Abstract
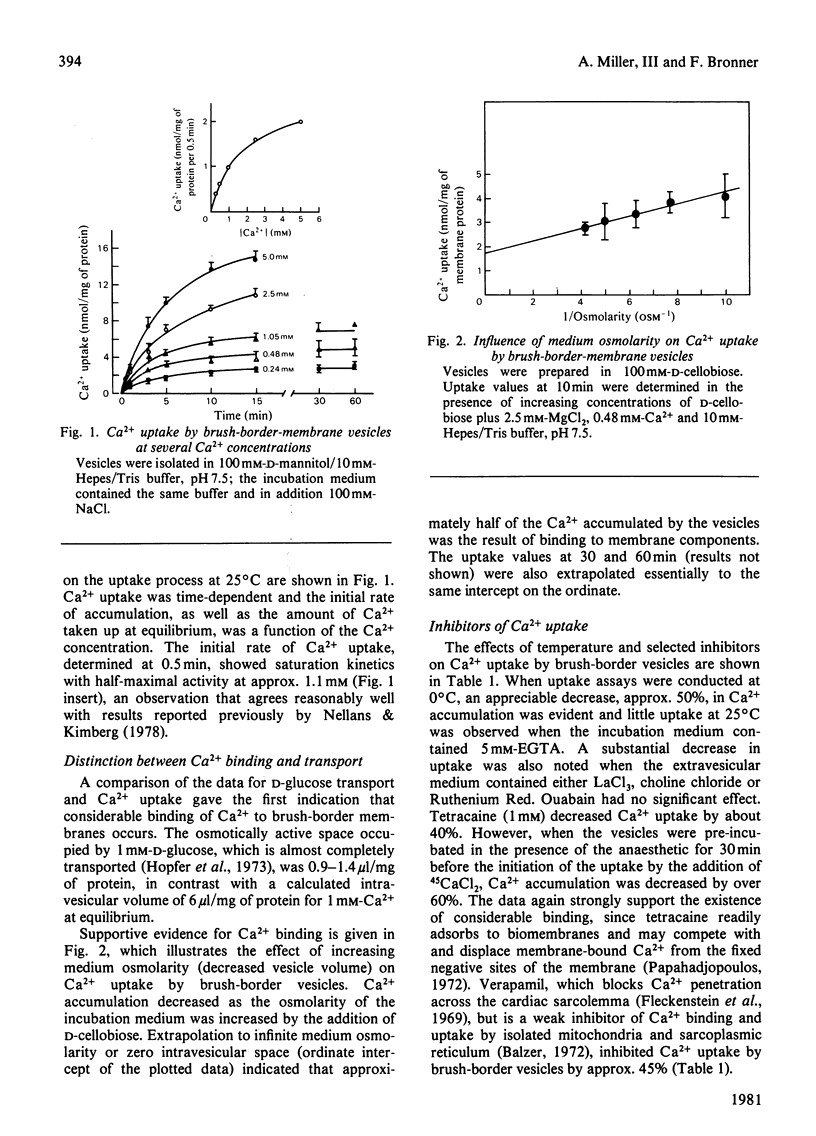
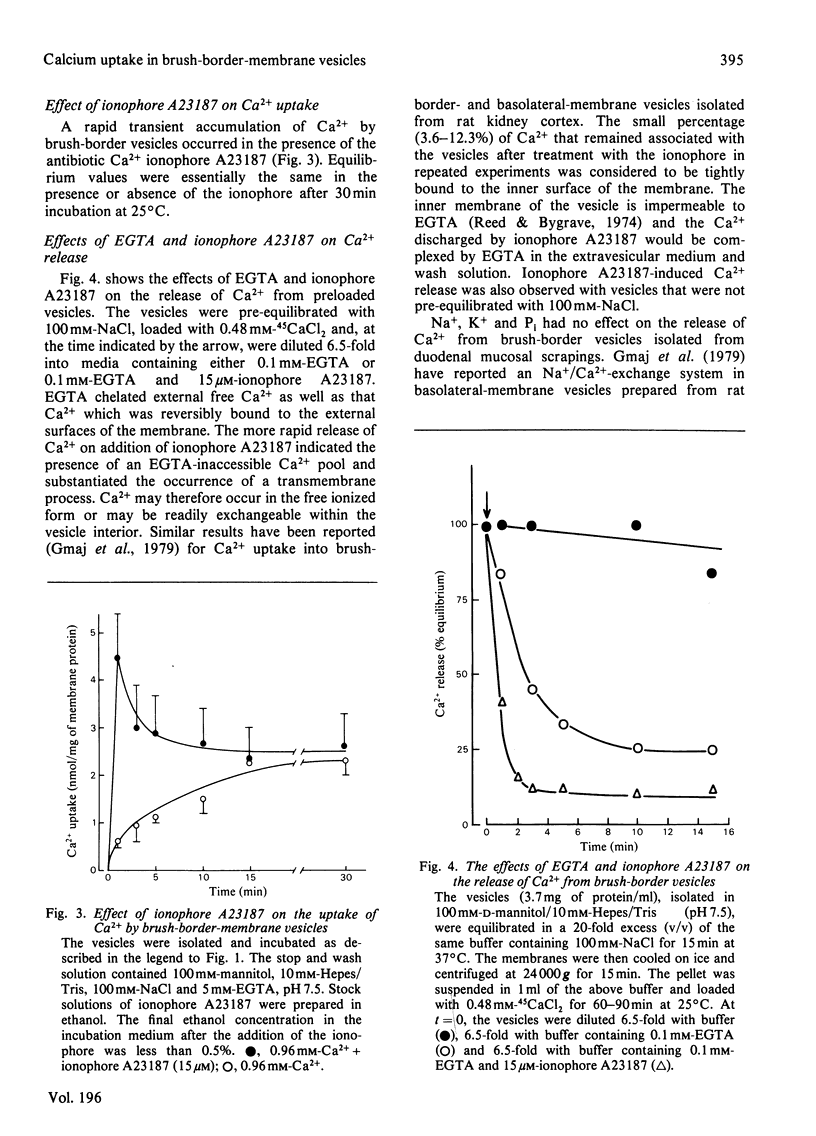
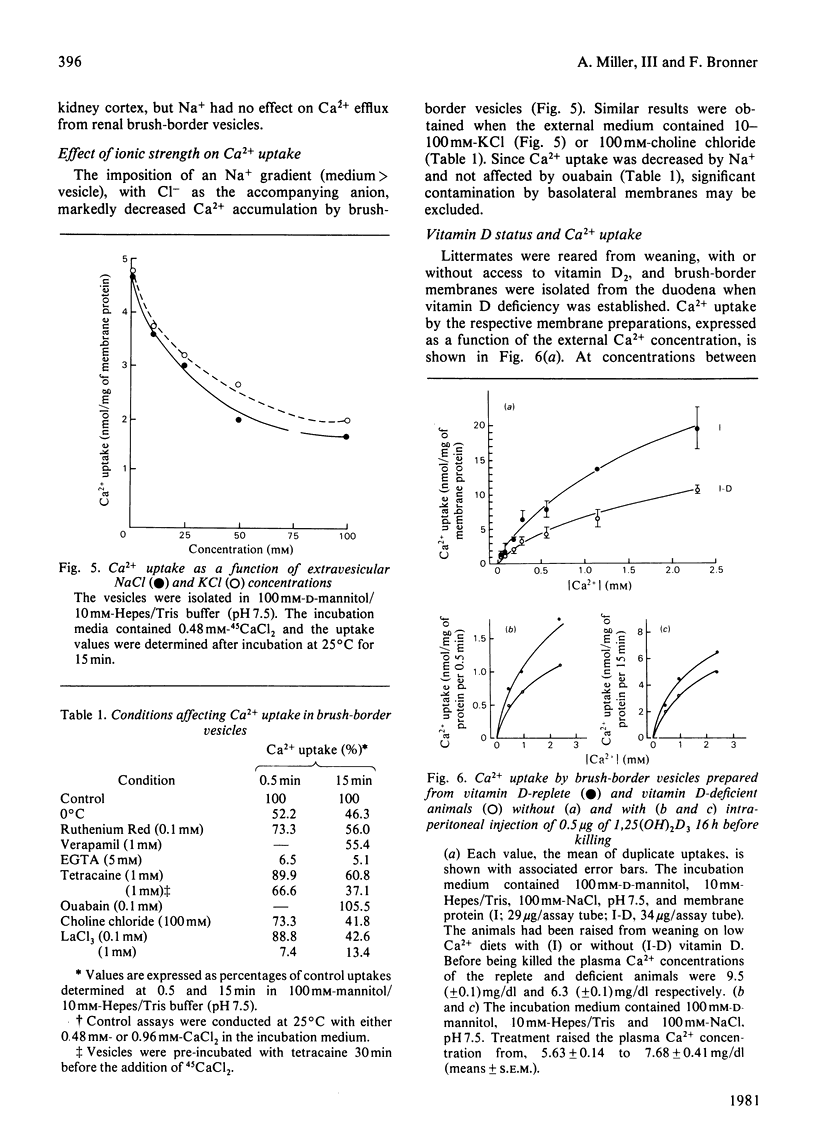
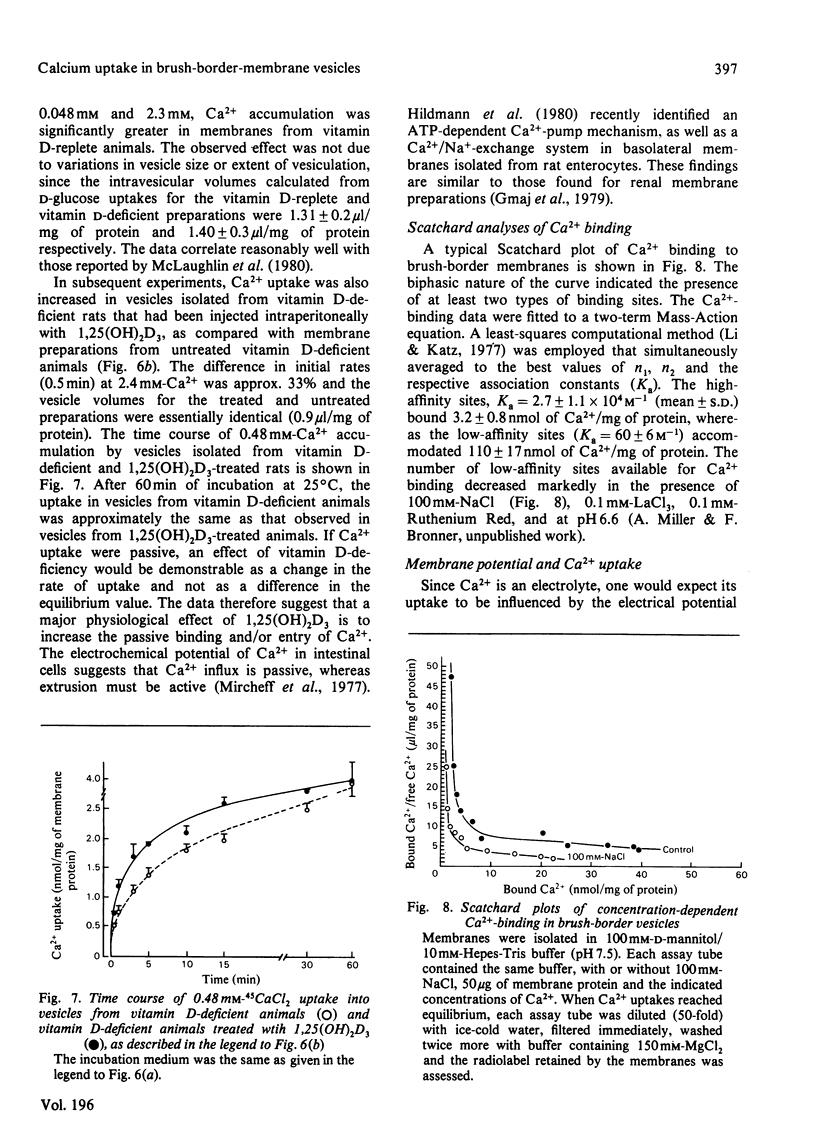
Ca2+ uptake in brush-border vesicles isolated from rat duodena was studied by a rapid-filtration technique. Ca2+ uptake showed saturation kinetics, was dependent on the pH and ionic strength of the medium and was independent of metabolic energy. Uptake activity was readily inhibited by Ruthenium Red, La3+, tetracaine, EGTA, choline chloride and Na+ or K+. The effect of variations in medium osmolarity on Ca2+ uptake and the ionophore A23187-induced efflux of the cation from preloaded vesicles indicated that the Ca2+-uptake process involved binding to membrane components, as well as transport into an osmotically active space. Scatchard-plot analyses of the binding data suggested at least two classes of Ca2+-binding sites. The high-affinity sites, Ka = (2.7 +/- 1.1) x 10(4) M-1 (mean +/- S.D.) bound 3.2 +/- 0.8 nmol of Ca2+/mg of protein, whereas the low-affinity sites (Ka = 60 +/- 6 M-1) bound 110 +/- 17 nmol of Ca2+/mg of protein. In the presence of 100 mM-NaCl, 1.7 and 53 nmol of Ca2+/mg of protein were bound to the high- and low-affinity sites respectively. Decreased Ca2+-uptake activity was observed in vesicles isolated from vitamin D-deficient as compared with vitamin D-replete animals and intraperitoneal administration of 1,25-dihydroxycholecalciferol to vitamin D-deficient rats 16 h before membrane isolation stimulated the initial rate of Ca2+ uptake significantly. The data indicated that Ca2+ entry and/or binding was passive and may involve a carrier-mediated Ca2+-uptake component that is associated with the brush-border membrane. Altering the electrochemical potential difference across the membrane by using anions of various permeability and selected ionophores appeared to increase primarily binding to the membrane rather than transport into the intravesicular space. Since there is considerable binding of Ca2+ to the vesicle interior, a comprehensive analysis of the transport properties of the brush-border membrane remains difficult at present.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balzer H. The effect of quinidine and drugs with quinidine-like action (propranolol, verapamil and tetracaine) on the calcium transport system in isolated sarcoplasmic reticulum vesicles of rabbit skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(3):256–272. doi: 10.1007/BF00501935. [DOI] [PubMed] [Google Scholar]
- Berner W., Kinne R., Murer H. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem J. 1976 Dec 15;160(3):467–474. doi: 10.1042/bj1600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner F., Freund T. Intestinal CaBP: a new quantitive index of vitamin D deficiency in the rat. Am J Physiol. 1975 Sep;229(3):689–694. doi: 10.1152/ajplegacy.1975.229.3.689. [DOI] [PubMed] [Google Scholar]
- Caroni P., Gazzotti P., Vuilleumier P., Simon W., Carafoli E. Ca2+ transport mediated by a synthetic neutral Ca2+ -ionophore in biological membranes. Biochim Biophys Acta. 1977 Nov 1;470(3):437–445. doi: 10.1016/0005-2736(77)90134-1. [DOI] [PubMed] [Google Scholar]
- Chevallier J., Butow R. A. Calcium binding to the sarcoplasmic reticulum of rabbit skeletal muscle. Biochemistry. 1971 Jul 6;10(14):2733–2737. doi: 10.1021/bi00790a012. [DOI] [PubMed] [Google Scholar]
- DOWDLE E. B., SCHACHTER D., SCHENKER H. Requirement for vitamin D for the active transport of calcium by the intestine. Am J Physiol. 1960 Feb;198:269–274. doi: 10.1152/ajplegacy.1960.198.2.269. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Tanaka K., Isselbacher K. J. Lipid composition of the isolated rat intestinal microvillus membrane. Biochem J. 1968 Aug;109(1):51–59. doi: 10.1042/bj1090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. A., Weiser M. M., Isselbacher K. J. Calcium translocation by Golgi and lateral-basal membrane vesicles from rat intestine: decrease in vitamin D-deficient rats. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3612–3616. doi: 10.1073/pnas.74.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T., Bronner F. Stimulation in vitro by 1,25-dihydroxy-vitamin D3 of intestinal cell calcium uptake and calcium-binding protein. Science. 1975 Dec 26;190(4221):1300–1302. doi: 10.1126/science.1198113. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Peterlik M. Vitamin D-induced phosphate transport in intestinal brush border membrane vesicles. Biochem Biophys Res Commun. 1980 Mar 13;93(1):87–92. doi: 10.1016/s0006-291x(80)80249-x. [DOI] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Gmaj P., Murer H., Kinne R. Calcium ion transport across plasma membranes isolated from rat kidney cortex. Biochem J. 1979 Mar 15;178(3):549–557. doi: 10.1042/bj1780549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U. Isolated membrane vesicles as tools for analysis of epithelial transport. Am J Physiol. 1977 Dec;233(6):E445–E449. doi: 10.1152/ajpendo.1977.233.6.E445. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Hopfer U., Sigrist-Nelson K., Ammann E., Murer H. Differences in neutral amino acid and glucose transport between brush border and basolateral plasma membrane of intestinal epithelial cells. J Cell Physiol. 1976 Dec;89(4):805–810. doi: 10.1002/jcp.1040890447. [DOI] [PubMed] [Google Scholar]
- Isselbacher K. J. The intestinal cell surface: properties of normal, undifferentiated, and malignant cells. Harvey Lect. 1973;(69):197–221. [PubMed] [Google Scholar]
- Katz A. M., Repke D. I. Sodium and potassium sensitivity of calcium uptake and calcium binding by dog cardiac microsomes. Circ Res. 1967 Nov;21(5):767–775. doi: 10.1161/01.res.21.5.767. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Li S. T., Katz E. P. On the state of anionic groups of demineralized matrices of bone and dentine. Calcif Tissue Res. 1977 Feb 11;22(3):275–284. doi: 10.1007/BF02010366. [DOI] [PubMed] [Google Scholar]
- MacLaughlin J. A., Weiser M. M., Freedman R. A. Biphasic recovery of vitamin D-dependent Ca2+ uptake by rat intestinal Golgi membranes. Gastroenterology. 1980 Feb;78(2):325–332. [PubMed] [Google Scholar]
- Max E. E., Goodman D. B., Rasmussen H. Purification and characterization of chick intestine brush border membrane. Effects of 1alpha(OH) vitamin D3 treatment. Biochim Biophys Acta. 1978 Aug 4;511(2):224–239. doi: 10.1016/0005-2736(78)90316-4. [DOI] [PubMed] [Google Scholar]
- Miller A., 3rd, Ueng T. H., Bronner F. Isolation of a vitamin D-dependent, calcium-binding protein from brush borders of rat duodenal mucosa. FEBS Lett. 1979 Jul 15;103(2):319–322. doi: 10.1016/0014-5793(79)81353-8. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U. Demonstration of electrogenic Na+-dependent D-glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):484–488. doi: 10.1073/pnas.71.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- NICOLAYSEN R., EEG-LARSEN N., MALM O. J. Physiology of calcium metabolism. Physiol Rev. 1953 Jul;33(3):424–444. doi: 10.1152/physrev.1953.33.3.424. [DOI] [PubMed] [Google Scholar]
- Palmer R. F., Posey V. A. Calcium and adenosine triphosphate binding to renal membranes. J Gen Physiol. 1970 Jan;55(1):89–103. doi: 10.1085/jgp.55.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D. Studies on the mechanism of action of local anesthetics with phospholipid model membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):169–186. doi: 10.1016/0304-4157(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Fontaine O., Max E. E., Goodman D. B. The effect of 1alpha-hydroxyvitamin D3 administration on calcium transport in chick intestine brush border membrane vesicles. J Biol Chem. 1979 Apr 25;254(8):2993–2999. [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER D., ROSEN S. M. Active transport of Ca45 by the small intestine and its dependence on vitamin D. Am J Physiol. 1959 Feb;196(2):357–362. doi: 10.1152/ajplegacy.1959.196.2.357. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Shlatz L., Marinetti G. V. Calcium binding to the rat liver plasma membrane. Biochim Biophys Acta. 1972 Dec 1;290(1):70–83. doi: 10.1016/0005-2736(72)90053-3. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Murer H., Hopfer U. Active alanine transport in isolated brush border membranes. J Biol Chem. 1975 Jul 25;250(14):5674–5680. [PubMed] [Google Scholar]
- Ueng T. H., Golub E. E., Bronner F. The effect of age and 1,25-dihydroxyvitamin D3 treatment on the intestinal calcium-binding protein of suckling rats. Arch Biochem Biophys. 1979 Sep;196(2):624–630. doi: 10.1016/0003-9861(79)90316-3. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Woodrow M. L., Scarpa A. Calcium binding to cardiac sarcolemma. Recent Adv Stud Cardiac Struct Metab. 1975;5:61–71. [PubMed] [Google Scholar]


