Abstract
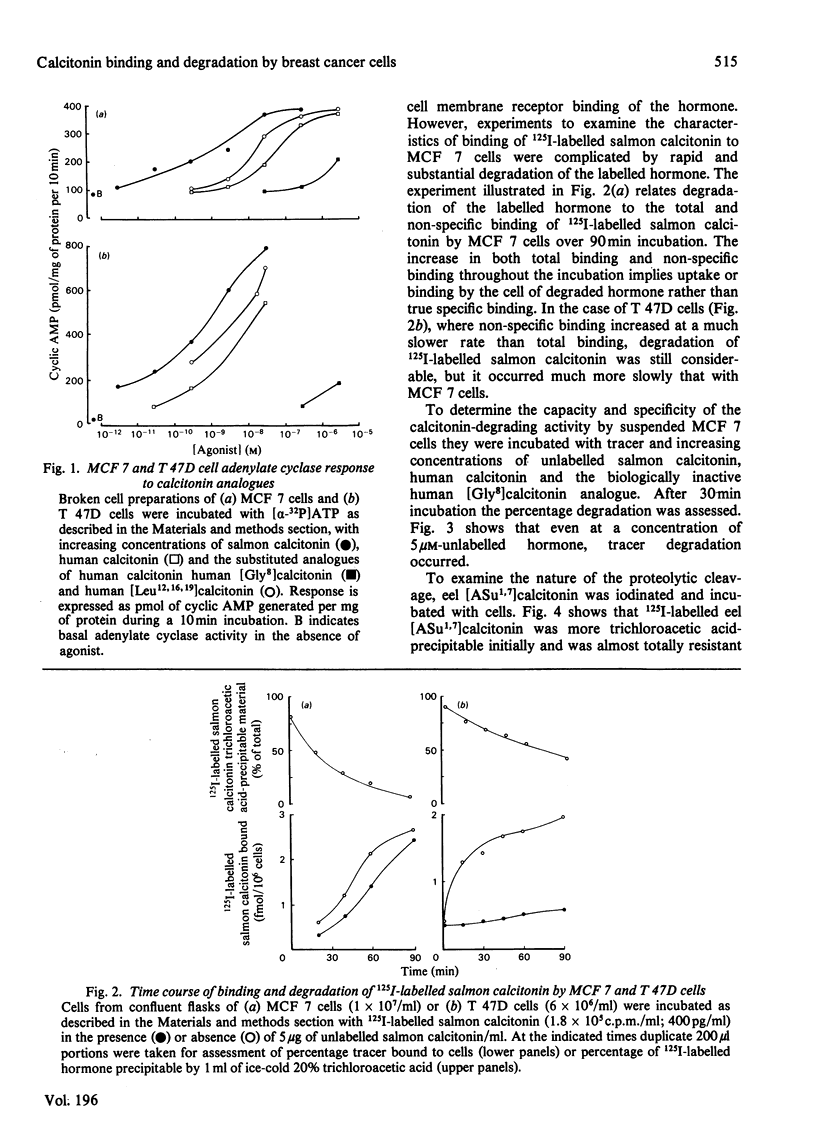
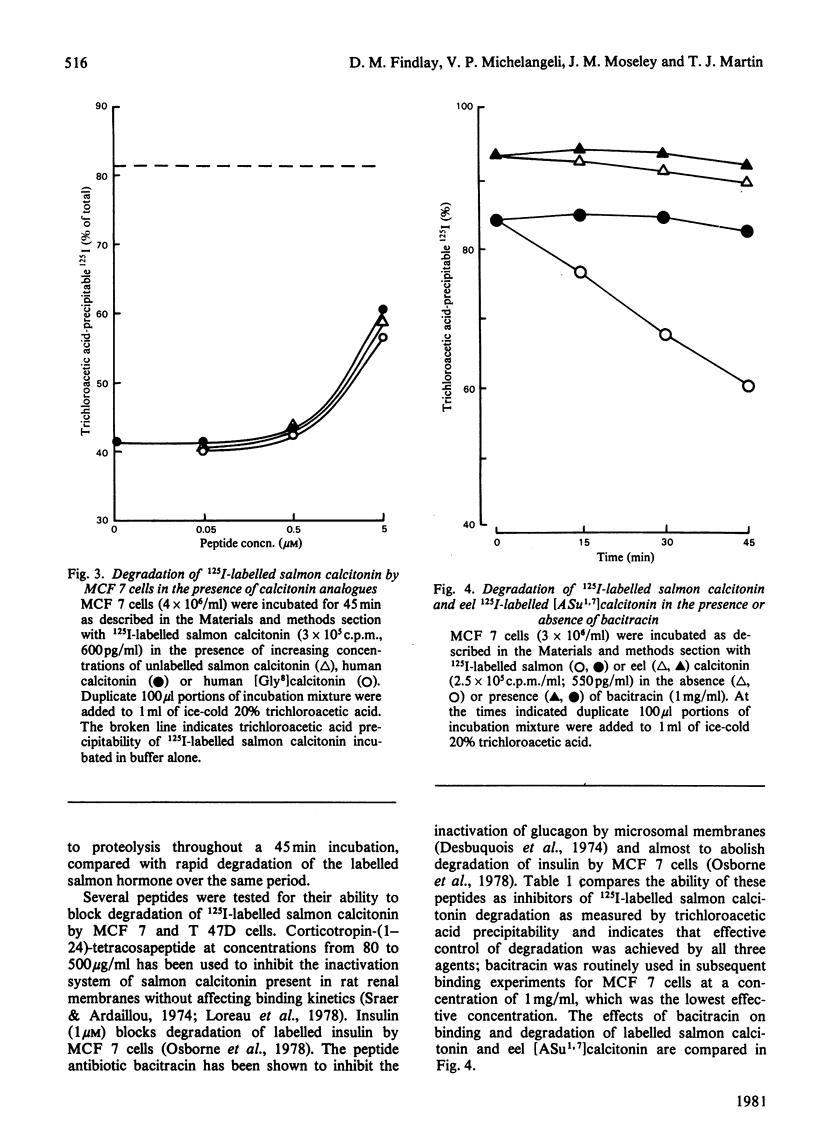
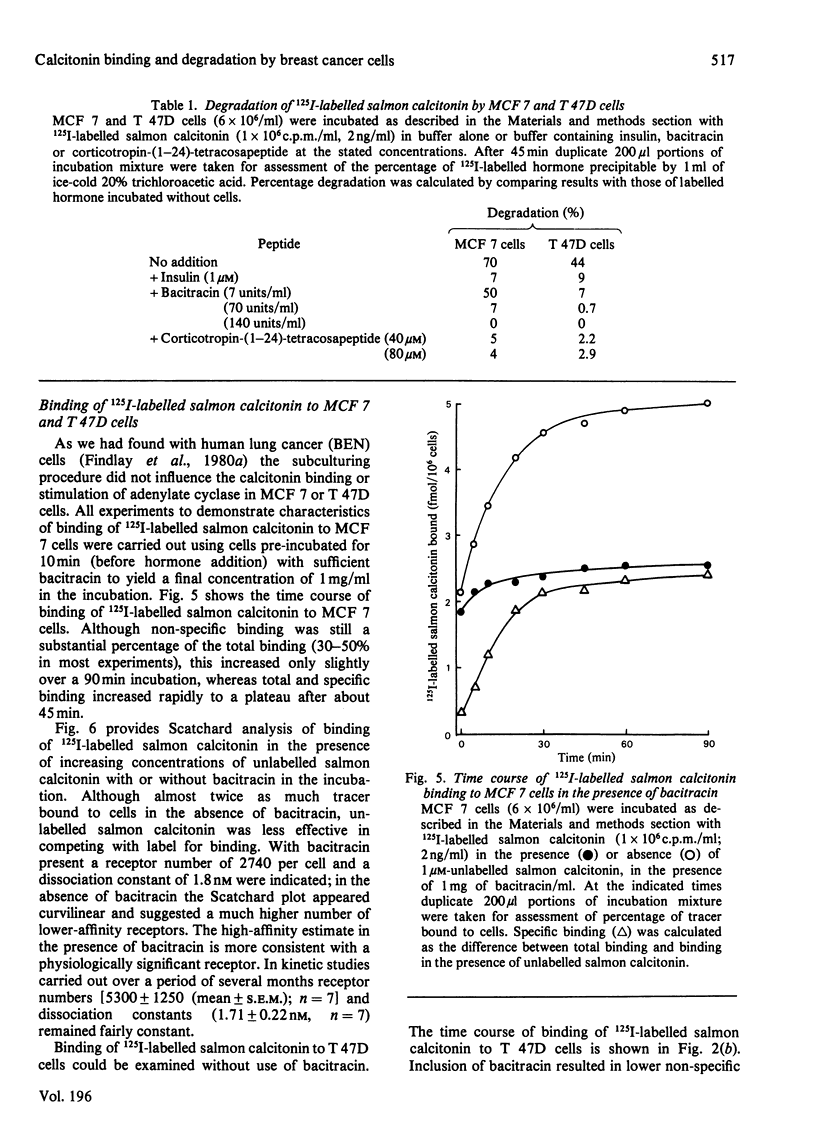
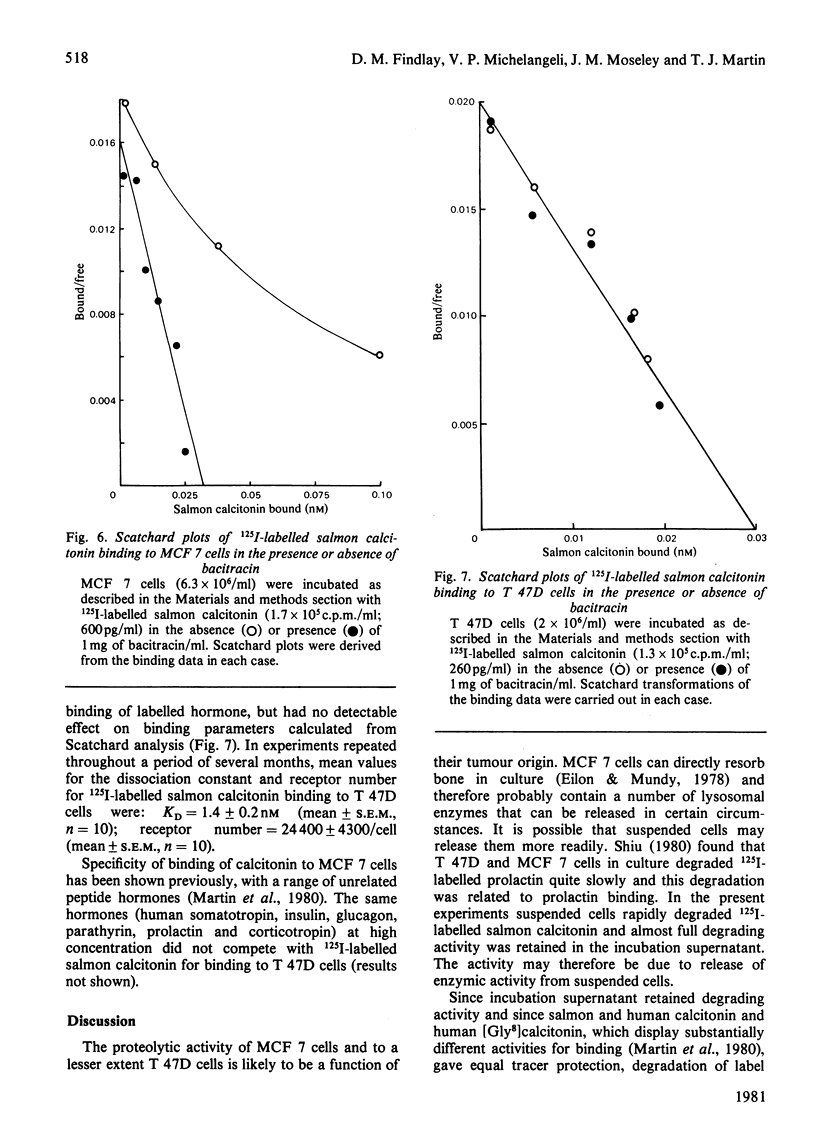
Two human breast cancer cell lines (MCF 7 and T 47D) possess calcitonin-responsive adenylate cyclase systems. Suspended cells of both lines specifically bound 125I-labelled salmon calcitonin with mean dissociation constants of 1.7 nM (MCF 7) and 1.4 nM (T 47D); mean receptor numbers were 5300 and 24400 per cell respectively. Measurement of specific binding to MCF 7 cells was obscured by rapid and substantial degradation of the labelled hormone. Degradation of 125I-labelled salmon calcitonin: (i) was of high capacity; (ii) lacked the specificity displayed by 125I-labelled salmon calcitonin binding to the same cells; and (iii) was not related to binding since cell incubation supernatants retained full degrading activity. The degrading activity was inhibited by corticotropin (1-24)-tetracosapeptide, insulin and bacitracin. Inclusion of bacitracin in the incubation resulted in apparently fewer numbers of lower affinity receptors on MCF 7 cells, whereas these parameters were identical to T 47D cells incubated in the presence or absence of bacitracin. Eel [2-aminosuberic acid 1,7]-calcitonin was resistant to proteolysis in the presence of either cell line. Analysis of hormone-receptor interactions with calcitonin-responsive cells should take account of potent calcitonin-degrading activities in some cell lines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Growth of a human mammary tumour cell line in a serum-free medium. Nature. 1979 Oct 4;281(5730):388–389. doi: 10.1038/281388a0. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Krug F., Cuatrecasas P. Inhibitors of glucagon inactivation. Effect on glucagon--receptor interactions and glucagon-stimulated adenylate cyclase activity in liver cell membranes. Biochim Biophys Acta. 1974 Mar 20;343(1):101–120. doi: 10.1016/0304-4165(74)90242-6. [DOI] [PubMed] [Google Scholar]
- Eilon G., Mundy G. R. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978 Dec 14;276(5689):726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Martin T. J., MacIntyre I., Moseley J. M. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979 Dec 22;2(8156-8157):1335–1336. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Eisman J. A., Frampton R. J., Moseley J. M., MacIntyre I., Whitehead R., Martin T. J. Calcitonin and 1,25-dihydroxyvitamin D3 receptors in human breast cancer cell lines. Cancer Res. 1980 Dec;40(12):4764–4767. [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Ellison M., Martin T. J. Properties of a calcitonin receptor and adenylate cyclase in BEN cells, a human cancer cell line. Cancer Res. 1980 Apr;40(4):1311–1317. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Zava D. T., Thilagar A. K., Jensen E. M., McGuire W. L. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978 Aug;38(8):2434–2437. [PubMed] [Google Scholar]
- Hunt N. H., Ellison M., Underwood J. C., Martin T. J. Calcitonin-responsive adenylate cyclase in a calcitonin-producing human cancer cell line. Br J Cancer. 1977 Jun;35(6):777–784. doi: 10.1038/bjc.1977.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N. H., Martin T. J., Michelangeli V. P., Eisman J. A. Effect of guanyl nucleotides on parathyroid hormone-responsive adenylate cyclase in chick kidney. J Endocrinol. 1976 Jun;69(3):401–412. [PubMed] [Google Scholar]
- Loreau N., Lajotte C., Wahbe F., Ardaillou R. Effects of guanyl nucleotides on calcitonin-sensitive adenylate cyclase and calcitonin binding in rat renal cortex. J Endocrinol. 1978 Mar;76(3):533–545. doi: 10.1677/joe.0.0760533. [DOI] [PubMed] [Google Scholar]
- Maier R., Kamber B., Riniker B., Rittel W. Analogues of human calcitonin. II. Influence of modifications in amino acid positions 1, 8 and 22 on hypocalcemic activity in the rat. Horm Metab Res. 1975 Nov;7(6):511–514. doi: 10.1055/s-0028-1093715. [DOI] [PubMed] [Google Scholar]
- Martin T. J., Findlay D. M., MacIntyre I., Eisman J. A., Michelangeli V. P., Moseley J. M., Partridge N. C. Calcitonin receptors in a cloned human breast cancer cell line (MCF 7). Biochem Biophys Res Commun. 1980 Sep 16;96(1):150–156. doi: 10.1016/0006-291x(80)91193-6. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Fedak S. A., Aurbach G. D. Preparation and characterization of a hormone-responsive renal plasma membrane fraction. J Biol Chem. 1972 Nov 10;247(21):6913–6918. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Woodward C., Aurbach G. D., Glossmann H., Keutmann H. T. Renal receptors for calcitonin. Binding and degradation of hormone. J Biol Chem. 1973 Jul 10;248(13):4797–4802. [PubMed] [Google Scholar]
- Moran J., Hunziker W., Fischer J. A. Calcitonin and calcium ionophores: cyclic AMP responses in cells of a human lymphoid line. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3984–3988. doi: 10.1073/pnas.75.8.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C. K., Hamilton B., Titus G., Livingston R. B. Epidermal growth factor stimulation of human breast cancer cells in culture. Cancer Res. 1980 Jul;40(7):2361–2366. [PubMed] [Google Scholar]
- Osborne C. K., Monaco M. E., Lippman M. E., Kahn C. R. Correlation among insulin binding, degradation, and biological activity in human breast cancer cells in long-term tissue culture. Cancer Res. 1978 Jan;38(1):94–102. [PubMed] [Google Scholar]
- Shafie S., Brooks S. C. Effect of prolactin on growth and the estrogen receptor level of human breast cancer cells (MCF-7). Cancer Res. 1977 Mar;37(3):792–799. [PubMed] [Google Scholar]
- Shiu R. P. Processing of prolactin by human breast cancer cells in long term tissue culture. J Biol Chem. 1980 May 10;255(9):4278–4281. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Wright D. R., Ivey J. L., Pont A. Calcitonin binding sites in bone: relationships to biological response and "escape". Recent Prog Horm Res. 1978;34:285–334. doi: 10.1016/b978-0-12-571134-0.50012-0. [DOI] [PubMed] [Google Scholar]
- Tonelli Q. J., Sorof S. Epidermal growth factor requirement for development of cultured mammary gland. Nature. 1980 May 22;285(5762):250–252. doi: 10.1038/285250a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi H., Shiraki M., Otani M., Matsuo M., Orimo H. Stability of [Asu1,7]-eel calcitonin and eel calcitonin in vitro and in vivo. Endocrinol Jpn. 1977 Jun;24(3):281–285. doi: 10.1507/endocrj1954.24.281. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Guzman R., Imagawa W., Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]


