Introduction
Ozanimod is an oral selective sphingosine 1-phosphate (S1P) receptor modulator that is approved in multiple countries for the treatment of adults with moderately to severely active ulcerative colitis (UC) and relapsing multiple sclerosis (RMS).1,2 Ozanimod is also currently being studied in Crohn’s disease (CD).3 S1P receptors are involved in regulating events during embryogenesis, such as angiogenesis, cardiogenesis, limb development, and neurogenesis.4 Currently, there are no adequate and well-controlled clinical studies on developmental risks associated with the use of ozanimod in pregnant women.1 However, animal studies have shown that administration of S1P receptor modulators, including ozanimod at doses resulting in clinically relevant exposures, during pregnancy resulted in adverse developmental effects, such as embryolethality, fetal malformations and skeletal variations, and neurobehavioral changes.1,5-7 Based on these findings, the prescribing information for all S1P receptor modulators contains general statements about potential fetal risk and recommendations for effective contraception use.1,5-7 This analysis evaluated pregnancy outcomes in the ozanimod clinical development program in patients with UC, CD, or RMS and in healthy volunteers.
Methods
Patients with UC, CD, or RMS and healthy volunteers who received ozanimod from all controlled and uncontrolled phase 1, 2, and 3 studies in the ozanimod clinical development program were followed prospectively in a clinical trial setting. These studies included 1 phase 2 (NCT01647516) and 2 phase 3 (NCT02435992 and NCT02531126) trials in UC; 1 phase 2 (NCT02531113) and 3 phase 3 (NCT03440372, NCT03464097, and NCT03467958) trials in CD; 1 phase 1 (NCT02797015), 1 phase 2/3 (NCT01628393), and 3 phase 3 (NCT02047734, NCT02294058, and NCT02576717) trials in RMS; and 2 phase 1 studies in healthy volunteers (RPC01-102, NCT04978298). These studies were conducted in 50 countries in North America (United States, Canada, Mexico), South America (Argentina, Chile, Colombia), Europe (Austria, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Moldova, the Netherlands, Poland, Portugal, Romania, Russia, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, United Kingdom, Ukraine), Asia-Pacific (Australia, China, India, Israel, New Zealand, South Korea, Saudi Arabia, Taiwan, Turkey), and Africa (Senegal, South Africa). All study protocols were reviewed and approved by the institutional review board at each study site. All patients provided written informed consent before study entry.
In all studies, female patients of reproductive potential were required to use effective contraception while receiving ozanimod and for up to 3 months after discontinuing ozanimod. Pregnancy testing occurred in female patients at screening (with serum beta-human chorionic gonadotropin test), at each study visit (with urine beta-human chorionic gonadotropin test), and monthly between study visits (with home urine test; serum test was performed during follow-up appointment for confirmation, if needed). Female patients were required to discontinue ozanimod if their pregnancy was confirmed unless they elected to terminate the pregnancy, in which case they were permitted to restart ozanimod. Male patients were to notify the investigator if their female partners became pregnant.
Patient and partner pregnancy outcomes were assessed through November 19, 2022. Outcomes were summarized descriptively and included live birth with or without congenital abnormality, premature birth, spontaneous abortion, elective termination, ongoing pregnancy, or no information despite follow-up.
Results
Patient Pregnancy Outcomes
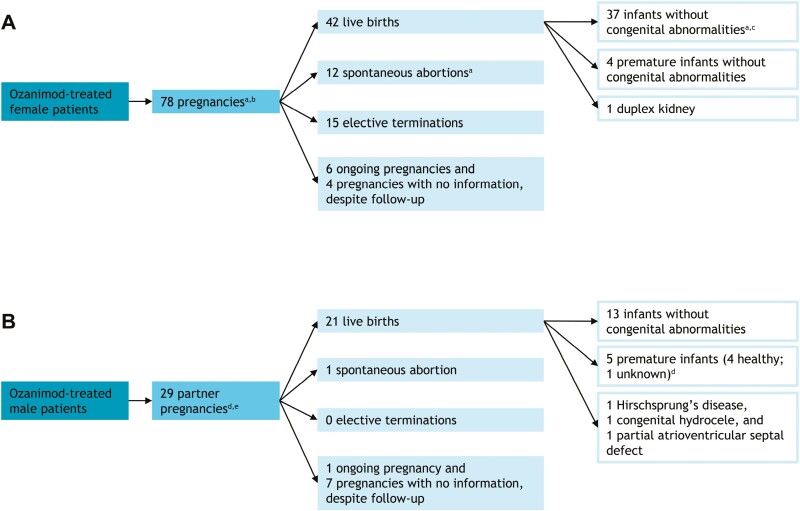
Of the 6057 patients with UC (n = 1271), CD (n = 954), or RMS (n = 2973) and healthy volunteers (n = 859) who received ozanimod, 78 pregnancies occurred; the median patient age was 29 years (Figure 1A). Among the 42 live births, 4 were premature (10% of live births) and 1 had a congenital abnormality (duplex kidney). There were 12 spontaneous abortions (15% of pregnancies) and 15 elective terminations (none due to known congenital abnormalities). The average duration of pregnancy until discontinuation of ozanimod was 5 to 6 weeks; in cases of spontaneous abortion in which dates were available, ozanimod had been discontinued within 7 weeks of the last menstrual period. Six pregnancies were ongoing and 4 pregnancies were without information despite follow-up.
Figure 1.
Pregnancy outcomes during the ozanimod clinical development program. (A) Patient pregnancy outcomes. (B) Partner pregnancy outcomes. aSeventy-nine outcomes resulted from 78 pregnancies because a twin pregnancy led to 1 live birth without congenital abnormality and 1 spontaneous abortion (ie, vanishing twin). bSome of the CD studies are still blinded, so it is unknown whether 3 patients received ozanimod or placebo. cIncludes 1 late intrauterine growth retardation with subsequent normal progress. dThirty outcomes resulted from 29 pregnancies because of a twin pregnancy that led to 2 premature births. eOne partner pregnancy was from a Crohns’ disease study, which is still blinded; it is unknown whether this patient received ozanimod or placebo.
There were 14 pregnancies in patients with UC (Table 1). There were no premature births or congenital abnormalities among the 7 live births, and other outcomes included 3 spontaneous abortions and 4 elective terminations. There were 6 pregnancies in patients with CD (Table 1). There were no premature births or congenital abnormalities among the 2 live births, and other outcomes included 1 spontaneous abortion. One pregnancy was ongoing and 2 were without information despite follow-up. There were 57 pregnancies in patients with RMS (Table 1). There were 4 premature births, 1 birth with late intrauterine growth retardation but subsequent normal progress, and 1 congenital abnormality (duplex kidney) among the 33 live births, and other outcomes included 8 spontaneous abortions and 10 elective terminations. Five pregnancies were ongoing and 2 were without information despite follow-up. The 57 pregnancies resulted in 58 outcomes due to a twin pregnancy; the outcome of that pregnancy was 1 live birth without congenital abnormality and 1 spontaneous abortion (ie, vanishing twin). There was 1 pregnancy in a healthy volunteer (Table 1); the outcome of that pregnancy was elective termination.
Table 1.
Patient pregnancy outcomes during the ozanimod clinical development program by population.
| UC | CDa | RMS | Healthy volunteer | Total | |
|---|---|---|---|---|---|
| Pregnancies | 14 | 6 | 57b | 1 | 78 |
| Live birth without congenital abnormality | 7 | 2 | 28b,c | 0 | 37 |
| Live birth with congenital abnormality | 0 | 0 | 1d | 0 | 1 |
| Premature live birth | 0 | 0 | 4 | 0 | 4 |
| Ongoing | 0 | 1 | 5 | 0 | 6 |
| Spontaneous abortion | 3 | 1 | 8b | 0 | 12 |
| Elective termination | 4 | 0 | 10 | 1 | 15 |
| No information | 0 | 2 | 2 | 0 | 4 |
Abbreviations: CD, Crohn’s disease; RMS, relapsing multiple sclerosis; UC, ulcerative colitis.
aSome of the CD studies are still blinded, so it is unknown whether 3 patients received ozanimod or placebo.
bFifty-eight outcomes resulted from 57 pregnancies because a twin pregnancy led to 1 live birth without congenital abnormality and 1 spontaneous abortion (ie, vanishing twin).
cIncludes 1 late intrauterine growth retardation with subsequent normal progress.
dThe congenital abnormality was a duplex kidney.
All exposures to ozanimod during pregnancy occurred during the first trimester. All patients discontinued study medication promptly after pregnancy was confirmed, except for those who elected pregnancy termination and remained on study medication.
Partner Pregnancy Outcomes
In partners of patients with UC, CD, or RMS and healthy volunteers who received ozanimod, 29 pregnancies were reported (Figure 1B). Among the 21 live births, 5 were premature and 3 had a congenital abnormality (Hirschsprung’s disease, congenital hydrocele, and partial atrioventricular septal defect). The congenital abnormalities were not suspected to be related to ozanimod. There was 1 spontaneous abortion and no elective terminations reported. One pregnancy was ongoing and 7 pregnancies were without information despite follow-up. The 29 pregnancies resulted in 30 outcomes due to a twin pregnancy; the outcome of that pregnancy was 2 premature births.
Discussion
In patients in the ozanimod clinical development program who had ozanimod exposure during early pregnancy, incidences of spontaneous abortion, preterm birth, and congenital abnormalities were comparable to the expected ranges within the general population.8-10 Pregnancy numbers were higher in patients with RMS compared with those with UC and CD. This is likely due to greater numbers of patients treated with ozanimod and longer study durations in RMS. Additionally, while several congenital abnormalities were reported in pregnancies of partners of patients in the ozanimod clinical development program, none were suspected to be related to ozanimod.
As recommended in the ozanimod prescribing information, pregnancy should be avoided when taking ozanimod and for 3 months after discontinuing ozanimod to allow for drug elimination.1 However, while clinical experience with ozanimod during pregnancy is limited, this analysis in a small cohort of patients from the ozanimod clinical development program, including those with inflammatory bowel disease and multiple sclerosis, demonstrated no increased incidence of fetal abnormalities or adverse pregnancy outcomes with ozanimod exposure during early pregnancy. These findings were observed despite evidence that active inflammatory bowel disease increases the risk of adverse pregnancy outcomes.11 It is possible that disease was controlled with ozanimod at the time of pregnancy, but future analyses are needed to confirm this interpretation. It is important to note that no study participant was exposed to ozanimod after the first trimester. Thus, the safety of ozanimod later in pregnancy remains unclear.
There did not appear to be a reproductive risk in partners of patients treated with ozanimod in this analysis. However, the sample size was small, and it is possible that partner pregnancies were underreported, as some partners of patients in the ozanimod clinical development program declined to give consent. Therefore, this analysis may not accurately represent pregnancy outcomes in partners of patients treated with ozanimod, although partner exposure to ozanimod is predicted to be negligible.
Future studies in larger cohorts will further examine pregnancy outcomes in patients treated with ozanimod and in partners of those treated with ozanimod. Studies analyzing pregnancy outcomes of patients exposed to ozanimod later in pregnancy are also needed to provide a more complete profile of ozanimod safety during pregnancy. There is currently a registry that monitors pregnancy outcomes in patients with multiple sclerosis exposed to ozanimod during pregnancy; a similar registry for patients with UC is planned.1
Acknowledgments
The authors thank the patients who participated in these clinical trials. The authors also thank Avideh Bahri, MPH, BS, of Bristol Myers Squibb, for her contributions in maintaining and updating the database used in this analysis. Writing and editorial assistance was provided by Anny Wu, PharmD, of Peloton Advantage, LLC, an OPEN Health company.
Contributor Information
Marla C Dubinsky, Susan and Leonard Feinstein Inflammatory Bowel Disease Clinical Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Lorna Charles, Bristol Myers Squibb, Princeton, NJ, USA.
Krzysztof W Selmaj, Center for Neurology, Lodz, Poland; Department of Neurology, Collegium Medicum, University of Warmia and Mazury, Olsztyn, Poland.
Giancarlo Comi, Department of Neurorehabilitation Sciences, Vita-Salute San Raffaele University and Casa di Cura Igea, Milan, Italy.
Anthony Krakovich, Bristol Myers Squibb, Princeton, NJ, USA.
Melissa Rosen, Bristol Myers Squibb, Princeton, NJ, USA.
C Janneke van der Woude, Department of Gastroenterology and Hepatology, Erasmus University Medical Center, Rotterdam, Netherlands.
Uma Mahadevan, Gastroenterology Division, Department of Medicine, University of California, San Francisco, San Francisco, CA, USA.
Funding
These clinical trials were sponsored by Bristol Myers Squibb (Princeton, NJ, USA).
Conflicts of Interest
M.C.D. has served as a consultant for AbbVie, Abivax, Arena, AstraZeneca, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech, Gilead, Janssen, Pfizer, Prometheus Labs, and Takeda. L.C., A.K., and M.R. are employees and/or shareholders of Bristol Myers Squibb. K.W.S. has served as a consultant for Biogen, Celgene, Genzyme, Merck, Novartis, Ono Pharma, Roche, Synthon, and Teva. G.C. has received compensation for consulting and/or speaking activities from Almirall, Biogen, Celgene, EXCEMED, Forward Pharma, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. C.J.v.d.W. has received grants and/or fees for advisory boards and presentations from AbbVie, Celltrion, Dr. Falk Pharma Benelux, Ferring, Galapagos, Janssen, Pfizer, and Takeda. U.M. has served as a consultant for AbbVie, Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Eli Lilly, Gilead, Janssen, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, and Takeda.
Data Availability
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1. Zeposia [Package Insert]. Bristol Myers Squibb; 2023. [Google Scholar]
- 2. Zeposia [Summary of Product Characteristics]. Celgene Distribution B.V.; 2023. [Google Scholar]
- 3. Feagan BG, Sandborn WJ, Danese S, et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5(9):819-828. [DOI] [PubMed] [Google Scholar]
- 4. Mendelson K, Evans T, Hla T.. Sphingosine 1-phosphate signalling. Development. 2014;141(1):5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilenya [Package Insert]. Novartis; 2023. [Google Scholar]
- 6. Mayzent [Package Insert]. Novartis Pharmaceuticals Corporation; 2023. [Google Scholar]
- 7. Ponvory [Package Insert]. Janssen Pharmaceuticals, Inc.; 2023. [Google Scholar]
- 8. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37-e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Privett JT, Jeans WD, Roylance J.. The incidence and importance of renal duplication. Clin Radiol. 1976;27(4):521-530. [DOI] [PubMed] [Google Scholar]
- 10. Rossen LM, Ahrens KA, Branum AM.. Trends in risk of pregnancy loss among US women, 1990-2011. Paediatr Perinat Epidemiol. 2018;32(1):19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akiyama S, Steinberg JM, Kobayashi M, Suzuki H, Tsuchiya K.. Pregnancy and medications for inflammatory bowel disease: an updated narrative review. World J Clin Cases. 2023;11(8):1730-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.