Abstract
Background
In the present study, early response assessment by o-(2-[18F]fluoroethyl)-l-tyrosine (FET) positron emission tomography (PET) and contrast-enhanced magnetic resonance imaging (MRI) were investigated in a phase II open-label single-center study of nivolumab plus bevacizumab for recurrent high-grade astrocytic glioma.
Methods
Twenty patients with nonresectable first recurrence of high-grade astrocytic glioma after EORTC/NCIC protocol underwent [18F]FET PET/MRI at baseline and after 2 cycles of treatment. Whole brain values of contrast-enhancing volume on MRI (CEV), of the mean (TBRmean) and maximal tumor-to-background ratio (TBRmax), and of metabolically active volume (MTV) on [18F]FET PET were obtained. Regional changes in [18F]FET uptake were assessed by parametric response mapping (PRM). Prediction of overall survival (OS) and response (OS > 11 months) were assessed by Cox and receiver operating characteristic (ROC) analysis, respectively. Also, MRI (response assessment in neuro-oncology [RANO] 2.0) and PET-based (PET RANO 1.0) response assessment criteria were compared.
Results
In ROC analysis responders were separated (P < .05) from nonresponders by lower MTV at follow-up (AUC 0.771, cutoff 18.3 mL), larger decrease in MTV (AUC 0.757, cutoff −5.3 mL), larger decrease in both TBRmax (AUC 0.814, cutoff −0.53) and relative TBRmax (AUC 0.829, cutoff −11%) and smaller PRM progressive volume (AUC 0.843, cutoff 4.0 mL). Change in CEV did not predict response. RANO 2.0 and PET RANO response assessment criteria had similar and only borderline prognostic values.
Conclusions
The study indicates that [18F]FET PET is superior to contrast-enhanced MRI for early response assessment in patients with recurrent high-grade astrocytic glioma treated with nivolumab and bevacizumab.
Keywords: antiangiogenic treatment, high-grade astrocytic glioma, immunotherapy, positron emission tomography, response assessment
Key Points.
We investigated the response assessment of bevacizumab + nivolumab in recurrent high-grade glioma.
[18F]FET PET was superior to contrast-enhanced MRI for prediction of survival.
MRI RANO 2.0 and PET RANO criteria have only borderline predictive value.
Importance of the Study.
Although prognosis in recurrent glioblastoma remains poor and studies of second-line therapies have failed to demonstrate overall survival benefits, some patients may show long-term response. The present study compared early response assessment using contrast-enhanced MRI and amino acid PET using [18F]FET for prediction of response (defined as overall survival >11 months) in patients with recurrent high-grade astrocytic glioma treated with a nivolumab and bevacizumab. The study showed that only [18F]FET PET parameters predicted response, while contrast-enhanced MRI did not. Recent MRI and PET response assessment (RANO) criteria had similar and only borderline prognostic value, and neither were predictive of response overall. The study adds to the evidence supporting the advantage of amino acid PET over contrast-enhanced MRI for response assessment in brain tumors treated with blood-brain-barrier modifying treatments.
Glioblastoma (GBM) WHO grade 4 is the most common primary brain tumor accounting for 63% of all glial tumors in Europe.1 The prognosis remains poor with a median survival of 14.6 months despite standard treatment consisting of maximal safe resection, radiotherapy and concomitant and adjuvant temozolomide (TMZ), often referred to as EORTC/NCIC protocol (or Stupp’s regime).2 All patients will eventually experience recurrence during or after standard treatment with a median survival at recurrence of 6–8 months.3,4
Antiangiogenic treatment with vascular endothelial growth factor (VEGF) antibodies targeting neoangiogenesis, such as bevacizumab, is approved and widely used for various cancers. In recurrent GBM, bevacizumab has been shown to prolong progression-free survival (PFS) but not to improve overall survival (OS).5 Therefore bevacizumab’s role in the management of recurrent GBM remains controversial and only a few countries approve of this treatment for recurrent GBM.6
Immunotherapy with check-point inhibitors aims to disrupt the check-point pathway which suppresses the antitumor response of the immune system. The use of check-point inhibitors has proven effective for example, in malignant melanoma and non-small lung cancers7 but has failed to show survival benefits for GBM.8–10 Preclinical and clinical data indicate that direct VEGF signaling may contribute to the recruitment, trafficking, and activation of the CD8 + T-cell response and reduce tumor-associated immunodeficiency11 and a single arm clinical trial showed that a tumor vaccine combined with bevacizumab was associated with a high rate of T-cell specific immune response.12 This serves as a rationale for combining a PD-1 inhibitor, nivolumab, with a VEGF inhibitor, bevacizumab, for recurrent GBM.
Although the results of these clinical trials are discouraging, some patients become long-term survivors and may benefit from immunotherapy. Given the paucity of treatments for recurrent GBM, there is an urgent need for biomarkers that may identify patients more likely to respond. Conventional magnetic resonance imaging (MRI) has low specificity for the detection of progressive disease after standard therapy13,14 due to post-treatment related effects. Activation of the immune response may additionally induce pseudo-progression with increasing contrast enhancement on MRI difficult to separate from true progression. In contrast, patients receiving antiangiogenic treatment may show pseudo-response with resolution of enhancement due to normalization of tumor vessel permeability despite progressive disease. Conventional anatomical post-contrast MRI may thus not be helpful for early response assessment of either treatment. Various functional imaging modalities have been introduced to supplement conventional MRI for response assessment in the post-treatment setting.15,16 The uptake of amino acid positron emission tomography (PET) tracers is generally believed to depend on l-amino acid transporters expressed on glioma cells independent of perfusion and permeability and more closely related to viable tumor tissue.17 Amino acid PET tracer such as [11C]-methyl-l-methionine (MET) or o-(2-[18F]fluoroethyl)-l-tyrosine (FET) have been reported to be of value for response assessment in patients treated with bevacizumab with retained ability to identify viable tumor tissue also in a pseudo-response setting18,19 and have been suggested to differentiate true progression from pseudo-progression in patients with cerebral metastases receiving nivolumab.20 Studies of response assessment in nivolumab for recurrent GBM are few, retrospective, and have not included amino acid PET.21–23
Response assessment with amino acid PET is usually based on changes in standard imaging metrics, that is, the metabolically active tumor volume (MTV) and tumor-to-background uptake ratio (TBR).24 However, these global metrics evaluated in isolation may have limitations in estimating tumor response in patients with mixed response or mixed pathology. Parametric response maps (PRM) may allow assessment of regional changes and have been investigated for MRI-based technique, that is, diffusion-weighted imaging or perfusion imaging, but experience with PRM for analysis of amino-acid PET in brain tumors is sparse.25,26
Recently, MRI criteria for response assessment in neuro-oncology (RANO 2.0) have been updated, and similar criteria for amino acid PET (PET RANO 1.0) have been introduced and are in the early phase of validation.27
The present study analyses [18F]FET PET and MRI imaging data obtained at baseline and after 8 weeks/2 cycles of combined nivolumab and bevacizumab treatment in patients with first recurrence of high-grade astrocytic glioma. The analysis aimed to identify [18F]FET PET imaging metrics that may allow for the separation of patients who may benefit from treatment in terms of survival benefit (responders) from those who do not (nonresponders), and further assess the potential of PRM analysis compared to standard PET metrics.
Methods
Study Design
Imaging data was obtained in the treatment arm (n = 20) of CA209-9UP, a phase II open-label single-center translational study of nivolumab in combination with bevacizumab for recurrent GBM (Eudra CT no. 2017-003925-13). Patients were included from October 2018 to 2020. Eligible patients all had a nonresectable first recurrence of GBM (WHO 2016 classification) during or after treatment including maximal safe surgery (n = 18), radiotherapy (photon n = 19, proton n = 1), and concomitant and adjuvant temozolomide (n = 20). Measurable disease according to RANO guidelines criteria within 2 weeks of treatment was required. Two patients (#25 and #29) did not fulfill RANO criteria for contrast-enhancing measurable lesions but were included in the clinical trial on the basis of metabolic active tumor on [18F]FET PET. Additional inclusion criteria were performance status (PS) of 0–2 and age >18 years. Full in- and exclusion criteria can be found in Supplementary Table S1. Neoadjuvant nivolumab (240 mg) and bevacizumab (10 mg/kg) was administered every 2 weeks, or until unacceptable toxicity, progression, or death. The trial (EudraCT 2017-003925-13) was approved The Capital Region of Denmark Committee on Health Research Ethics (ref. H-17040888) and written consents were obtained with the possibility to withdraw consent at any time. Rationale and primary clinical and immunological results of the study have been reported previously.28
Pathology
All patients had histology verified glioblastoma WHO IV, defined by the 2016 WHO classification which was used at the time of the study. Five patients with IDH mutation would no longer be reclassified as glioblastoma according to the current 2021 classification, but as astrocytoma WHO 4, IDH mutant and, thus, collectively referred to as high grade astrocytic glioma. In the exploratory analysis, we could not demonstrate any influence of IDH mutation status and have not stratified the analysis according to IDH status.
Image Acquisition
Imaging was intended to be performed on a Siemens Biograph mMR 3T hybrid PET/MRI system equipped with a 16-channel head-neck coil (Siemens). However, in 3 patients (ID #1. #25 and #29) very recent [18F]FET PET had been performed on a PET/CT system (Biograph mCT, Siemens, Erlangen, Germany) and used as a baseline along with same-day or most recent MRI acquired within 2 weeks in order not to delay initiation of treatment. Scan and reconstruction parameters were standardized across scanners. PET imaging was performed according to recent guidelines.24
The hybrid PET/MRI protocol included a single-bed 20-minute simultaneous PET/MRI acquisition performed 20 min after intravenous injection of approximately 200 MBq [18F]FET. The MRI protocols included a minimum axial T2 (0.7 × 0.7 × 5 mm3), T2 FLAIR (1.2 × 0.9 × 5 mm3), and post-contrast 3D-T1 (1 × 1 × 1 mm3) weighted sequences.
The PET images were reconstructed into a 344 × 344 matrix (voxel-size 0.8 × 0.8 × 2 mm3) using 3D OP-OSEM (4 iterations, 21 subsets) and applying a 5 mm Gaussian filter. The spatial resolution of the system is approximately 5 mm.29 Attenuation correction was performed either using a separately obtained low-dose CT (120 kV, 30 mAs, 5 mm slice width, Siemens Biograph PET/CT system) as previously described30 or MRI based attenuation correction from a region-specific optimization of a UTE sequence (RESOLUTE).31
Image Analysis
For lesion contouring, PET and structural MRI were registered, displayed and analyzed using Mirada RTx software (version 1.8, Mirada Medical Ltd.). To ensure consistency, image analysis was for the purpose of the study performed by a single author (OH).
Conventional MRI was read as a part of the clinical imaging report by an experienced neuroradiologist unless reporting was not deemed relevant due to a very recent diagnostic MRI. Guided by the MR report, the contrast-enhancing volume (CEV) was delineated by isocontouring and adjusted manually. Qualitative assessment (decrease/stable/increase) of nonenhancing T2/T2-FLAIR hyperintensities at follow-up as stated in the MRI report was noted.
The metabolically active [18F]FET volume (MTV) was defined according to guidelines as tissue with [18F]FET uptake (tumor-to-background ratio, TBR) exceeding 1.6 of the mean activity of a background region drawn in normal appearing cortex of the contralateral hemisphere.32
For each parameter absolute and relative (%) changes from baseline were calculated.
Parametric Response Maps
The rationale behind parametric response maps (PRM) is to isolate metabolic changes that clearly exceed noise level. Using in-house developed software each [18F]FET PET image was rigidly registered to each other and the mean PET image was registered to T1 weighted post contrast MRI. The PET images were voxel-wise subtracted (follow-up minus baseline) after normalization to mean value of an identical background region similar to manual analysis described above. From a separate set of test-retest [18F]FET PET images33 the normal distribution of the voxel-wise variability in tracer uptake (TBR) in gray and white matter in the normal-appearing hemisphere contralateral to the tumor was estimated and the 95% confidence interval calculated. Values within this range corresponding to +/− 0.2 TBR values were excluded from the PRM, and values outside this range were interpreted as tumor growth or shrinkage.34 Only voxels within the union of MTV at baseline and at follow-up were considered in the PRM analysis. As exploratory PRM metrics of treatment response the volumes measured in absolute values (mL) and relative to the union of MTV (%) with either an increase in uptake (PRMplus and rPRMplus) or a decrease (PRMminus and rPRMminus), were determined. Furthermore, since PRM is based on image subtraction, we also calculated the net absolute or relative change, i.e. PRMplus—-PRMminus (PRMnet and rPRMnet) to partly off-set changes in opposite directions in the event of larger shifts in tumor activity caused by for example, differences in the contribution of edema or resection cavity collapse. Example of PRM analysis is shown in Figure 1.
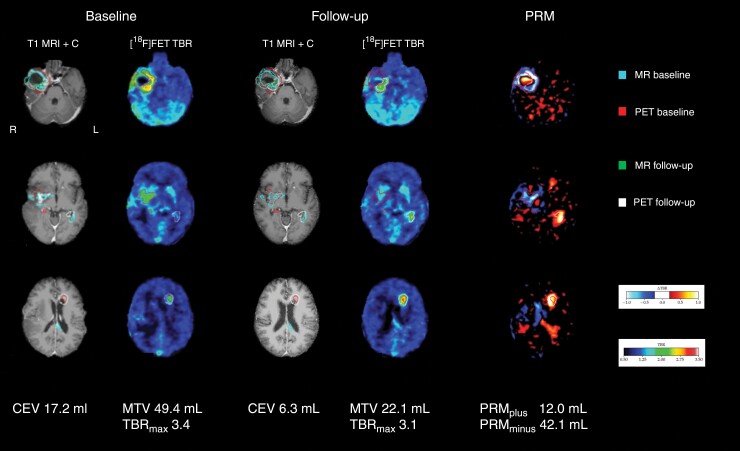
Figure 1.
Example of standard and PRM analysis. Contrast enhanced MRI (T1 MRI + C) show overall decreasing enhancing volume (−63%), and metabolically active volume of the large tumor in the right temporal lobe in patient ID #36, but with increasing activity in the satellite tumors in the left hemisphere. Response according to MRI RANO is stable disease, but progressive according to PET RANO. Areas of increasing and decreasing uptake is clearly visible on parametric response mapping (PRM) image. Also note apparent increase on PRM image in the partially collapsed cavity in the right temporal component. Images are shown in MNI space. Tumor volume contours are shown overlaid.
Standard MRI and PET Response Assessment
Standard MRI response assessment was adapted from the recently updated RANO 2.0 criteria.35 Based on perpendicular measurements of contrast-enhancing lesions (minimum > 10 × 10 minutes), partial response was defined as a decrease in the sum of products of >50%, progression as >25% increase in the sum of products or new measurable lesion (>10 × 10 mm), or as stable disease. RANO response was based on measurements as stated in the MRI reports and subsequently corroborated by an experienced neuroradiologist (VAL). An initial MRI progression at first follow-up that subsequently resolved was considered MRI pseudo-progression, while an initial regression with discordant clinical deterioration or progression assessed by [18F]FET PET with subsequent clinico-radiographical progression leading to exit of protocol was considered MRI pseudo-response.
For [18F]FET PET, the newly presented PET based response assessment criteria36 were applied. According to these, response/progressive disease is defined as decrease/increase in any target lesion (MTV > 0.5 ml) of >30% in TBRmax, > 10% in TBRmean or > 40% in MTV. New measurable lesions (MTV > 0.5 mL) are also considered progression, while complete response is defined as disappearance of all measurable disease. Both MRI RANO and PET RANO response were converted to an ordinal response score: complete or partial response (−1), stable disease (0) and progression (+1) for Cox and receiver operating characteristic (ROC) analyses.
As some patients experienced clinical progression before scheduled follow-up imaging and did not undergo the second PET scan, clinical criteria of progression were not included in the analysis and only patients with complete data were included in comparisons of MRI RANO and PET RANO.
Definition of Treatment Response
A beneficial response to treatment (responder) was defined as overall survival (OS) longer than the median survival in a historical control group treated with bevacizumab and irinotecan upfront for recurrent nonresectable GBM. Controls were identified from an on-site database.3 Controls were matched to the study cohort using propensity scores accounting for sex, age, steroid use, MGMT status, and multifocal disease in order to account for patient demographics. Adjusted predicted median OS was 11.0 months (n = 60) which was used to define response in the present study. Actual survival data showed 2 clearly separated patient groups with OS < 7.3 months and OS > 14.1 months, respectively, and a single patient (#1) with OS of 10.6 months, that is, just below the prespecified cutoff (Supplementary Table S2).
Statistics
For continuous parameters, the median value [range] is reported and group differences are tested using the Mann–Whitney test or Wilcoxon signed rank test for difference between follow-up and baseline. Categorical variables were analyzed using Fischer’s exact test.
ROC analysis with determination of area under the curve (AUC) was applied to identify imaging metrics predictive of response. Optimal cutoffs for the prediction of nonresponder were determined by maximization of Youden’s index. The Equality of ROC AUC was assessed using the DeLong test. Associations of clinical variables and imaging metrics with OS were investigated in univariate Cox proportional hazard models, and metrics identified by ROC analysis were further combined pairwise in bivariate Cox models to assess if the effects were independent. Survival functions according to response criteria were analyzed by Kaplan–Meier survival curves and compared by Log-rank test. All statistical analysis was performed in STATA 15 (Stata Corp). A two-tailed significance level of .05 was applied.
Results
Baseline [18F]FET PET was obtained on hybrid PET/MRI in 17 patients and on separate MRI and PET/CT systems in 3 patients. Due to clinical progression before scheduled follow-up PET/MRI, 2 patients had only MRI performed prior to completing the second series of treatment, and one patient did not have any follow-up imaging. Information on single subjects and scan results along with group summary statistics are provided in Supplementary Table S2.
None of the clinical risk factors or baseline metrics were different between responders and nonresponders, and did not predict response in ROC analysis or overall survival (OS) in univariate Cox analysis (for detailed results see Supplementary Table S3).
Treatment was associated with an overall decrease in median CEV both in responders (−84%) and nonresponders (−47%), although CEV at follow-up was not statistically significantly different from baseline in either group. Statistically significant decreases in median MTV (−74%), TBRmean (−9%), and TBRmax (−30%), were found in responders, but no significant changes were observed in nonresponders (Supplementary Table S2).
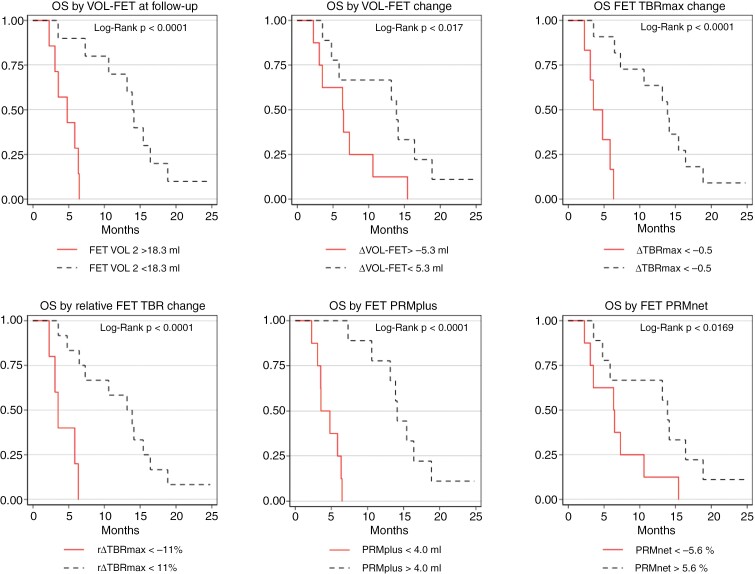
ROC analysis identified MTV at follow-up, MTV change, PRMplus, PRMnet, and both absolute and relative change in TBRmax as predictors of response. ROC AUC and optimal cutoff are shown in Table 1 and ROC curves in Supplementary Figure S1. The metabolically progressive volume (PRMplus) yielded the highest ROC AUC of 0.843 providing sensitivity of 80% and specificity of 100% at the optimal cutoff of 4 mL, although ROC AUC was not significantly different between metrics. Combining any of the metrics did not statistically significantly increase ROC AUC.
Table 1.
ROC Analysis for Prediction of Response (OS > 11 Months)
| ROC AUC [asymptotic 95% CI] |
P-value | Optimal cutoff | Sensitivity (%) | Specificity (%) | Overall survival HR [95% CI] |
P-value | |
|---|---|---|---|---|---|---|---|
| MTV follow-up (mL) | 0.771 [0.511–1.000] | (.041) | 18.3 | 70 | 100 | 1.031 [1.011–1.052] | (.002) |
| ΔMTV (mL) | 0.757 [0.515–0.999] | (.037) | −5.3 | 70 | 86 | 1.023 [1.005–1.041] | (.006) |
| ΔTBRmax (a.u.) | 0.814 [0.595–1.000] | (.005) | −0.53 | 70 | 100 | 2.807 [1.173–4.714] | (.020) |
| rΔTBRmax (%) | 0.829 [0.613–1.000] | (.003) | −11 | 70 | 100 | 1.034 [1.008–1.062] | (.012) |
| PRMplus (mL) | 0.843 [0.634–1.000] | (.001) | 4.0 | 80 | 100 | 1.040 [1.013–1.068] | (.003) |
| PRMnet (mL) | 0.757 [0.512–1.000] | (.040) | −5.6 | 70 | 86 | 1.019 [1.000–1.035] | (.019) |
Abbreviations: MTV, [18F]FET metabolically active volume; TBRmax, maximal [18F]FET tumor to background ratio; PRM, parametric response mapping; RANO, response assessment in neuro-oncology; ROC, receiver operating characteristics; AUC, area under curve. Δ and rΔ denote the absolute and relative change from the baseline.
Univariate hazard ratios (HR) for OS of the metrics identified by ROC analysis are also shown in Table 1. In bi-variate models, only PRMplus and MTV at follow-up had independent prognostic effects (except when combined). In univariate Cox analysis, shorter OS was also associated with increasing contrast-enhancing volume at follow-up (HR 1.073, P = .002), larger relative PRMplus (HR 1.026, P = .014), and larger relative PRMnet (HR 1.010, P = .029), but none of these predicted response at the .05 significance level in ROC analysis (Supplementary Table S3).
Table 2 shows the median OS when stratifying patients according to response criteria compared to RANO criteria and clinical risk factors. Corresponding Kaplan–Meier curves for OS according to cutoff are shown in Figure 2.
Table 2.
Median Survival According to Response Criteria and Clinical Risk Factors
| Threshold | Criterion present OS (months) |
Criterion not present OS (months) |
P-value Mann–Whitney |
|
|---|---|---|---|---|
| Optimal metrics | ||||
| MTV at follow-up (mL) | <18.3 | 14.0 | 4.2 | <.001 |
| ΔMTV (mL) | <−5.3 | 13.9 | 4.9 | .025 |
| ΔTBRmax (a.u.) | <−0.5 | 13.9 | 3.5 | <.001 |
| rΔTBRmax (%) | <−11 | 13.5 | 3.3 | .001 |
| PRMplus (mL) | <4 | 14.1 | 3.6 | <.001 |
| PRMnet (mL) | −5.6 | 13.9 | 4.9 | .025 |
| RANO response | ||||
| PET RANO a | PR or SD (vs PD) | 12.1 | 6.3 | .056 |
| PR (vs SD or PD) | 13.3 | 7.1 | .054 | |
| MRI RANO a | PR or SD (vs PD) | 11.4 | 6.6 | .140 |
| PR (vs SD or PD) | 12.9 | 8.0 | .118 | |
| Clinical risk factors | ||||
| IDH | Mutant | 11.2 | 8.2 | .407 |
| MGMT | Methylated | 10.0 | 7.7 | .569 |
| Steroid use | No | 9.7 | 6.6 | .513 |
| Multifocal, n (%) | No | 10.2 | 6.0 | .248 |
| PS 1, n (%) | Yes | 9.3 | 5.4 | .614 |
| Age | <55 years | 8.1 | 10.9 | .367 |
Abbreviations: IDH, isocitrate dehydrogenase; MGMT, methylguanine-DNA-methyltransferase; MTV [18F]FET, metabolically active volume; TBRmax, maximal [18F]FET tumor to background ratio; PRM, parametric response mapping; RANO, response assessment in neuro-oncology; OS, overall survival. Δ and rΔ denote the absolute and relative change from baseline, respectively; PR, partial (or complete) response; SD, stable disease; PD, progressive disease.
a n = 17 (for details see also Supplementary Table S3).
Figure 2.
Kaplan–Meier plots overall survival. Patients are grouped according to response criteria determined by ROC analysis.
No cases of MRI pseudo-progression at the first follow-up were noted. Using MRI-RANO criteria 2 cases could be considered as MRI pseudo-response of which one (#34) was correctly classified as PD according to PET-RANO and the other (#15) did not undergo follow-up PET imaging.
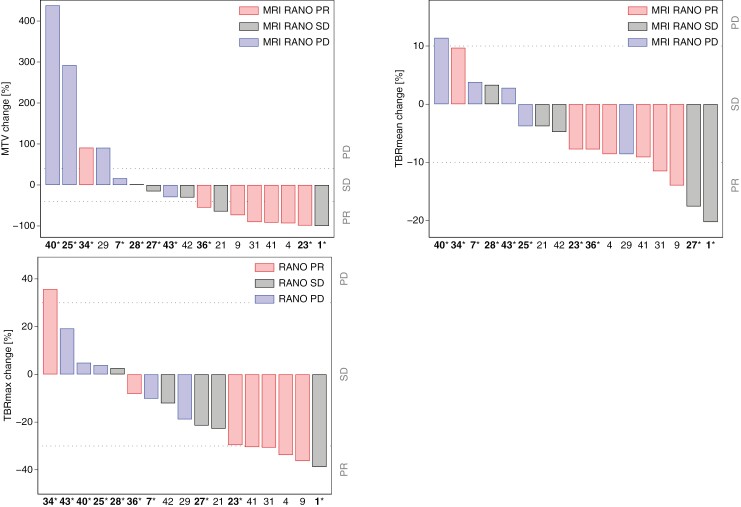
Of the 17 patients with PET also at follow-up PET RANO and MRI RANO classification were in agreement in 13 of the 17 patients (6 with response, 3 with stable disease, and 4 with progression), while 2 patients (both nonresponders) with progression according to PET RANO were classified as response on MRI, and the 2 patients (one responder and one nonresponder) with response on PET RANO was classified as stable (n = 2) disease on MRI. Waterfall plots showing PET RANO response metrics according to MRI RANO response and outcome are provided in Figure 3.
Figure 3.
Waterfall plots of [18F]FET response metrics. Each bar shows percent change in MTV (A), in TBRmean (B), and TBRmax (C) of individual patients with [18F]FET also at follow-up. Dotted lines show PET RANO upper and lower limits for stable disease (SD). Bars are color coded according to MRI RANO response. Subject ID is shown below with font color indicating responder (OS > 11 months) or nonresponder (OS < 11 months, marked with asterisk). Patients #25 and #29 did not fulfill RANO criteria for contrast-enhancing measurable lesions at baseline. PR, partial response; PD, progressive disease; OS, overall survival.
Distribution of neither MRI RANO nor PET RANO differed between responders and nonresponders (Fischer’s exact test: P = .604 and .178, respectively) and ordinal scores did not predict response in ROC analysis (ROC AUC 0.671, P = .188 vs 0.693, P = .143). PET RANO classification showed borderline significant median OS in patients with progression vs nonprogression (P = .056) and response versus nonresponse (P = .054), see also Table 2. Omitting the 2 patients with nonmeasurable disease according to MRI RANO improved performance of both MRI RANO and PET RANO with also MRI progression predictive of nonresponse (ROC AUC 0.667, P = .046; see Supplementary Table S4 for details).
T2/T2-FLAIR increase was noted in 2 nonresponders (#15 and #27) with nonprogression on contrast enhanced MRI of which one showed partial response on PET and one had clinical progression. In contrast, the 2 patients with pseudoresponse defined by PET progression (#34 and #36) both showed decrease in T2/T2-FLAIR. No association of PET RANO progression and qualitative T2/T2-FLAIR increase was observed (P = .280, Fischer’s exact).
Discussion
In the present study we investigated the value of [18F]FET PET for early response assessment in patients with recurrent high grade astrocytic glioma treated with a check-point inhibitor, nivolumab, in combination with a VEGF inhibitor, bevacizumab. The main findings are that only [18F] FET-derived metrics at follow-up were predictive of response to treatment, whereas baseline clinical characteristics and PET and MRI imaging metrics including contrast enhancing volume were not. We further applied PRM analysis and identified the metabolic progressive volume as a predictor of nonresponse to treatment. Recommended PET and MRI response assessment criteria were only borderline predictive of survival and not of response overall.
To the best of our knowledge, this is the first study to apply amino acid PET for response assessment for checkpoint inhibitors for glioma, here in combination with bevacizumab. In a retrospective analysis of patients with brain metastases, it was reported that a 10% decrease in TBRmean predicted long-term response to immunotherapies with either a check point inhibitor or targeted therapies, often in combination with radiotherapy.20 Previous studies of response assessment of bevacizumab treatment using amino acid PET in recurrent high-grade glioma have recently been reviewed.19 Only a few prospective studies with both pre- and post-treatment amino acid PET have been published. One study included 21 patients with recurrent IDH wildtype GBM treated with bevacizumab and lomustine.37 The authors found that an MTV < 5 mL at follow-up (8 weeks) was the best predictor of treatment response (defined as OS > 9 months). Two studies using the same patient group (n = 20–24), applied [11C]MET for recurrent GBM treated with bevacizumab + temozolomide showed that patients with concordant response on MRI and PET (tumor to normal tissue [T/N] ratio < 1.6) at 8 weeks had longer progression-free survival (PFS) than those with only MRI response (considered pseudo-response).38 and that the T/N ratio at 8 weeks and the change in T/N ratio at 4 weeks were the best predictors of PFS.39 In a study of 30 patients with recurrent high grade glioma with 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (DOPA) PET performed at baseline and at 2, 4 and 6 weeks after initiation of bevacizumab,40 an MTV < 18 mL at 2 weeks was the best predictor of survival associated with a 3.5 time longer OS. In summary, although these previous studies differ in terms of imaging time points, metrics and tracer, they overall show that early metabolic response was associated with a longer survival and usually superior or at least additive to MRI response assessment, and that the metabolic tumor burden at follow-up may be more predictive than both baseline metrics and changes from baseline to follow-up. Our findings are overall in agreement with these previous studies.
The 2 patient populations were clearly separated by median OS of 15.4 months and 4.8 months for responders and nonresponders, respectively, (Supplementary Table S2). Our 11 months cutoff for separation into the 2 classes were based on a historical control group treated with bevacizumab and irinotecan for recurrent nonresectable GBM. However, as treatment effects may not be comparable there can be some uncertainties in the cutoff, and a lower value, for example, 9 months, as used in other studies,27,37 would have reclassified patient ID #1 with OS 10.6 months as responder with an overall improvement in predictive performance. In this patient [18F]FET PET showed the disappearance of a relatively small lesion of MTV = 2.8 mL and, thus, complete metabolic response according to PET RANO. Another outlier was patient ID #29 classified as a responder with OS of 15.4 months that showed PET and MR RANO progressive disease with a 91 % increase in MTV from 1.1 to 2.1 mL, but with a paradoxical 19 % decrease in TBRmax from 2.8 to 2.3. This patient showed steady PD despite reinduction with TMZ after 4 cycles underlining the limitations of using a fixed OS-defined response criterium. Patients ID #1 and ID #29 also had the smallest initial tumor volumes in the cohort, which could indicate that for tumors with MTV in the lower ranges, PET RANO metrics are less accurate. The patients were fasting, but there could nevertheless be fluctuating uptake by for example, drug effects or amino acid levels,33 leading to small changes in the calculated MTV defining thresholds.
Radiological response assessment according to RANO 2.0 criteria are mainly based on perpendicular measurements of the diameter or the contrast enhancement on T1 weighted imaging,35 or the total volume of contrast enhancement. Our data showed that response assessments based on the contrast enhancement, either by RANO criteria or by volume changes were of limited value for the prediction of responders and at best borderline statistically significantly associated with OS. Of note, we did not apply T1 subtraction which may allow a more accurate assessment of the contrast-enhancing tumor in the presence of reduced permeability induced by antiangiogenic treatment.41
The criteria defined in PET RANO was also of only borderline predictive value and did not perform better than MRI RANO criteria, although PET progression tended to be more sensitive for prediction of nonresponse than MRI RANO in direct comparison.
Three cases could be considered as MRI pseudo-response (clinical progression in ID #15 and discordant PET progression in ID #34 and #36) while no cases of pseudo-progression were observed. This suggests that imaging during follow-up in combined bevacizumab and nivolumab in our study may be dominated by effects of bevacizumab rather than those of nivolumab. Pseudo-progression in clinical trials with check-point inhibitors for glioblastoma is a relatively rarely occurring event with a rate of 8.4% among treated patients who had PD.42
In addition to standard metrics recommended in guidelines24 we also applied PRM analysis. PRM was originally described for analysis of diffusion weighted MRI.43 Two studies have investigated the potential of PRM analysis of amino acid PET. One study retrospective analyzed [11C]MET scans from 14 patients with recurrent glioma treated with WT1 immunotherapy and found relative progressive volume to predict OS.25 The other study analyzed [18F]DOPA scans obtained 1 week before and at 2 time-points (1–2 weeks and 5–7 weeks) after initiation of bevacizumab in 24 patients with recurrent glioma. The authors reported that relative progressive volume (rPRMplus) between the 2 post-treatment time-point predicted 3 months PFS and 6 months OS.26 Our analysis identified both absolute and relative progressive volume (PRMplus) and net change (PRMnet) as prognostic factors for OS. We also showed the predictive value of PRMplus performing better than that of rΔTBRmax. All 3 studies suggest that the progressive volume has higher impact on prognosis than the often much larger regressive volume. This is in line with the study of [18F]FET PET for response evaluation of Lomustine in recurrent grades 3–4 glioma suggesting that appearance on new hot-spots was most potent in predicting nonresponse.44
A caveat of PRM analysis is related to gross tumor morphology changes, for example, due to alterations in peritumoral edema or changes in the volume of the resection cavity (as shown in Figure 1). This probably explains the counterintuitive finding that PRMplus values above 0 mL were observed also in responders, although usually offset by a larger PRMminus. In these situations, a rigid registration as applied in the present analysis, may lead to erroneous results when subtracting images and performing calculations restricted to the VOI intersection of TBR > 1.6 of the 2 [18F]FET PET scans. Thus, there may be relevant metabolic changes occurring outside this volume and irrelevant changes within. Furthermore, as tissue edema derived from a defective blood-brain barrier may reduce activity uptake, antiangiogenic treatment may lead to functional restitution in healthy tissue and increase uptake above the PRMplus threshold that may be misinterpreted as progression. Conversely, a progressive lesion may lead to secondary peritumoral functional reductions that may explain why the average PRMminus was not different in the 2 groups (Supplementary Table S2) and not predictive of response.
PRM may be most useful as a reading support in patients with a relatively short follow-up period in the absence of larger anatomical changes and should be approached with the above limitations in mind. An advantage of PRM analysis in daily routine is the ease of visualization and quantification of regional changes when analyzing follow-up [18F]FET PET scans. This may be very helpful not only for response assessment but also in other situation, for example, suspicion of progressive disease. PRM may also potentially allow identification of metabolic progression and earlier identification of progressive disease that is above the threshold for noise, but below the accepted threshold (TBR 1.6) for tumor tissue. Thus, the quantitative PRM metrics and identified cutoff’s are best understood as an adjunct to reading and should be interpreted cautiously and examined further. Standardized whole tumor [18F]FET metrics are the selection of choice for now.
Response assessment using physiological MRI techniques such as diffusion-weighted imaging (DWI) reflecting cellularity and oedema45 or dynamic susceptibility perfusion MRI, that are more directly associated with the effect on the vessels46 have been reported to predict survival in recurrent gliomas treated with bevacizumab. An advantage over amino acid PET is that MRI based techniques may be included in routine MRI protocols and obviate the need for separate and more costly PET examinations. A review of these techniques is outside the scope of this paper, but findings are inconsistent and more recent publications failed to confirm the prognostic values of pre- to post-treatment changes in patients treated with bevacizumab despite clear effects of treatment when compared to patients not treated with bevacizumab.47,48 Possible caveats of these techniques are the dependence on tumor delineation based on conventional MRI and more sophisticated analyses (eg, histogram analysis or PRM). Studies directly comparing amino acid PET and advanced MRI for response assessment in recurrent high-grade gliomas are sparse. A small study showed significant correlation of amino acid uptake by [11C]-methionine PET and cerebral blood flow measurements by arterial spin labeling, but superior accuracy of amino acid PET for predicting progression free survival.39
A key advantage of amino acid PET is identification of the nonenhancing tumor. Using T2 or T2-FLAIR, the differentiation of nonenhancing tumor from gliosis and oedema may be difficult. The value of assessing the nonenhancing component in recurrent glioblastoma has been a matter of controversies and not recommended in enhancing high grade gliomas in RANO 2.0.49 In recurrent GBM treated with bevacizumab, T2/T2-FLAIR increase may allow for earlier detection of progression, but is not predictive of survival.48,50 Accordingly, we did not include a detailed analysis of the nonenhancing tumor. Based on qualitative assessment of the neuroradiologist we observed poor agreement of T2/T2-FLAIR increase with PET RANO progression, both overall and for identification of MRI RANO pseudoresponse, suggesting that information from amino acid PET is also additive to T2/T2-FLAIR for assessing nonenhancing tumor.
Early response assessment, whether by MRI or PET, aims to identify patients who do not benefit from continued treatment. As we did not observe cases of pseudoprogression, which might otherwise lead to premature discontinuation of in fact effective treatment, the benefit of PET was mainly related to the identification of pseudo-response with survival benefit only in patients with a substantial metabolic response. Lacking alternative treatments, the discontinuation of salvage therapies is a major decision, and treating physicians may be reluctant to stop salvage treatments in the absence of clear clinical-radiographic progression or severe side effects—and not on the basis of a lack of sufficient response. Here, it is worth noticing that response was based on longer or shorter survival than expected from a reference group, and the study design does not allow us to assess the survival benefit of individual patient. In case of a only minor metabolic responses below guideline suggested cutoffs, it may be prudent to follow the patient more closely with continued treatment, which may be futile in patients with over metabolic progression exceeding test–retest variability.33
A number of limitations should be mentioned. First of all, a larger sample would have improved statistics and allowed for more elaborate statistical analysis accounting for multiple co-variates. Still, the size is not very different from that previous prospective studies including between 10 and 30 patients each.19,27 Due to the exploratory nature of the study aiming to identify optimal metrics for a new treatment combination, we assessed a relatively large number of metrics, and the findings need to be confirmed in future larger studies.
We also included patients with IDH mutations that would no longer be classified as GBM. IDH mutated tumors may differ both in terms of prognosis and imaging characteristic and, thus, bias results, but within the aforementioned limitations of the statistical analysis no influence of IDH status was demonstrated.
Conclusion
The present study suggests standard [18F]FET PET metrics was superior to contrast enhanced MRI for early response assessment in patients with recurrent high grade astrocytic glioma treated with nivolumab and bevacizumab. The study further showed that the biological tumor volume at follow-up was the best predictor of overall survival, and that judicious use of parametric response mapping may be a useful addition to standard whole tumor metrics. The findings and response assessment criteria need to be confirmed in larger studies.
Supplementary Material
Acknowledgments
We thank The John and Birthe Meyer Foundation who generously donated the Siemens Biograph mMR PET/MR scanners to Copenhagen University Hospital Rigshospitalet. We acknowledge the contribution of L. Anderberg for programming of in-house software for parametric response mapping. The authors would also like to thank all technologists and radiographers for scanner assistance. Bristol-Myers Squibb generously provided the drug Nivolumab, however, had no influence on the trial design, methods, or results.
Contributor Information
Otto Mølby Henriksen, Department of Clinical Physiology and Nuclear Medicine, Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark.
Simone Maarup, Department of Oncology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Benedikte Hasselbalch, Department of Oncology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Hans Skovgaard Poulsen, The DCCC Brain Tumor Center, Danish Comprehensive Cancer Center, Copenhagen, Denmark; Department of Oncology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Ib Jarle Christensen, The DCCC Brain Tumor Center, Danish Comprehensive Cancer Center, Copenhagen, Denmark.
Karine Madsen, Department of Clinical Physiology and Nuclear Medicine, Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark.
Vibeke Andrée Larsen, Department of Radiology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Ulrik Lassen, Department of Oncology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Ian Law, Department of Clinical Medicine, Faculty of Health and Medical Science, University of Copenhagen, Copenhagen, Denmark; Department of Clinical Physiology and Nuclear Medicine, Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark.
Funding
The CA209-9UP study was supported by grants from The Danish Cancer society (Grant R204-A12416) and from Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis’ Legat.
Conflicts of interest statement
The authors declare no competing interests.
Authorship statement
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Otto M. Henriksen, Simone Maarup, Benedikte Hasselbalch, Karine Madsen, Vibeke A. Larsen, Ib J Christensen, and Ian Law. Hans S. Poulsen provided clinical information from the on-site clinical glioblastoma database. The first draft of the manuscript was written by Otto M. Henriksen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by The Capital Region of Denmark Committee on Health Research Ethics (ref. H-17040888).
Data availability
Most relevant meta-data are provided in Supplementary Table S2. Additional data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of data.
References
- 1. Crocetti E, Trama A, Stiller C, et al. ; RARECARE working group. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012;48(10):1532–1542. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Urup T, Dahlrot RH, Grunnet K, et al. Development and validation of a prognostic model for recurrent glioblastoma patients treated with bevacizumab and irinotecan. Acta Oncol. 2016;55(4):418–422. [DOI] [PubMed] [Google Scholar]
- 4. van Linde ME, Brahm CG, de Witt Hamer PC, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: A retrospective multicenter analysis. J Neurooncol. 2017;135(1):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 6. Schiff D, Wen PY.. The siren song of bevacizumab: Swan song or clarion call? Neuro Oncol. 2018;20(2):147–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajan A, Kim C, Heery CR, Guha U, Gulley JL.. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum Vaccin Immunother. 2016;12(9):2219–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khasraw M, Reardon DA, Weller M, Sampson JH.. PD-1 inhibitors: Do they have a future in the treatment of glioblastoma? Clin Cancer Res. 2020;26(20):5287–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Aguiar RB, de Moraes JZ.. Exploring the immunological mechanisms underlying the anti-vascular endothelial growth factor activity in tumors. Front Immunol. 2019;10(May):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rini BI, Weinberg V, Fong L, et al. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107(1):67–74. [DOI] [PubMed] [Google Scholar]
- 13. Rachinger W, Goetz C, Popperl G, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–11. [DOI] [PubMed] [Google Scholar]
- 14. van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A.. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27(10):4129–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henriksen OM, Del Mar Alvarez-Torres M, Figueiredo P, et al. High-grade glioma treatment response monitoring biomarkers: A position statement on the evidence supporting the use of advanced MRI techniques in the clinic, and the latest bench-to-bedside developments. part 1: Perfusion and diffusion techniques. Front Oncol. 2022;12(Mar):810263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Booth TC, Wiegers EC, Warnert EAH, et al. High-grade glioma treatment response monitoring biomarkers: A position statement on the evidence supporting the use of advanced mri techniques in the clinic, and the latest bench-to-bedside developments. Part 2: Spectroscopy, chemical exchange saturation, multiparametric imaging, and radiomics. Front Oncol. 2021;11(Feb):811425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galldiks N, Lohmann P, Fink GR, Langen KJ.. Amino Acid PET in Neurooncology. J Nucl Med. 2023;64(5):693–700. [DOI] [PubMed] [Google Scholar]
- 18. Albert NL, Weller M, Suchorska B, et al. Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes KL, O’Neal CM, Andrews BJ, et al. A systematic review of the utility of amino acid PET in assessing treatment response to bevacizumab in recurrent high-grade glioma. Neurooncol. Adv.. 2021;3(1):vdab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galldiks N, Abdulla DSY, Scheffler M, et al. Treatment monitoring of immunotherapy and targeted therapy using (18)F-FET PET in patients with melanoma and lung cancer brain metastases: Initial experiences. J Nucl Med. 2021;62(4):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Lim-Fat MJ, Qin L, et al. A comparative retrospective study of immunotherapy RANO versus standard RANO criteria in glioblastoma patients receiving immune checkpoint inhibitor therapy. Front Oncol. 2021;11(Jun):679331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song J, Kadaba P, Kravitz A, et al. Multiparametric MRI for early identification of therapeutic response in recurrent glioblastoma treated with immune checkpoint inhibitors. Neuro Oncol. 2020;22(11):1658–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagiwara A, Oughourlian TC, Cho NS, et al. Diffusion MRI is an early biomarker of overall survival benefit in IDH wild-type recurrent glioblastoma treated with immune checkpoint inhibitors. Neuro Oncol. 2022;24(6):1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiba Y, Kinoshita M, Okita Y, et al. Use of (11)C-methionine PET parametric response map for monitoring WT1 immunotherapy response in recurrent malignant glioma. J Neurosurg. 2012;116(4):835–842. [DOI] [PubMed] [Google Scholar]
- 26. Harris RJ, Cloughesy TF, Pope WB, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaban A, Waschulzik B, Bernhardt D, et al. Amino acid PET vs. RANO MRI for prediction of overall survival in patients with recurrent high grade glioma under bevacizumab therapy. Eur J Nucl Med Mol Imaging. 2024;51(6):1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skadborg SK, Maarup S, Draghi A, et al. Nivolumab reaches brain lesion in patients with recurrent glioblastoma and induces T-cell activity and upregulation of checkpoint pathways. Cancer Immunol Res. 2024;12(9):1202–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delso G, Furst S, Jakoby B, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52(12):1914–1922. [DOI] [PubMed] [Google Scholar]
- 30. Andersen FL, Ladefoged CN, Beyer T, et al. Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone. Neuroimage. 2014;84(Jan):206–216. [DOI] [PubMed] [Google Scholar]
- 31. Ladefoged CN, Andersen FL, Kjaer A, Hojgaard L, Law I.. RESOLUTE PET/MRI attenuation correction for O-(2-(18)F-fluoroethyl)-L-tyrosine (FET) in brain tumor patients with metal implants. Front Neurosci. 2017;11(Aug):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 33. Chehri S, Henriksen OM, Marner L, et al. A prospective clinical study of the influence of oral protein intake on [18F]FET-PET uptake and test-retest repeatability in glioma. EJNMMI Res. 2024;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellingson BM, Chen W, Harris RJ, et al. PET parametric response mapping for clinical monitoring and treatment response evaluation in brain tumors. PET Clinics. 2013;8(2):201–217. [DOI] [PubMed] [Google Scholar]
- 35. Wen PY, van den Bent M, Youssef G, et al. RANO 2.0: update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. 2023;41(33):5187–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Albert NL, Galldiks N, Ellingson BM, et al. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): A report of the RANO group. Lancet Oncol. 2024;25(1):e29–e41. [DOI] [PubMed] [Google Scholar]
- 37. Galldiks N, Dunkl V, Ceccon G, et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur J Nucl Med Mol Imaging. 2018;45(13):2377–2386. [DOI] [PubMed] [Google Scholar]
- 38. Beppu T, Terasaki K, Sasaki T, et al. MRI and 11C-methyl-L-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clin Nucl Med. 2016;41(11):852–857. [DOI] [PubMed] [Google Scholar]
- 39. Beppu T, Sato Y, Sasaki T, et al. Comparisons between PET with 11C-Methyl-L-methionine and arterial spin labeling perfusion imaging in recurrent glioblastomas treated with bevacizumab. Clin Nucl Med. 2019;44(3):186–193. [DOI] [PubMed] [Google Scholar]
- 40. Schwarzenberg J, Czernin J, Cloughesy TF, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: Contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim M, Weller M, Idbaih A, et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24(11):1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: A noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005;102(15):5524–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wollring MM, Werner JM, Bauer EK, et al. Prediction of response to lomustine-based chemotherapy in glioma patients at recurrence using MRI and FET PET. Neuro Oncol. 2023;25(5):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ellingson BM, Cloughesy TF, Lai A, et al. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(10):1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmainda KM, Zhang Z, Prah M, et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: Results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 2015;17(8):1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park JE, Kim HS, Park SY, et al. Identification of early response to anti-angiogenic therapy in recurrent glioblastoma: Amide proton transfer-weighted and perfusion-weighted MRI compared with diffusion-weighted MRI. Radiology. 2020;295(2):397–406. [DOI] [PubMed] [Google Scholar]
- 48. Kickingereder P, Brugnara G, Hansen MB, et al. Noninvasive characterization of tumor angiogenesis and oxygenation in bevacizumab-treated recurrent glioblastoma by using dynamic susceptibility MRI: Secondary analysis of the European organization for research and treatment of cancer 26101 trial. Radiology. 2020;297(1):164–175. [DOI] [PubMed] [Google Scholar]
- 49. Wen PY, van den Bent M, Youssef G, et al. RANO 2.0: Update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. 2023;41(33):5187–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang RY, Rahman R, Ballman KV, et al. The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res. 2016;22(3):575–581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most relevant meta-data are provided in Supplementary Table S2. Additional data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.