated tOVA per follicular stage before and after culture. The results shown are mean ± standard deviation from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001. (H) Oocyte diameter was measured in pr/p follicles and sc follicles in freshly isolated tOVA before and after culture. The results shown are mean ± standard deviation; *P < 0.05, compared to sc follicles at D0. Mc_D8, culture after McLaughlin et al. (2018) for 8 days; Xu_W1/W3, culture after Xu et al. (2021) for 1/3 weeks; pr, primordial; p, primary; sc, secondary; ea, early atretic; sa, secondary atretic.
After isolation on day 0 (D0), we counted a total of 504 follicles in fresh tOVA ovarian cubes (6–8× 1 mm3 cubes/donor, n = 3 donors), and from those the large majority were unilaminar follicles (average of 81% primordial, 17% primary and 2% atretic follicles) (Fig. 1B and C). At D0, from the 504 follicles, only 5 were secondary follicles (1%). As expected, some ovarian cortex cubes did not contain follicles, however, none of the ovarian cubes with follicles contained only atretic follicles after 7–8 days of culture (Fig. 1D), and each cube with follicles contained between 16 and 64 follicles (Supplementary Table S2). Moreover, after 7–8 days in culture, we observed secondary follicles in fresh tOVA using either Mc medium or Xu medium, however, the granulosa cells in both primary and secondary follicles were less compacted than those observed at D0 (Fig. 1B and E). Interestingly, the distribution of follicular stages in fresh tOVA differed considerably between the two types of culture media (Fig. 1F and G). Using Mc medium, we quantified a total of 427 follicles (7–8× 1 mm3 cubes/donor, n = 3 donors) and observed on average 26% primordial, 53% primary, 8% secondary, 9% early atretic, and 4% secondary atretic follicles, whereas using Xu medium we quantified a total of 415 follicles (7–8× 1 mm3 cubes/donor, n = 3 donors) and observed 37% primordial, 27% primary, 4% secondary, 30% early atretic, and 2% secondary atretic follicles. Completing the W3 culture period of Step1 using Xu medium resulted in the majority of the follicles entering atresia (the total number of follicles quantified was 421, 7–8× 1 mm3 cubes/donor, n = 3 donors), with on average 4% primordial, 2% primary, 0% secondary, 83% early atretic, and 11% secondary atretic follicles in all the ovarian cubes that contained follicles (Fig. 1D, F, and G).
In conclusion, culturing fresh tOVA ovarian cortical tissue for 7–8 days resulted in a significantly increased percentage of secondary follicles compared to D0, with a higher percentage obtained using Mc medium (Fig. 1F and G). However, not only the morphology of the granulosa cells in secondary follicles was abnormal, but the diameter of the oocytes in (non-atretic) secondary follicles after culture remained comparable to that of the oocytes in (non-atretic) unilaminar follicles and were significantly smaller than oocytes in secondary follicles at D0 (Fig. 1H), suggesting perhaps either a delayed or compromised development.
In vitro growth of follicles from cryopreserved-thawed tOVA (cryo tOVA)
Next, we investigated the distribution of follicular stages in the cortical tissue from the same tOVA ovaries (6–8× 1 mm3 cubes/donor, n = 3 donors) after the cryopreservation-thawing procedure (Fig. 2A). We counted a total of 500 follicles at D0 after thawing and observed that the percentage of atretic follicles increased to an average of 21%, on average 69% primordial and 10% primary follicles (Fig. 2B and 2C), and from the 500 follicles only four were secondary follicles (1%), suggesting that the cryopreservation-thawing procedure had a strong impact on the follicular quality of tOVA ovarian cortical tissue prior to culture.
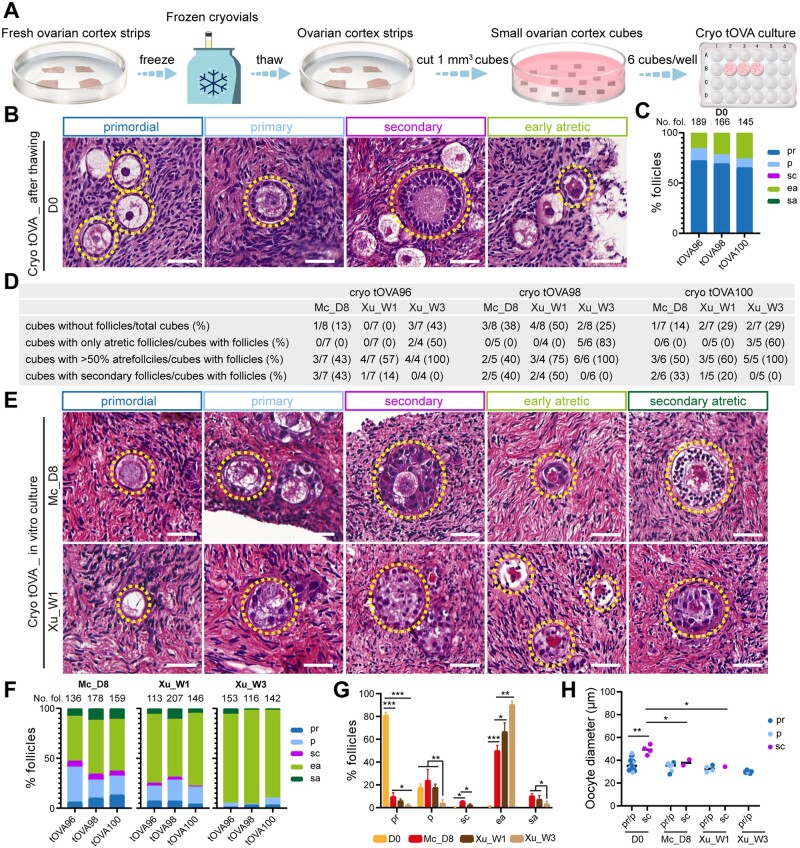
Figure 2.
Follicular distribution in cryopreserved (cryo) trans masculine ovarian cortex before and after culture. (A) Schematic workflow used to culture cryo-thawed trans masculine ovarian cortex (tOVA). (B) Hematoxylin and eosin (HE) staining of cryo-thawed tOVA prior to culture (day 0, D0), showing different types of follicles (dashed line). Scale bars are 50 µm. (C) Percentage of follicular distribution in cryo-thawed tOVA per donor prior to culture (D0). In the top of the graph are the total number of follicles counted per donor. (D) Characteristics of the tOVA tissue cubes analyzed per donor regarding follicles. (E) HE staining of cryo-thawed tOVA after culture, showing different types of follicles (dashed line). Scale bars are 50 µm. (F) Percentage of follicular distribution in cryo-thawed tOVA per donor after culture. In the top of the graph are the total number of follicles counted per donor. (G) Percentage of follicular distribution in cryo-thawed tOVA per follicular stage before and after culture. The results shown are mean ± standard deviation from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001. (H) Oocyte diameter was measured in pr/p follicles and sc follicles in cryo-thawed tOVA before and after culture. The results shown are mean ± standard deviation; *P < 0.05, **P < 0.01, compared to sc follicles at D0. Mc_D8, culture after McLaughlin et al. (2018) for 8 days; Xu_W1/W3, culture after Xu et al. (2021) for 1/3 weeks; pr, primordial; p, primary; sc, secondary; ea, early atretic; sa, secondary atretic.
After 7–8 days in culture, several ovarian cortical cubes containing follicles showed secondary follicles (4 in 14 cubes in tOVA96; 4 in 9 cubes in tOVA98; 3 in 11 cubes in tOVA100) (Fig. 2D), however the secondary follicles observed in the cryo tOVA were not much larger than the primary follicles (Fig. 2E). Although none of the cubes with follicles contained only atretic follicles, the number of ovarian cubes containing more than 50% atretic follicles was high (7 in 14 cubes in tOVA96; 5 in 9 cubes in tOVA98; 6 in 11 cubes in tOVA100) (Fig. 2D) and the majority of follicles present in cryo tOVA were atretic (Fig. 2E, F, and G). Using Mc medium, we quantified 473 follicles (7–8× 1 mm3 cubes/donor, n = 3 donors) and observed on average 10% primordial, 24% primary, 6% secondary, 50% early atretic and 10% secondary atretic follicles, whereas using Xu medium we quantified 466 follicles (7–8× 1 mm3 cubes/donor, n = 3 donors) and observed 6% primordial, 18% primary, 2% secondary, 67% early atretic and 7% secondary atretic follicles. Completing the W3 culture period of Step1 using Xu medium, the majority of the follicles were atretic (the total number of follicles quantified was 411, 7–8× 1 mm3 cubes/donor, n = 3 donors), with on average 4% primordial, 2% primary, 0% secondary, 83% early atretic, and 11% secondary atretic follicles (Fig. 2F and 2G). In agreement with that observed after the culture of fresh tOVA, the culture of cryo tOVA with Mc medium yielded more secondary follicles than with Xu medium (Fig. 2G). Moreover, the diameter of the oocytes in secondary follicles after culture of cryo tOVA was still comparable with those in unilaminar follicles and therefore was significantly smaller than the diameter of the oocytes in secondary follicles at D0 (Fig. 2H).
In vitro growth of follicles from cryopreserved-thawed cis female ovarian cortex (cryo cOVA)
To compare the in vitro growth of follicles between cryo tOVA and cryo cOVA, we analyzed the distribution of follicular stages in cryo cOVA ovaries from cis females (6–8× 1 mm3 cubes/donor, n = 3 donors) after the cryopreservation-thawing procedure (Fig. 3A). We counted a total of 481 follicles at D0, after the cryopreservation-thawing procedure. Interestingly, the percentage of atretic follicles was 6% in cryo cOVA and on average 81% primordial, 13% primary follicles (Fig. 3B and C), much lower than the 21% atretic follicles detected at D0 in cryo tOVA (Fig. 2C). At D0, from the 481 follicles only 4 were secondary follicles (1%). After 7–8 days of culture, none of the ovarian cubes containing follicles showed only atretic follicles (Fig. 3D). There was considerably less atresia in cryo cOVA cultured with Mc medium, than with Xu medium (Fig. 3C and E).
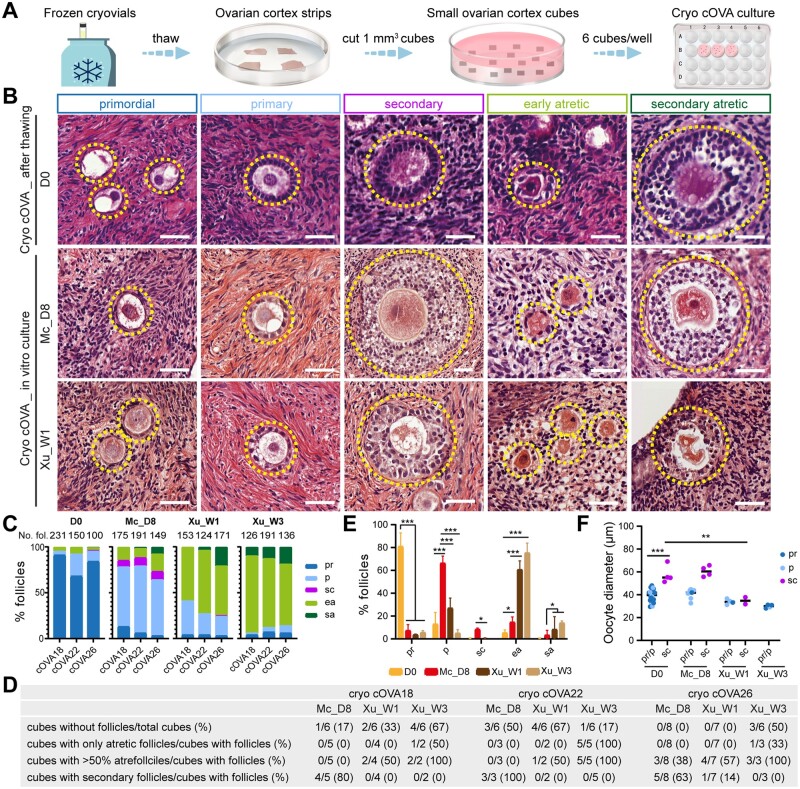
Figure 3.
Follicular distribution in cryopreserved (cryo) cis female ovarian cortex before and after culture. (A) Schematic workflow used to culture cryo-thawed cis female ovarian cortex (cOVA). (B) Hematoxylin and eosin (HE) staining of cryo-thawed cOVA before (D0) and after culture, showing different types of follicles (dashed line). Scale bars are 50 µm. (C) Percentage of follicular distribution in cryo-thawed cOVA per donor before (D0) and after culture. In the top of the graph are the total number of follicles counted per donor. (D) Characteristics of the tissue cubes analyzed per donor regarding follicles. (E) Percentage of follicular distribution in cryo-thawed cOVA per follicular stage before and after culture. The results shown are mean ± standard deviation from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001. (F) Oocyte diameter was measured in pr/p follicles and sc follicles in cryo-thawed cOVA before and after culture. The results shown are mean ± standard deviation; **P < 0.01, ***P < 0.001, compared to sc follicles at D0. Mc_D8, culture after McLaughlin et al. (2018) for 8 days; Xu_W1/W3, culture after Xu et al. (2021) for 1/3 weeks; pr, primordial; p, primary; sc, secondary; ea, early atretic; sa, secondary atretic.
After D8 culture using Mc medium, we quantified 515 follicles (6–8× 1 mm3 cubes/donor, n = 3 donors) and observed on average 7% primordial, 66% primary, 8% secondary, 16% early atretic, and 3% secondary atretic follicles, whereas after W1 using Xu medium, we quantified 488 follicles (6–7× 1 mm3 cubes/donor, n = 3 donors) and there were on average 4% primordial, 27% primary, 1% secondary, 60% early atretic and 8% secondary atretic follicles. After the W3 culture period of Step1 using Xu medium, the large majority of the follicles were atretic (the total number of follicles quantified was 448, 6× 1 mm3 cubes/donor, n = 3 donors), with on average 5% primordial, 0% primary, 0% secondary, 75% early atretic, and 14% secondary atretic follicles (Fig. 3C and E).
Regarding the diameter of the oocytes in secondary follicles after culture of cryo cOVA, we observed that using Mc medium resulted in oocytes with comparable diameter to oocytes in secondary follicles at D0, in contrast to oocytes cultured for W1 using Xu medium that yields oocytes in secondary follicles with diameters comparable to that in unilaminar follicles at D0 (Fig. 3F). Concluding, using Mc medium, we observed the presence of secondary follicles in the majority of the ovarian cubes with follicles (4 in 5 cubes in cOVA18; 3 in 3 cubes in cOVA22; 5 in 8 cubes in cOVA26) (Fig. 3D) and those secondary follicles contained normal-sized oocytes, suggesting that this medium may be suitable to produce high quality secondary follicles after in vitro culture.
Granulosa cells and theca cell development in the secondary follicles after in vitro growth
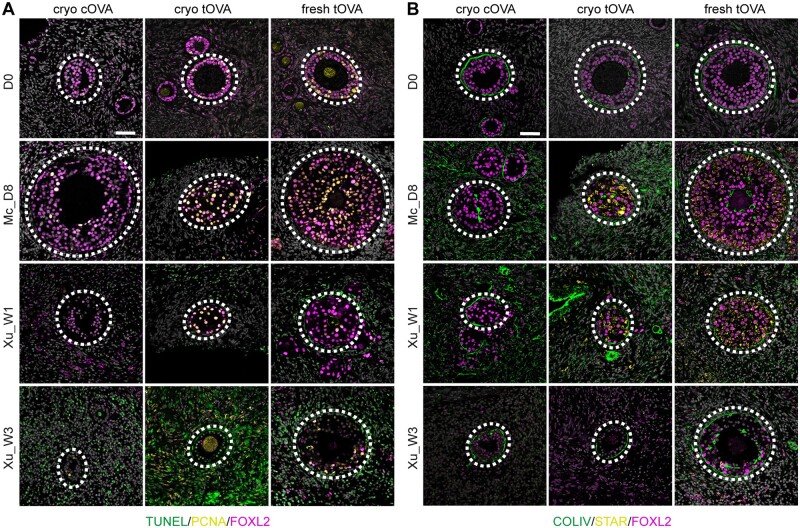
To evaluate further the quality of the secondary follicles obtained after in vitro culture of ovarian cortex, we investigated the expression of two complementary markers of granulosa cells, the secreted factor anti-Müllerian hormone (AMH) that is expressed from primary follicles onwards and the intermediate filament keratin 19 (KRT19) that is exclusively expressed in unilaminar follicles, whereas all oocytes express DDX4 (Loffler et al., 2000; Del Valle et al., 2022). In contrast to granulosa cells in secondary follicles at D0, the granulosa cells in secondary follicles after culture expressed low/no levels of AMH, and granulosa cells in secondary follicles from tOVA in particular continued to express abnormally high levels of KRT19, independently of the culture method used (Fig. 4A and Supplementary Table S3), suggesting developmental arrest.
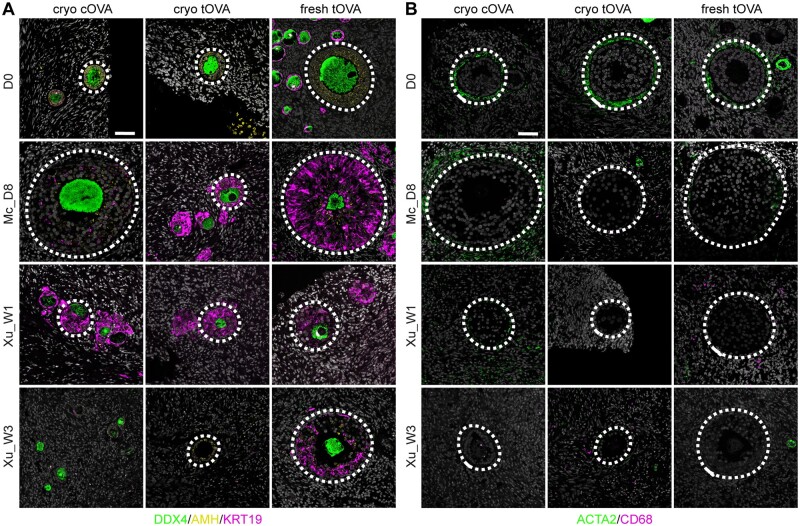
Figure 4.
Characterization of cell types associated with secondary follicles. (A) Immunofluorescence for DDX4 (green), AMH (yellow), and KRT19 (magenta) in secondary follicles present in cryo-thawed cOVA, cryo-thawed tOVA, and freshly isolated tOVA before (D0) and after culture. Dashed line highlights secondary follicles. Scale bar is 50 µm. (B) Immunofluorescence for ACTA2 (green) and CD68 (magenta) in the stromal tissue surrounding secondary follicles present in cryo-thawed cOVA, cryo-thawed tOVA, and freshly-isolated tOVA before (D0) and after culture. Dashed line highlights secondary follicles. Scale bar is 50 µm. Mc_D8, culture after McLaughlin et al. (2018) for 8 days; Xu_W1/W3, culture after Xu et al. (2021) for 1/3 weeks.
We also investigated the presence of another ovarian-specific cell type important for folliculogenesis, the theca cells, using ACTA2 (also known as smooth muscle actin), that marks theca cell progenitors emerging at the periphery of the follicular basement membrane during the transition from primary to secondary follicles (Guahmich et al., 2023). Notably, although theca cells could be observed in secondary follicles of both tOVA and cOVA at D0, after culture the secondary follicles only few tOVA contained ACTA2+ theca cells, whereas those were detected in most secondary follicles of cOVA (Fig. 4B and Supplementary Table S3). CD68 macrophages, that could be involved in atresia, were not present in either tOVA or cOVA after culture (Fig. 4B).
Together, we concluded that the secondary follicles in tOVA (cryo and fresh tOVA) after culture showed abnormal granulosa cells expressing high levels of KRT19 and absence of ACTA2+ theca cell progenitors. This contrasted with secondary follicles in cryo cOVA that showed granulosa cells that downregulated KRT19, although the levels of AMH remained low; and ACTA2+ theca cell progenitors, particularly when using Mc medium.
Ovarian stromal development in the ovarian cortical tissue after in vitro growth
Next, we investigated the levels of late apoptosis using TUNEL to detect DNA fragmentation, confirming high levels of apoptosis in particular in the stromal cells of both cOVA and tOVA using Xu medium, whereas apoptotic stromal cells were less prominent using Mc medium (Fig. 5A and Supplementary Fig. S2 and Table S3). Granulosa cells (FOXL2+) in the secondary follicles seemed proliferative (PCNA+) after culture using both types of media (Fig. 5A), suggesting that tOVA granulosa cells although developmental arrested may show seemingly normal proliferation rates. After culture, we observed collagen type IV (COLIV) deposition in the basement membrane of the secondary follicles comparable to D0, but COLIV was also abnormally expressed throughout the ovarian stromal compartment in both tOVA and cOVA (Fig. 5B).
Figure 5.
Characterization of stromal cells in the vicinity of secondary follicles. (A) Immunofluorescence for PCNA (yellow) and FOXL2 (magenta) together with TUNEL staining (green) in cryo-thawed cOVA, cryo-thawed tOVA, and freshly-isolated tOVA before (D0) and after culture. Dashed line highlights secondary follicles. Scale bar is 50 µm. (B) Immunofluorescence for COLIV (green), STAR (yellow), and FOXL2 (magenta) in cryo-thawed cOVA, cryo-thawed tOVA, and freshly-isolated tOVA before (D0) and after culture. Dashed line highlights secondary follicles. Scale bar is 50 µm. Mc_D8, culture after McLaughlin et al. (2018) for 8 days; Xu_W1/W3, culture after Xu et al. (2021) for 1/3 weeks.
Another striking difference between the granulosa cells in secondary follicles of cOVA and tOVA after culture was the fact that the granulosa cells of cultured tOVA were strongly positive for the steroidogenic acute regulatory protein (STAR), a key factor involved in steroidogenesis. Interestingly, STAR was not expressed in the granulosa cells in secondary follicles of tOVA at D0 (Fig. 5B and Supplementary Table S3). This suggested that long-term exposure to testosterone in tOVA may prime granulosa cells to luteinize (Kiriakidou et al., 1996) using the culture media tested here. Concluding, the culture of cryo cOVA using Mc medium seemed to yield high-quality secondary follicles, although the stromal compartment showed signs of fibrosis. Unexpectedly, the culture of either fresh tOVA or cryo tOVA resulted in abnormal secondary follicles, with granulosa cells expressing high levels of KRT19 and STAR, and the absence of the initial (ACTA2+) theca cell layer. Although the number of donors used was small, our results indicated caution when using tOVA to optimize in vitro growth protocols, as inhibition of premature luteinization may be necessary to promote folliculogenesis in tOVA.
Discussion
It would be highly beneficial to develop fertility preservation strategies that allow for the maturation in the laboratory of functionally mature MII oocytes, starting from the patient ovarian cortex, that could be directly used in medically assisted reproduction (MAR) (ESHRE Guideline Group on Female Fertility Preservation et al., 2020). However, this has proved rather challenging (Del Valle and Chuva de Sousa Lopes, 2023), particularly as the availability of ovarian tissue to optimize culture protocols is very limited. First, we tested the effect of fragmentation (strips versus cubes; 1 cube versus 6–8 cubes) on fresh tOVA ovarian cortex as it has been shown that fragmentation into ovarian cubes promoted follicular growth (Kawamura et al., 2013). In the material used in this study, the degree of fragmentation and group culture influenced neither the percentage of secondary follicles obtained after 1-week culture nor the percentage of atretic versus healthy follicles.
Here, we used two culture media (McLaughlin et al., 2018; Xu et al., 2021) on cryopreserved-thawed ovarian cortex from cOVA and assessed the generation of secondary follicles after approximately one week in culture. In the cOVA tested, the Mc medium performed better than the Xu medium. The observed differences compared to the original protocols (McLaughlin et al., 2018; Xu et al., 2021) could have resulted, for example, from differences in the source of human serum albumin or recombinant follicle-stimulating hormone, but could also have resulted from technical differences or donor heterogeneity. Using the first step of a culture protocol introduced by McLaughlin et al. (2018), we obtained secondary follicles from cryopreserved-thawed cOVA that contained oocytes with the expected diameter, proliferating granulosa cells with an expected molecular signature and emerging ACTA2+ theca cell progenitors surrounding the secondary follicles (Guahmich et al., 2023). Although the stroma of the cryopreserved-thawed ovarian cortex from cOVA showed increased collagen deposition, these results are encouraging to further investigate the next steps leading to the maturation of MII oocytes that could be used clinically.
Surprisingly, there was a striking difference in culture efficiency between the use of cryopreserved-thawed ovarian cortex from cOVA and tOVA, suggesting that optimization of culture conditions using exclusively tOVA needs to be considered, as the results may not reflect outcomes when using cOVA. For example, after culture of tOVA, the diameter of the oocytes in the secondary follicles remained similar to that in unilaminar follicles and the granulosa cells in the secondary follicles showed a mixed molecular signature, expressing markers of the granulosa cells in unilaminar follicles (Del Valle et al., 2022) as well as steroidogenic-marker STAR, upregulated in luteinized granulosa cells (Kiriakidou et al., 1996). Moreover, ACTA2+ theca cell progenitors were few or did not form in cultured tOVA, together suggesting either impaired or delayed folliculogenesis. The number of tOVA donors used in this study was limited, but the results suggested that prolonged testosterone treatment until gender-affirming surgery may impact the ability of tOVA to undergo folliculogenesis using culture medium tested in cOVA donors (McLaughlin et al., 2018; Xu et al., 2021). In agreement, Bailie et al. (2023) have recently also reported that increased exposure to testosterone associated with reduced follicular growth and increased DNA damage in freshly isolated tOVA. The impact of prolonged testosterone treatment on the ovaries is not well understood but may include stromal hyperplasia and a thickened tunica albuginea (Kinnear and Moravek, 2023). Hence, using tOVA ovarian cortex to optimize folliculogenesis in culture may require additional adaptations to the culture medium to allow the maturation of high-quality secondary follicles. Importantly, in case it is proven that in tOVA (from donors undergoing testosterone treatment) not only in vitro matured MII oocytes show low rates of fertilization and embryo development (Lierman et al., 2021; Christodoulaki et al., 2023), but also in vitro growth of unilaminar follicles is compromised, it may be advisable to discuss fertility preservation options either before initiating testosterone treatment or to interrupt testosterone treatment prior to gender-affirming surgery to revert any adverse effects on the tOVA.
Comparing the results obtained from freshly isolated and cryopreserved-thawed tOVA, it is clear that the cryopreservation and thawing procedure resulted in a significant increase of follicular atresia even prior to culture, but it did not impact the generation of secondary follicles after culture. Anderson et al. (2014) showed that the morphology, percentage, follicular diameter, and oocyte diameter of cultured secondary follicles in pre-pubertal and pubertal ovarian cortex differed from those in the adult ovarian cortex. Although they reported similar results between freshly isolated and cryopreserved-thawed pre-puberal and puberal cOVA, they pointed out that current culture systems are not suitable for all sources of ovarian tissue (Anderson et al., 2014), which is also supported by our findings.
Finally, both culture media resulted in a pronounced upregulation of COLIV in the stromal compartment. As the stromal compartment plays an important role in folliculogenesis (Kinnear et al., 2020; Shen et al., 2023), it may be relevant to optimize culture conditions that could prevent upregulation of COLIV, as this may have beneficial effects in the growth of high-quality secondary follicles. Notably, Grosbois et al. (2023) reported an overall decrease in collagen, using Picrosirius red to quantify Collagen I (COLI) and Collagen III (COLIII), in freshly isolated cOVA after Step1 in Mc medium. This discrepancy between results may be the result of the different methods to detect different collagens, the different types of ovarian tissue used (fresh cOVA), the size of the ovarian pieces, or the use of specific medium components from specific brands.
In conclusion, our study provides evidence of follicular growth from cryopreserved-thawed cOVA and tOVA after in vitro culture using two types of culture medium previously tested on freshly isolated cOVA. These findings represent a critical first step towards optimizing in vitro growth and maturation of oocytes from cryopreserved-thawed cOVA and tOVA for potential clinical use.
Supplementary Material
Acknowledgements
We thank the patients who donated tissue for this study, the members of the Chuva de Sousa Lopes group, Dr Xueying FAN in particular, for useful discussions and technical advice and Huan CHENG for drawing the schematic workflows. We would like to dedicate this manuscript to Dr Leoni LOUWE, who contributed significantly to this manuscript but unfortunately passed away before submission.
Contributor Information
Hui Cheng, Department of Anatomy and Embryology, Leiden University Medical Center, Leiden, The Netherlands; The Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW), Leiden University Medical Center, Leiden, The Netherlands.
Fu Wei, Department of Anatomy and Embryology, Leiden University Medical Center, Leiden, The Netherlands; The Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW), Leiden University Medical Center, Leiden, The Netherlands.
Julieta S Del Valle, Department of Anatomy and Embryology, Leiden University Medical Center, Leiden, The Netherlands; The Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW), Leiden University Medical Center, Leiden, The Netherlands.
Tessa H R Stolk, Department of Obstetrics and Gynecology, Amsterdam UMC Location Vrije University Amsterdam, Amsterdam, The Netherlands; Amsterdam UMC, Centre of Expertise on Gender Dysphoria, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
Judith A Huirne, Department of Obstetrics and Gynecology, Amsterdam UMC Location Vrije University Amsterdam, Amsterdam, The Netherlands; Amsterdam UMC, Centre of Expertise on Gender Dysphoria, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
Joyce D Asseler, Department of Obstetrics and Gynecology, Amsterdam UMC Location Vrije University Amsterdam, Amsterdam, The Netherlands; Amsterdam UMC, Centre of Expertise on Gender Dysphoria, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
Gonneke S K Pilgram, Department of Gynecology, Leiden University Medical Center, Leiden, The Netherlands.
Lucette A J Van Der Westerlaken, Department of Gynecology, Leiden University Medical Center, Leiden, The Netherlands.
Norah M Van Mello, Department of Obstetrics and Gynecology, Amsterdam UMC Location Vrije University Amsterdam, Amsterdam, The Netherlands; Amsterdam UMC, Centre of Expertise on Gender Dysphoria, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
Susana M Chuva De Sousa Lopes, Department of Anatomy and Embryology, Leiden University Medical Center, Leiden, The Netherlands; The Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW), Leiden University Medical Center, Leiden, The Netherlands; Ghent-Fertility and Stem Cell Team (G-FAST), Department of Reproductive Medicine, Ghent University Hospital, Ghent, Belgium.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
H.C. and S.M.C.D.S.L. conceived the study. H.C., F.W., J.S.D.V., T.H.R.S., J.A.H., J.D.A., G.S.K.P., L.A.J.V.D.W., N.M.V.M., and S.M.C.D.S.L. contributed to the material collection. J.D.A., L.A.J.V.D.W., N.M.V.M., and S.M.C.D.S.L. arranged the ethical permits. H.C., F.W., and J.S.D.V. generated data. All authors contributed to data analysis. All authors contributed to manuscript writing. All authors approved the submitted version. All authors provided the final approval of the version to be published.
Funding
This research was funded by the European Research Council Consolidator Grant OVOGROWTH (ERC-CoG-2016-725722 to J.S.D.V. and S.M.C.D..S.L.), the Novo Nordisk Foundation (reNEW NNF21CC0073729 to H.C., F.W., J.S.D.V., S.M.C.D.S.L.) and China Scholarship Council (CSC 202008320362 and CSC 202008450034 to H.C. and F.W.), respectively.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Anderson RA, Baird DT.. The development of ovarian tissue cryopreservation in Edinburgh: Translation from a rodent model through validation in a large mammal and then into clinical practice. Acta Obstet Gynecol Scand 2019;98:545–549. [DOI] [PubMed] [Google Scholar]
- Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE.. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod 2014;29:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA.. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 2012;18:73–91. [DOI] [PubMed] [Google Scholar]
- Bailie E, Maidarti M, Jack S, Hawthorn R, Watson N, Telfer E, Anderson RA.. The ovaries of transgender men indicate effects of high dose testosterone on the primordial and early growing follicle pool. Reprod Fertil 2023;4:e220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, Braat DD, Peek R.. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update 2013;19:483–506. [DOI] [PubMed] [Google Scholar]
- Christodoulaki A, He H, Zhou M, Cardona Barberan A, De Roo C, Chuva De Sousa Lopes SM, Baetens M, Menten B, Van Soom A, De Sutter P. et al. Characterization of ovarian tissue oocytes from transgender men reveals poor calcium release and embryo development, which might be overcome by spindle transfer. Hum Reprod 2023;38:1135–1150. [DOI] [PubMed] [Google Scholar]
- Coleman E, Radix AE, Bouman WP, Brown GR, de Vries ALC, Deutsch MB, Ettner R, Fraser L, Goodman M, Green J. et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health 2022;23:S1–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T'Sjoen G, Cornelissen R. et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online 2017;34:557–566. [DOI] [PubMed] [Google Scholar]
- Del Valle JS, Chuva de Sousa Lopes SM.. Bioengineered 3D ovarian models as paramount technology for female health management and reproduction. Bioengineering (Basel) 2023;10:832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle JS, Mancini V, Laverde Garay M, Asseler JD, Fan X, Metzemaekers J, Louwe LA, Pilgram GSK, van der Westerlaken LAJ, van Mello NM. et al. Dynamic in vitro culture of cryopreserved-thawed human ovarian cortical tissue using a microfluidics platform does not improve early folliculogenesis. Front Endocrinol (Lausanne) 2022;13:936765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson RA, Amant F, Braat D, D'Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020;2020:hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraison E, Huberlant S, Labrune E, Cavalieri M, Montagut M, Brugnon F, Courbiere B.. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: a systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum Reprod 2023;38:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris) 2010;71:132–143. [DOI] [PubMed] [Google Scholar]
- Grosbois J, Bailie EC, Kelsey TW, Anderson RA, Telfer EE.. Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture. Hum Reprod 2023;38:444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guahmich NL, Man L, Wang J, Arazi L, Kallinos E, Topper-Kroog A, Grullon G, Zhang K, Stewart J, Schatz-Siemers N. et al. Human theca arises from ovarian stroma and is comprised of three discrete subtypes. Commun Biol 2023;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S. et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear HM, Moravek MB.. Reproductive capacity after gender-affirming testosterone therapy. Hum Reprod 2023;38:1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear HM, Tomaszewski CE, Chang FL, Moravek MB, Xu M, Padmanabhan V, Shikanov A.. The ovarian stroma as a new frontier. Reproduction 2020;160:R25–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidou M, McAllister JM, Sugawara T, Strauss JF 3rd. Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. J Clin Endocrinol Metab 1996;81:4122–4128. [DOI] [PubMed] [Google Scholar]
- Lierman S, Tilleman K, Braeckmans K, Peynshaert K, Weyers S, T’Sjoen G, De Sutter P.. Fertility preservation for trans men: frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J Assist Reprod Genet 2017;34:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lierman S, Tolpe A, De Croo I, De Gheselle S, Defreyne J, Baetens M, Dheedene A, Colman R, Menten B, T'Sjoen G. et al. Low feasibility of in vitro matured oocytes originating from cumulus complexes found during ovarian tissue preparation at the moment of gender confirmation surgery and during testosterone treatment for fertility preservation in transgender men. Fertil Steril 2021;116:1068–1076. [DOI] [PubMed] [Google Scholar]
- Loffler S, Horn LC, Weber W, Spanel-Borowski K.. The transient disappearance of cytokeratin in human fetal and adult ovaries. Anat Embryol (Berl) 2000;201:207–215. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE.. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod 2018;24:135–142. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–1033. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Evidence-based outcomes after oocyte cryopreservation for donor oocyte in vitro fertilization and planned oocyte cryopreservation: a guideline. Fertil Steril 2021;116:36–47. [DOI] [PubMed] [Google Scholar]
- Rosendahl M, Greve T, Andersen CY.. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet 2013;30:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liu J, Luo A, Wang S.. The stromal microenvironment and ovarian aging: mechanisms and therapeutic opportunities. J Ovarian Res 2023;16:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei FU, Fan X, Del Valle JS, Asseler JD, Van Der Meeren LE, Cheng HUI, Roelen BAJ, Louwe LA, Pilgram GSK, Van Der Westerlaken LAJ. et al. Classification of atretic small antral follicles in the human ovary. IJMS 2023;24:16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Lawson MS, Bean Y, Ting AY, Pejovic T, De Geest K, Moffitt M, Mitalipov SM, Xu J.. Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Hum Reprod 2021;36:1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yding Andersen C, Mamsen LS, Kristensen SG.. Fertility preservation: freezing of ovarian tissue and clinical opportunities. Reproduction 2019;158:F27–F34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.