Abstract
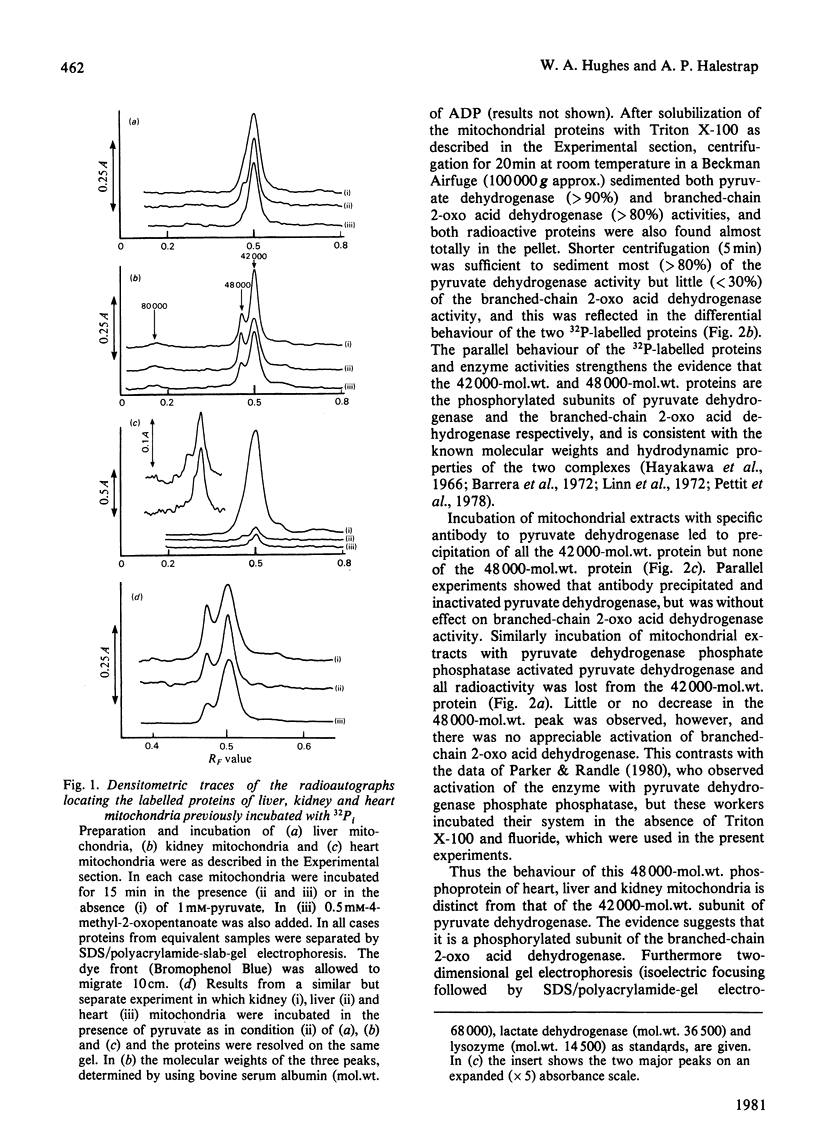
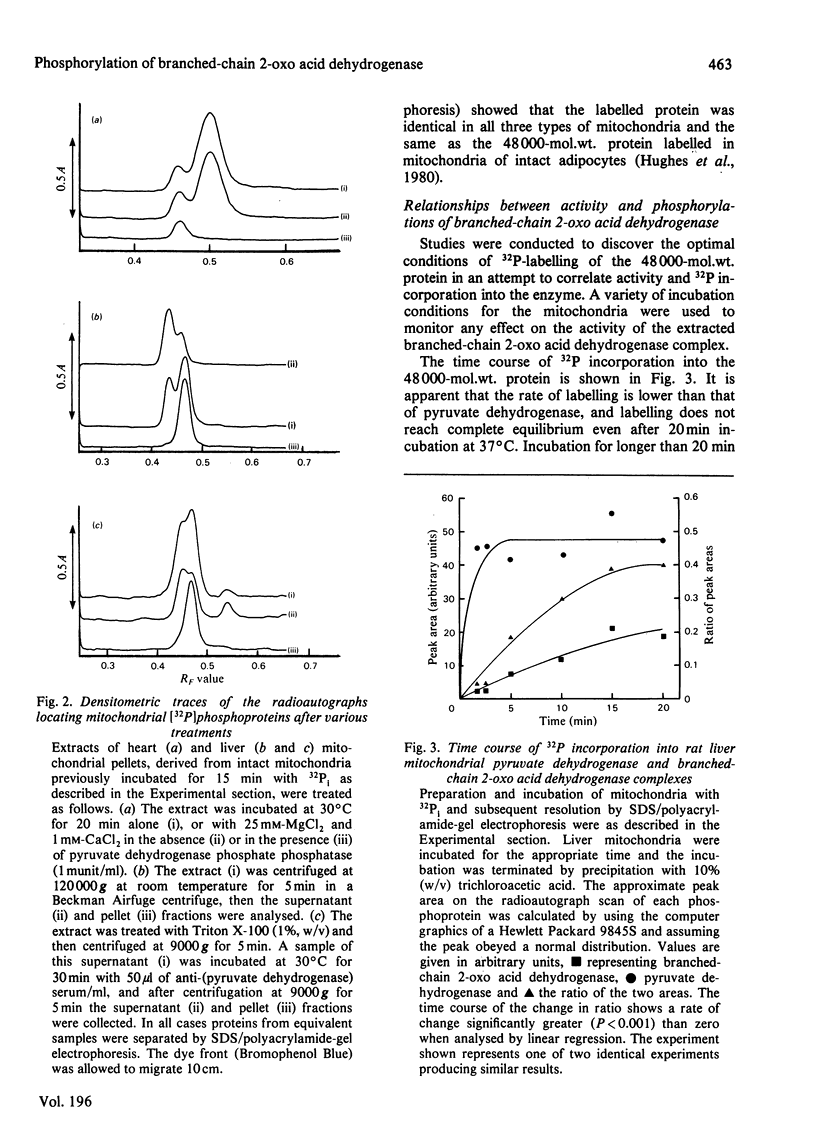
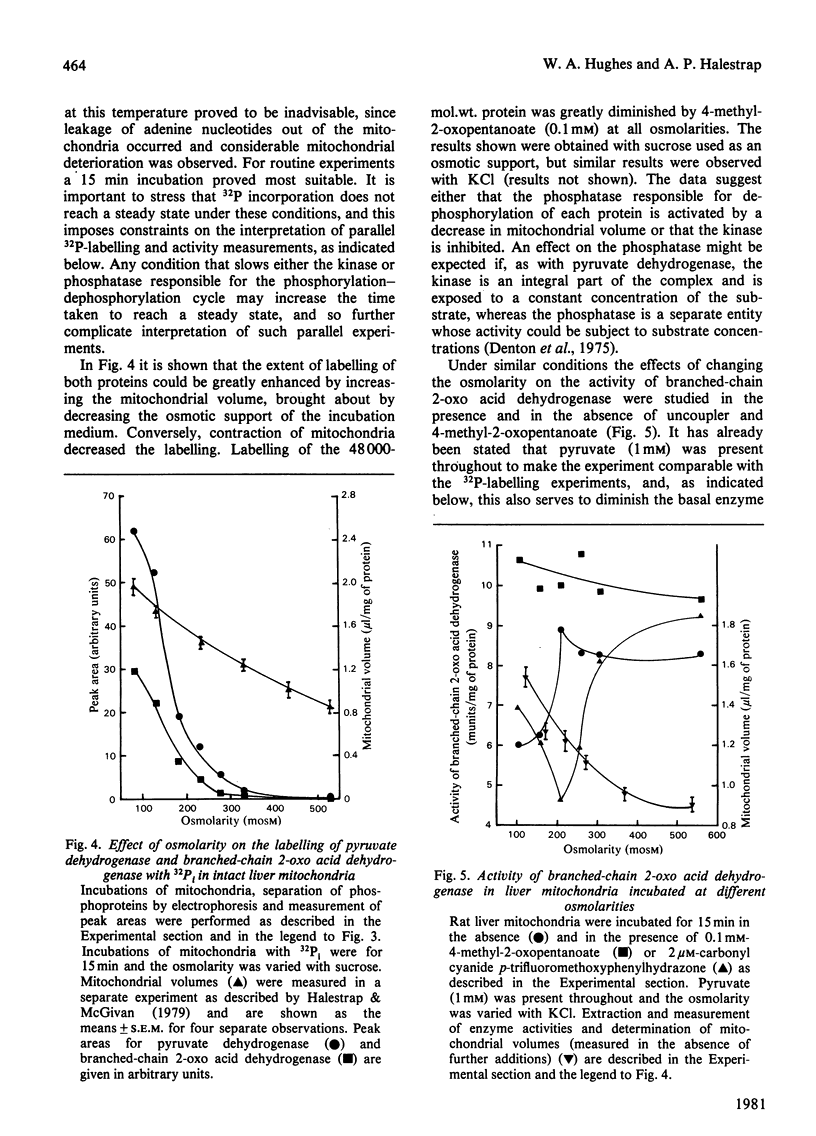
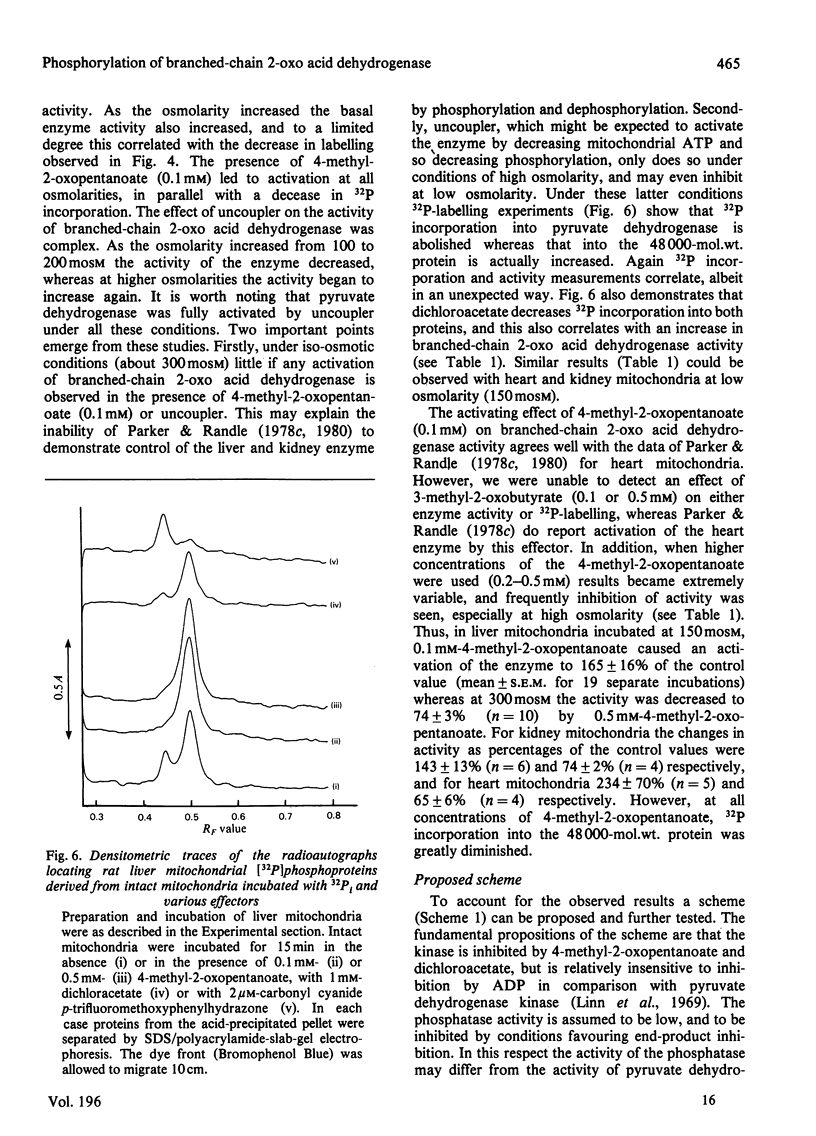
1. Incubation of mitochondria from heart, liver and kidney with [32P]phosphate allowed 32P incorporation into two intramitochondrial proteins, the decarboxylase alpha-subunit of the pyruvate dehydrogenase complex (mol.wt 42000) and a protein of mol.wt. 48000. 2. This latter protein incorporated 32P more slowly than did pyruvate dehydrogenase, was not precipitated by antibody to pyruvate dehydrogenase and showed behaviour distinct from that of pyruvate dehydrogenase towards high-speed centrifugation and pyruvate dehydrogenase phosphate phosphatase. 3. 32P incorporation into the protein was greatly diminished by the presence of 0.1 mM-4-methyl-2-oxopentanoate, but enhanced by pyruvate (1 mM), hypo-osmotic treatment of mitochondria and, under some conditions, by uncoupler. 4. The activity of branched-chain 2-oxo acid dehydrogenase was assayed in parallel experiments. Under appropriate conditions the enzyme was inhibited when 32P incorporation was increased and activated when incorporation was decreased. The data suggest that the 48000-mol.wt. phosphorylated protein is identical with the decarboxylase subunit of branched-chain 2-oxo acid dehydrogenase and that this enzyme may be controlled by a phosphorylation-dephosphorylation cycle akin to that for pyruvate dehydrogenase. 5. Strict correlation between activity and 32P incorporation was not observed, and a scheme for the regulation of the enzyme is proposed to account for these discrepancies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A., Krzysik B. A., Morse E. L., Amin P. M., Allen E. R. Oxidative energy metabolism in the skeletal muscle: biochemical and ultrastructural evidence for adaptive changes. J Lab Clin Med. 1974 Apr;83(4):548–562. [PubMed] [Google Scholar]
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Bremer J., Davis E. J. The effect of acylcarnitines on the oxidation of branched chain alpha-keto acids in mitochondria. Biochim Biophys Acta. 1978 Mar 30;528(3):269–275. doi: 10.1016/0005-2760(78)90016-4. [DOI] [PubMed] [Google Scholar]
- Buffington C. K., DeBuysere M. S., Olson M. S. Studies on the regulation of the branched chain alpha-keto acid dehydrogenase in the perfused rat heart. J Biol Chem. 1979 Oct 25;254(20):10453–10458. [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Roche T. E. A unifying mechanism for stimulation of mammalian pyruvate dehydrogenase(a) kinase by reduced nicotinamide adenine dinucleotide, dihydrolipoamide, acetyl coenzyme A, or pyruvate. J Biol Chem. 1978 Jan 25;253(2):496–503. [PubMed] [Google Scholar]
- Chua B., Siehl D. L., Morgan H. E. Effect of leucine and metabolites of branched chain amino acids on protein turnover in heart. J Biol Chem. 1979 Sep 10;254(17):8358–8362. [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Frick G. P., Goodman H. M. Insulin regulation of branched chain alpha-keto acid dehydrogenase in adipose tissue. J Biol Chem. 1980 Jul 10;255(13):6186–6192. [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Goldberg A. L., Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol. 1972 Dec;223(6):1384–1391. doi: 10.1152/ajplegacy.1972.223.6.1384. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975 Apr;148(1):85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T., Hirashima M., Ide S., Hamada M., Okabe K., Koike M. Mammalian alpha-keto acid dehydrogenase complexes. I. Isolation, purification, and properties of pyruvate dehydrogenase complex of pig heart muscle. J Biol Chem. 1966 Oct 25;241(20):4694–4699. [PubMed] [Google Scholar]
- Hughes W. A., Brownsey R. W., Denton R. M. Studies on the incorporation of [32P]phosphate into pyruvate dehydrogenase in intact rat fat-cells. Effects of insulin. Biochem J. 1980 Nov 15;192(2):469–481. doi: 10.1042/bj1920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. A., Halestrap A. P. Phosphorylation of branched-chain 2-oxo acid dehydrogenase within intact mitochondria [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):374–374. doi: 10.1042/bst0080374. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Zapalowski C., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproic acid metabolism in skeletal muscle. Effects of starvation and insulin. J Biol Chem. 1980 Mar 25;255(6):2418–2426. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R. Direct evidence for the inactivation of branched-chain oxo-acid dehydrogenase by enzyme phosphorylation. FEBS Lett. 1980 Dec 1;121(2):306–308. doi: 10.1016/0014-5793(80)80369-3. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R. Reversible ATP-induced inactivation of branched-chain 2-oxo acid dehydrogenase. Biochem J. 1980 Oct 15;192(1):155–163. doi: 10.1042/bj1920155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Active and inactive forms of branched-chain 2-oxoacid dehydrogenase complex in rat heart and skeletal muscle. FEBS Lett. 1980 Apr 7;112(2):186–190. doi: 10.1016/0014-5793(80)80176-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Branched chain 2-oxo-acid dehydrogenase complex of rat liver. FEBS Lett. 1978 Jun 1;90(1):183–186. doi: 10.1016/0014-5793(78)80325-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Inactivation of rat heart branched-chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1978 Nov 1;95(1):153–156. doi: 10.1016/0014-5793(78)80072-6. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Partial purification and properties of branched-chain 2-oxo acid dehydrogenase of ox liver. Biochem J. 1978 Jun 1;171(3):751–757. doi: 10.1042/bj1710751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans R. M., Jolly W. W., Harris R. A. Studies on the regulation of leucine catabolism. III. Effects of dichloroacetate and 2-chloropropionate on leucine oxidation by the heart. J Mol Cell Cardiol. 1980 Jan;12(1):1–16. doi: 10.1016/0022-2828(80)90107-8. [DOI] [PubMed] [Google Scholar]
- Sans R. M., Jolly W. W., Harris R. A. Studies on the regulation of leucine catabolism. Mechanism responsible for oxidizable substrate inhibition and dichloroacetate stimulation of leucine oxidation by the heart. Arch Biochem Biophys. 1980 Apr 1;200(2):336–345. doi: 10.1016/0003-9861(80)90363-x. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W., Veerkamp J. H., Glatz J. F. 4-Methyl-2-oxopentanoate oxidation by rat skeletal-muscle mitochondria. Biochem J. 1979 Aug 15;182(2):353–360. doi: 10.1042/bj1820353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymack P. P., DeBuysere M. S., Olson M. S. Studies on the activation and inactivation of the branched chain alpha-keto acid dehydrogenase in the perfused rat heart. J Biol Chem. 1980 Oct 25;255(20):9773–9781. [PubMed] [Google Scholar]
- Wałajtys-Rode E., Coll K. E., Williamson J. R. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. II. Interactions with gluconeogenesis and urea synthesis. J Biol Chem. 1979 Nov 25;254(22):11521–11529. [PubMed] [Google Scholar]
- Williamson J. R., Wałajtys-Rode E., Coll K. E. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. I. Regulation of branched chain alpha-ketoacid metabolism. J Biol Chem. 1979 Nov 25;254(22):11511–11520. [PubMed] [Google Scholar]
- Wohlhueter R. M., Harper A. E. Coinduction of rat liver branched chain alpha-keto acid dehydrogenase activities. J Biol Chem. 1970 May 10;245(9):2391–2401. [PubMed] [Google Scholar]


