Abstract
Background
Antibiotics are a cornerstone in management of intra-abdominal abscesses in Crohn’s disease (CD). Yet, the optimal route of antibiotic administration is poorly studied. We aimed to compare surgical and nonsurgical readmission outcomes for patients hospitalized for intra-abdominal abscesses from CD discharged on oral (PO) or intravenous (IV) antibiotics.
Methods
Data for patients with CD hospitalized for an intra-abdominal abscess were obtained from 3 institutions from January 2010 to December 2020. Baseline patient characteristics were obtained. Primary outcomes of interest included need for surgery and hospital readmission within 1 year from hospital discharge. We used multivariable logistic regression models and Cox regression analysis to adjust for abscess size, history of prior surgery, history of penetrating disease, and age.
Results
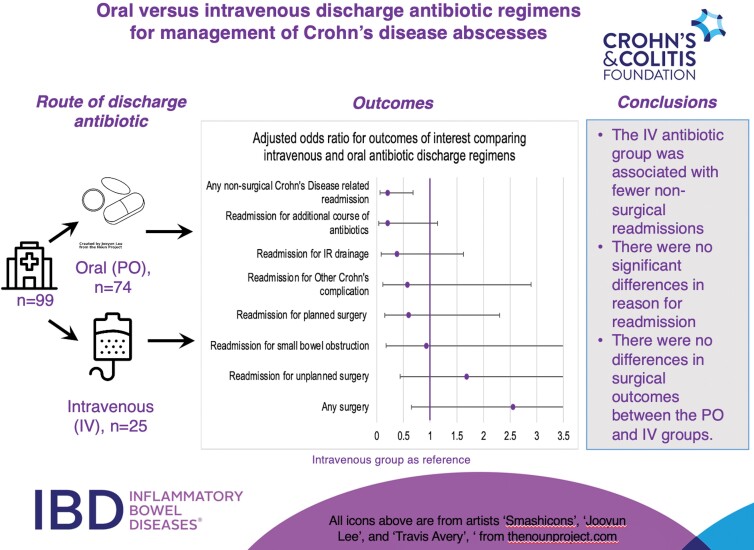
We identified 99 patients discharged on antibiotics (PO = 74, IV = 25). Readmissions related to CD at 12 months were less likely in the IV group (40% vs 77% PO, P = .01), with the IV group demonstrating a decreased risk for nonsurgical readmissions over time (hazard ratio, 0.376; 95% confidence interval, 0.176-0.802). Requirement for surgery was similar between the groups. There were no differences in time to surgery between groups.
Conclusions
In this retrospective, multicenter cohort of CD patients with intra-abdominal abscess, surgical outcomes were similar between patients receiving PO vs IV antibiotics at discharge. Patients treated with IV antibiotics demonstrated a decreased risk for nonsurgical readmission. Further prospective trials are needed to better delineate optimal route of antibiotic administration in patients with penetrating CD.
Keywords: intra-abdominal abscesses, Crohn’s disease, antibiotics
Graphical Abstract
Graphical Abstract.
Key Messages.
What is already known:
Intra-abdominal abscesses are a common presentation of penetrating Crohn’s disease.
The optimal route of discharge antibiotic administration in intra-abdominal abscesses is poorly described.
What is new here:
Those who receive oral antibiotics at discharge are more likely to be readmitted for nonsurgical Crohn’s disease-related admissions.
There are similar overall rates of surgery between those treated with oral and intravenous antibiotics.
How can this study help patient care:
For patients hospitalized with intra-abdominal abscesses secondary to Crohn’s disease, an oral discharge antibiotic regimen may be a safe option with similar outcomes to intravenous regimens. Outcomes of readmission for surgery, abscess drainage, small bowel obstruction, and other Crohn’s complications were similar between the 2 groups. However, those discharged on an oral antibiotic regimen were more likely to be readmitted for nonsurgical reasons overall.
Introduction
One-fifth of patients with penetrating Crohn’s disease (CD) present with intra-abdominal abscesses; however, optimal management remains controversial and understudied.1,2 Historically, optimal therapy was surgical management, but recent data reveal similar abscess recurrence rates between surgical and nonsurgical management.3–5
The use of antibiotics in intra-abdominal abscesses secondary to CD has also been poorly studied. Data from single-center retrospective studies have shown that antibiotics alone can be efficacious in preventing surgery in over half of abscess cases.6,7 The majority of clinical practice decisions regarding route of antibiotics for Crohn’s abscess treatment remains based on clinician instinct, with little to no data guiding use of oral (PO) vs intravenous (IV) antibiotic regimens in intra-abdominal abscesses secondary to CD.5
In patients without CD, data suggest that PO antibiotics or transition to PO from IV antibiotics can be effective in treating complicated intra-abdominal infections, diverticulitis, and appendicitis.8–13 Long-term IV antibiotics require indwelling catheters, which can have complications and worsen quality of life.14,15 Additionally, IV antibiotics are associated with increased costs.16,17 Given the patient-centered and systems-based benefits of PO antibiotics, it is critical to understand whether PO antibiotics are an effective option in treatment of intra-abdominal abscesses secondary to CD. In this retrospective, multicenter study, we compared the outcomes of surgery and nonsurgical readmission in patients with CD hospitalized with an intra-abdominal abscess based on route of antibiotics (PO vs IV) at discharge.
Materials and Methods
Study Design
This is a multicenter, retrospective cohort study of patients from NYU Langone Health, Massachusetts General Hospital (MGH), and the University of North Carolina at Chapel Hill. Patients aged 18 years and older with a diagnosis of CD and presence of intra-abdominal abscess were identified from radiology reports from January 2010 to December 2020 at each institution. Manual review by a clinician was performed to ensure diagnosis of CD and presence of intra-abdominal abscess on radiology report. Patients hospitalized primarily for Crohn’s intra-abdominal abscess were included. Exclusion criteria included prior history of intra-abdominal abscess, reason for admission other than intra-abdominal abscess, abscess as a complication of surgery, perianal abscess without concurrent abdominal abscesses, outpatient antibiotic treatment for abscess prior to hospitalization, surgery during index hospitalization prior to discharge, insufficient clinical records, or abscess secondary to alternative pathology (such as diverticulitis; Supplementary Figure 1). To ensure abscesses that could be attributed to postoperative complications were not included, we excluded any patient with abdominal surgery within 1 year of abscess diagnosis.
Patients were grouped into PO or IV antibiotic groups based on route of antibiotic administration at discharge from index abscess hospitalization.
Patient Characteristics
Baseline variables collected included age, sex, duration of CD, smoking history, history of prior bowel surgery for CD, history of penetrating disease prior to index abscess admission (prior abdominal/pelvic fistula or phlegmon), and history of perianal disease.
Clinical information at time of abscess diagnosis included abscess imaging data, size (0-3 cm, 3-6 cm, >6 cm), location, presence of multiple abscesses, CD-specific therapy within 3 months of abscess diagnosis (steroids, immunomodulators, class of biologic therapy), and clinical symptoms at abscess presentation. Clinical symptoms at time of abscess diagnosis were determined by chart review of clinical notes.
Hospitalization variables collected included evidence of drug microbiology data with drug-resistant organism (defined by resistance to at least 1 antibiotic on any culture data), laboratory data closest to discharge (C-reactive protein [CRP], hemoglobin, albumin, white blood cells [WBCs], and absolute neutrophil count [ANC]), length of hospitalization, route and class of inpatient antibiotics, repeat abscess imaging, and requirement for abscess drainage (laparoscopic or radiologic approach) during index abscess hospitalization.
Postdischarge variables collected included route and class of antibiotics on discharge from index abscess hospitalization, requirement for bowel resection or diversion surgery within 1 year of discharge, time to surgery, therapies for CD after discharge and prior to surgery, nonsurgical readmission data for 1 year after discharge (time to first readmission, reason for readmission), and length of follow-up. Reasons for nonsurgical readmission for CD included readmission for additional course of antibiotics, interventional radiology (IR) drainage, small bowel obstruction, or others such as peripherally inserted central venous catheter (PICC) line complications, IR drain site complications, persistent symptoms, readmission for IV steroids, or readmission for CD flare. Surgical requirement was further subdivided into planned or unplanned surgery; planned surgery was defined as the patient having a surgical consultation prior to surgery with direct admission for surgery.
Outcomes
The 2 coprimary outcomes of interest were nonsurgical hospital readmission for CD-related complication and need for surgery within 1 year of discharge from index hospitalization. Secondary outcomes evaluated included reason for readmission, defined as admission for additional course of antibiotics, IR drainage, small bowel obstruction, or other CD-related complication. Abscess recurrence, defined as redemonstration of abscess after initial resolution on repeat imaging, and type of surgery, categorized as planned or unplanned surgery, were additionally evaluated. Two additional analyses of outcomes were performed stratified by abscess size and need to switch antibiotic class at discharge in the oral antibiotic group.
Statistical Methods
Oral and IV groups were compared using χ2 and Mann-Whitney U tests for categorical and continuous variables, respectively. Logistic regression was performed to determine odds ratios (ORs), 95% confidence intervals (CIs), and P values for outcomes. Variables to include in the models were selected a priori based on clinical relevance and variables of significance on univariate analysis. The models were adjusted for abscess size >6 cm, history of prior surgery, history of penetrating disease, WBC closest to discharge, microbiologic evidence of antibiotic resistance, and age. Additional models were performed as sensitivity analyses, including length of hospitalization and use of biologics after discharge. Outcomes of interest were analyzed individually using logistic regression analyses. Cox regression analysis was performed to estimate hazard ratios (HRs) and 95% CI, for time to first readmission and time to surgery for the PO and IV groups, adjusting for the same variables as noted previously. A P value of < .05 was considered statistically significant. All analyses were conducted using SPSS software version 28 (IBM, Armonk, New York).
Institutional Review Board
Institutional review board (IRB) approval was obtained at each site with permissions for deidentified data transfer to the coordinating site (NYU Langone Health) for data analysis. The IRB number for the coordinating site was s20-01425.
Results
Cohort Characteristics
Ninety-nine individuals with CD and intra-abdominal abscess treated with antibiotics (PO, 74; IV, 25) were included (Table 1). The majority were men (60%), with a median age of 27 years (interquartile range [IQR], 17). Those treated with PO antibiotics were older (median age, PO = 28 years, IV = 22 years; P = <.01; Table 1). A higher proportion of patients treated with IV antibiotics had a history of penetrating CD prior to abscess diagnosis (PO = 18%, IV = 36%; P = .06), though this was not statistically significant. There was no significant difference between the groups with respect to history of prior CD-related surgery or perianal disease. The PO antibiotic group was more likely to have ileal disease alone, while the IV group was more likely to have ileocolonic disease. There were no statistically significant differences when comparing CD-specific medications prior to abscess diagnosis between the 2 groups. The most frequent clinical symptoms at the time of abscess diagnosis included abdominal pain, fever, nausea, and vomiting. Clinical symptoms (Supplementary Table 1) were similar between the 2 groups, with abdominal pain being the primary complaint (PO = 92%, IV = 100%; P = .18).
Table 1.
Characteristics of patients with Crohn’s disease hospitalized for intra-abdominal abscess.
| Total (n = 99) | PO Antibiotics (n = 74) | IV Antibiotics (n = 25) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (Q1-Q3) | 27 (21-38) | 28 (18) | 22 (8) | <.01 |
| Female Sex, n (%) | 40 (40) | 33 (45) | 7 (28) | .14 |
| Median duration of Crohn’s Disease, years (Q1-Q3) | 4 (0-13) | 2 (15) | 4 (9) | .69 |
| Current Smoker, n (%) | 16 (16) | 5 (7) | 1 (4) | .65 |
| Medical History | ||||
| Previous history of CD-related surgery, n (%) | 21 (21) | 17 (23) | 4 (16) | .46 |
| History of penetrating Crohn’s Disease, n (%) | 22 (22) | 13 (18) | 9 (36) | .06 |
| History of perianal Crohn’s Disease, n (%) | 18 (18) | 14 (19) | 4 (16) | .74 |
| Montreal classification of active disease at abscess diagnosis, n (%) | ||||
| L1 | 35 (35) | 31 (41) | 4 (16) | .02 |
| L2 | 5 (5) | 5 (7) | 0 (0) | .18 |
| L3 | 59 (60) | 38 (51) | 21 (84) | <.01 |
| Medications prior to abscess diagnosis, n (%) | ||||
| Steroids | 45 (46) | 31 (42) | 14 (56) | .22 |
| 5-aminosalicylic acid | 19 (19) | 12 (16) | 7 (28) | .19 |
| Immunomodulators | 18 (18) | 12 (16) | 6 (24) | .38 |
| Anti-Tumor Necrosis Factor (TNF) | 20 (20) | 13 (17) | 7 (28) | .23 |
| Combination Anti-TNF/immunomodulator | 5 (5) | 3 (4) | 2 (8) | .44 |
| Anti-Integrin | 3 (3) | 3 (4) | 0 (0) | .31 |
| Anti-Interleukin 12/23 | 5 (5) | 2 (3) | 3 (12) | .07 |
| Abscess Characteristics | ||||
| Abscess Size, n (%) | .59 | |||
| 0-3 cm | 34 (37) | 24 (34) | 10 (44) | |
| 3-6 cm | 45 (48) | 36 (51) | 9 (39) | |
| >6 cm | 14 (15) | 10 (14) | 4 (17) | |
| Abscess Location, n (%) | .47 | |||
| Ileum | 67 (68) | 49 (66) | 18 (72) | |
| Other small bowel | 13 (13) | 10 (14) | 3 (12) | |
| Large Bowel | 11 (11) | 7 (10) | 4 (16) | |
| Ileum and large bowel | 7 (7) | 7 (10) | 0 (0) |
Abscess Characteristics
Clinical characteristics, including abscess characteristics and Crohn’s disease history, are shown in Table 1. There were no significant differences in abscess characteristics when comparing presence of multiple abscesses, size, or location between the 2 groups.
Hospitalization
There were no significant differences between the groups regarding length of stay, IR drainage, or requirement for laparoscopic drainage during initial hospitalization (Table 2). Two patients underwent laparoscopic drainage in the NYU cohort, while 1 patient underwent laparoscopic drainage from the MGH cohort. Total median length of stay was 4 days (IQR = 3 days). Repeat imaging during index hospitalization was performed in 15 cases (15%), with no significant difference in prevalence of imaging between the PO and IV groups. For those with repeat imaging, there was a significant decrease in average size of abscess (4.8 cm on initial imaging, 3.4 cm on repeat imaging; P < .01).
Table 2.
Variables of interest for patients with Crohn’s disease hospitalized for intra-abdominal abscess.
| Total (n = 99) | PO Antibiotics (n = 74) | IV Antibiotics (n = 25) | P | |
|---|---|---|---|---|
| Median lab values closest to discharge | ||||
| CRP, mg/L (Q1-Q3) | 51.0 (17.9-84.1 | 34.0 (19.0-84.1) | 59.0 (24.0-114.3) | .14 |
| Hemoglobin, g/dL (Q1-Q3) | 10.5 (9.2-12.3) | 10.2 (9.0-11.8) | 11 (8.9-12.3) | .91 |
| Albumin, g/dL (Q1-Q3) | 3.3 (3.1-3.9) | 3.5 (3.0-4.0) | 3.3 (3.0-4.0) | .81 |
| White Blood Cell Count, cells × 109/L (Q1-Q3) | 7.8 (6.2-9.6) | 7.0 (5.4-8.3) | 7.8 (5.8-9.2) | .03 |
| Absolute Neutrophil Count, cells × 104/μl (Q1-Q3) | 5.7 (4.2-7.6) | 5.6 (3.5-6.3) | 5.3 (4.0-6.3) | .06 |
| Median length of hospitalization, days (Q1-Q3) | 4 (3-6) | 4 (3-5) | 5 (4-7) | .19 |
| IR drainage performed during hospitalization, n (%) | 42 (42) | 31 (42) | 11 (44) | .85 |
| Laparoscopic drainage performed during hospitalization, n (%) | 3 (3) | 3 (4) | 0 (0) | .31 |
| Repeat imaging performed during hospitalization, n (%) | 15 (15) | 11 (15) | 4 (16) | .89 |
| Evidence of microbiology data with drug-resistant organism, n (%) | 11 (11) | 5 (7) | 6 (24) | .02 |
There were no significant differences between groups when comparing median albumin, CRP, ANC, and hemoglobin levels at discharge (Table 2). Both groups had elevated CRP levels at discharge compared with a normal range of less than 10 mg/L (PO = 34.0 mg/L, IV = 59.0 mg/L; P = .14). The PO group had significantly lower median WBC values at discharge compared with the IV group (PO = 7.0, IV = 7.8; P = .03). Those in the IV group had higher rates of antibiotic-resistant organisms than the PO group (PO 7%, IV 24%; P = .02)
Surgery and Readmissions
The primary outcomes addressed in our study were nonsurgical readmission or surgery requirement within 1 year of discharge from index abscess hospitalization (Table 3). Those discharged on IV antibiotics were less likely to be readmitted for nonsurgical, CD-related readmissions (OR, 0.205; 95% CI, 0.062-0.679) compared with the PO group. On subgroup analysis for reasons for readmission, there was a trend towards fewer readmissions for an additional course of antibiotics in the IV group compared with the PO antibiotic group, but this did not reach statistical significance (PO = 24%, IV = 8%; OR, 0.203; 95% CI, 0.036-1.137). There were no other differences in reasons for readmission between the 2 groups. The majority of patients in both groups required surgery within 12 months of discharge (PO = 61%, IV = 76%), but there was no significant difference between planned, unplanned, or combined planned and unplanned surgery between the groups on univariate or multivariable analysis.
Table 3.
Postdischarge outcomes of interest for patients with Crohn’s disease hospitalized for intra-abdominal abscess stratified by route of discharge antibiotic.
| Univariate Analysis | Multivariable Analysisb | |||||
|---|---|---|---|---|---|---|
| PO Antibiotics (n = 74) | IV Antibiotics (n = 25) | OR (95% CI)d | P | OR (95% CI)c | P | |
| Abscess Recurrence (n, %) | 12 (16) | 5 (20) | 1.389 (0.432–4.469) | .58 | 1.901 (0.453–7.976 | .38 |
| Readmission for CD-related complication within 12 months (n, %) | 57 (77) | 10 (40) | 0.199 (0.076–0.523) | <.01 | 0.205 (0.062–0.679) | .01 |
| Reasons for nonsurgical CD-related readmission: | ||||||
| Readmission for additional course of antibiotics (n, %) | 18 (24) | 2 (8) | 0.271 (0.058–1.261) | .08 | 0.203 (0.036–1.137) | .07 |
| Readmission for IR drainage (n, %) | 19 (26) | 3 (12) | 0.395 (0.106–1.469) | .16 | 0.374 (0.086–1.627) | .19 |
| Readmission for small bowel obstruction (n, %) | 10 (14) | 3 (12) | 0.873 (0.220–3.463) | .94 | 0.93 (0.174–4.995) | .94 |
| Readmission for other Crohn’s complicationa (n, %) | 16 (22 | 4 (16) | 0.690 (0.207–2.302) | .55 | 0.574 (0.114–2.895) | .50 |
| Surgery (n, %) | 45 (61) | 19 (76) | 2.041 (0.729–5.714) | .17 | 2.550 (0.648–10.04) | .18 |
| Planned surgery (n, %) | 33 (45) | 12(48) | 0.623 (0.199–1.954) | .42 | 0.595 (0.154–2.302) | .45 |
| Unplanned surgery (n, %) | 12 (16) | 7 (28) | 1.604 (0.512–5.029) | .42 | 1.682 (0.434–6.512) | .45 |
| Readmission for postop complications (n, %) | 8 (11) | 2 (8) | 0.531 (1.01–2.793) | .45 | 0.661 (0.097–4.513) | .67 |
aDefined as admission for PICC line complication (ie, thrombosis, infection), IR drain site complication, persistent symptoms (poor oral intake, nausea, emesis, abdominal pain, etc), IV steroids, or Crohn’s disease flare.
bAdjusted for abscess size greater than 6 cm, history of prior surgery, history of penetrating disease, age, WBC on discharge, history of resistant strains.
cReference group is IV antibiotic group for analysis.
A minority of both groups had abscess recurrence within 12 months of discharge (PO = 16%, IV = 20%). Adjusting for age, abscess size >6 cm, history of prior CD-related surgery, WBC at discharge, history of antibiotic resistance, and history of penetrating disease, there was no significant difference in abscess recurrence when comparing PO to IV on multivariable analysis (OR, 1.901; 95% CI, 0.453-7.976). As an additional sensitivity analysis, an additional multivariable analysis was performed adding an additional variable of biologic use after discharge or length of stay with no significant change to any outcomes.
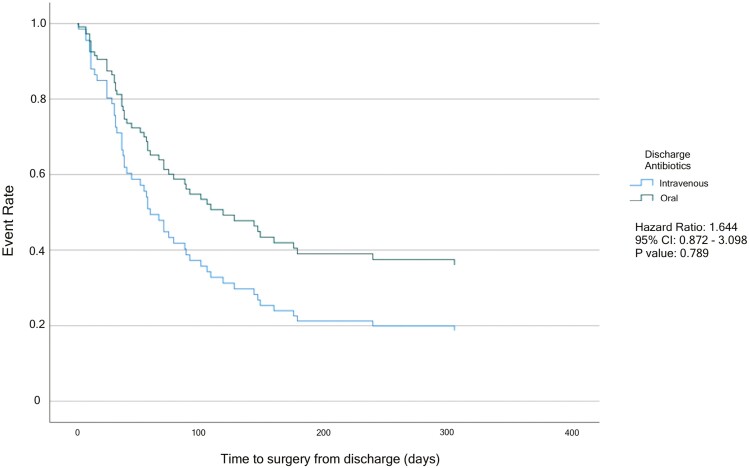
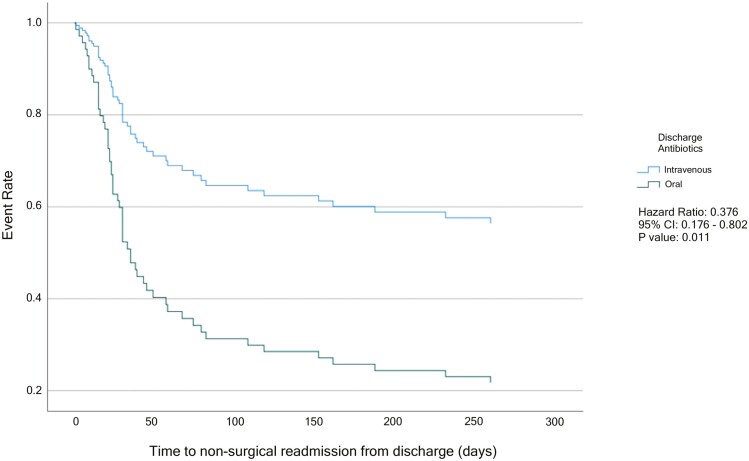
Cox regression models for time to surgery and time to readmission within 1 year postdischarge are shown in Figures 1 and 2. On multivariable analysis, there was no significant difference when comparing time to surgery between the PO and the IV group (HR, 1.644; 95% CI, 0.872-3.098; Figure 1). When analyzing time to first nonsurgical CD-related readmission on multivariable analysis, the risk for nonsurgical readmission was significantly decreased in the IV group when compared with the PO antibiotic group (HR, 0.376; 95% CI, 0.176-0.802; Figure 2).
Figure 1.
Multivariable Cox Regression for Time to Surgery from Discharge. Adjusted for abscess size greater than 6 cm, history of prior surgery, history of penetrating disease, age, WBC on discharge, and evidence of antibiotic resistance.
Figure 2.
Multivariable Cox Regression for Time to Readmission from Discharge. Adjusted for abscess size greater than 6 cm, history of prior surgery, history of penetrating disease, age, WBC on discharge, and evidence of antibiotic resistance.
When assessing outcomes stratified by abscess size alone (Supplementary Table 2), there were no significant differences in abscess recurrence, readmissions for any CD-related complication (including readmission for additional antibiotics, IR drainage, small bowel obstruction, or other), or surgery (including planned and unplanned). Of note, 6 patients did not have abscess size available. When comparing surgical requirement for those with abscesses >6 cm compared with those <6 cm, a higher percentage of those with abscesses >6 cm underwent surgery (86% vs 62%), though this trend was not statistically significant (P = .09).
Of note, amongst patients who underwent repeat abscess imaging during their hospitalization, 9 patients underwent surgery and 11 were readmitted for nonsurgical reasons.
Antibiotics
Assessment of antibiotic use during and after hospitalization is outlined in Table 4. During hospitalization, there were significant differences in fluoroquinolone (PO = 50%, IV 28%; P < .05), carbapenem (PO = 3%, IV = 24%; P < .01), and aminoglycoside (PO 0%, IV = 8.0%; P = .02) use between the 2 groups. The most commonly prescribed antibiotic during hospitalization was metronidazole (PO = 72%, IV = 52%). In the PO group, other commonly used inpatient antibiotics included fluoroquinolones (50%), penicillins with or without beta-lactamase inhibitors (30%), and cephalosporins (23%). In the IV group, other commonly prescribed inpatient classes of antibiotics included penicillins with or without beta-lactamase inhibitors (48%), cephalosporins (32%), fluoroquinolones (28%), and carbapenems (24%).
Table 4.
Medications during hospitalization and postdischarge for abdominal abscess.
| Total (n = 99) | PO Antibiotics (n = 74) | IV Antibiotics (n = 25) | P | |
|---|---|---|---|---|
| Hospitalization Antibiotics (n, %) | ||||
| Fluoroquinolones | 44 (44) | 37 (50) | 7 (28) | .05 |
| Metronidazole | 66 (67) | 53 (72) | 13 (52) | .06 |
| Penicillins +/- beta-lactamase inhibitors | 34 (34) | 22 (30) | 12 (48) | .11 |
| Cephalosporins | 25 (25) | 17 (23) | 8 (32) | .39 |
| Carbapenems | 8 (8) | 2 (3) | 6 (24) | <.01 |
| Lipopeptides | 0 (0) | 0 (0) | 0 (0) | |
| Glycopeptides | 7 (7) | 4 (5) | 3 (12) | .28 |
| Aminoglycosides | 2 (2) | 0 (0) | 2 (8) | .02 |
| Postdischarge Antibiotics (n, %) | ||||
| Fluoroquinolones | 51 (52) | 50 (68) | 1 (4) | <.01 |
| Metronidazole | 72 (73) | 59 (80) | 13 (52) | <.01 |
| Lincosamides | 3 (3) | 3 (3) | 0 (0) | .31 |
| Pencillins +/- beta-lactamase inhibitors | 18 (18) | 11 (15) | 7 (28) | .14 |
| Cephalosporins | 21 (21) | 13 (18) | 8 (32) | .13 |
| Carbapenems | 8 (8) | 0 (0) | 8 (32) | <.01 |
| Glycopeptides | 2 (2) | 2 (3) | 0 (0) | .41 |
| Medications within 6 months of discharge (n, %) | ||||
| Steroids | 39 (39) | 30 (41) | 9 (36) | .69 |
| 5-aminosalicylic acid | 11 (11) | 7 (10) | 4 (16) | .37 |
| Immunomodulators | 18 (18) | 14 (19) | 4 (16) | .74 |
| Anti-TNF | 39 (39) | 30 (41) | 9 (36) | .69 |
| Combination Anti-TNF/immunomodulator | 8 (8) | 6 (8) | 2 (8) | .99 |
| Anti-Integrin | 3 (3) | 3 (4) | 0 (0) | .31 |
| Anti-IL 12/23 | 4 (4) | 4 (5) | 0 (0) | .24 |
The most common initial postdischarge antibiotics in the PO group included metronidazole (80%), fluoroquinolones (68%), cephalosporins (18%), and penicillins with or without beta-lactamase inhibitors (15%). In the IV group, the most commonly prescribed initial postdischarge antibiotics were metronidazole (52%), carbapenems (32%), cephalosporins (32%), and penicillins with or without beta-lactamase inhibitors (28%). A higher proportion of patients in the PO antibiotic group was prescribed fluoroquinolones (PO = 68%, IV = 4%; P < .01) and metronidazole (PO = 80%, IV = 52%; P < .01), while a higher percentage of patients in the IV group received carbapenems (PO = 0%, IV = 32%; P < .01).
To determine if there was any impact of switching antibiotic class at discharge amongst those discharged on oral antibiotics, we assessed outcomes, stratifying by need to switch antibiotic class (Supplementary Table 3). There were no significant differences in abscess recurrence, readmissions for any CD-related complication (including readmission for additional antibiotics, IR drainage, small bowel obstruction or other), or surgery (including planned and unplanned).
Postdischarge Crohn’s Disease Treatment
Within 6 months of discharge, there were no differences between the PO and IV antibiotic groups in the use of steroids, immunomodulators, 5-aminosalicylic acid, or any biologic classes (Table 4). The most common class of biologics prescribed in both groups was antitumor necrosis factor (anti-TNF) agents, with 41% of the PO group and 36% of the IV group receiving anti-TNF therapy (P = .69). Both anti-integrin and anti-interleukin (IL)-12/23 biologic treatments were only prescribed in the PO antibiotic group, with 4% and 5% of the PO group receiving these medications, respectively. Overall, 8% of patients were treated with combination anti-TNF therapy and immunomodulator therapy (PO = 8%, IV = 8%; P = .99).
Discussion
This retrospective, multicenter study assessed outcomes of hospitalized patients with CD complicated by intra-abdominal abscess treated with PO vs IV antibiotics at the time of discharge. There was no significant difference in surgery between the 2 groups, with the majority of patients requiring surgery within 12 months; however, the patients in the IV group demonstrated a lower risk for nonsurgical CD-related readmission when compared with the PO group. This trend may have been driven by readmission for additional antibiotics. There were no other significant differences in the patterns of readmission. There were also no significant differences in length of stay, abscess drainage, abscess recurrence, or time to surgery between the 2 groups. Route of antibiotic administration did not yield significant differences in outcomes when stratified by size of abscess. There were no significant differences in outcomes when stratifying the oral group by need to switch antibiotic class at discharge. These data can be valuable to providers caring for patients presenting with intra-abdominal abscess in the setting of CD.
To our knowledge, this is the first study to compare outcomes between routes of antibiotic administration at hospital discharge for Crohn’s patients diagnosed with intra-abdominal abscesses. Studies of other infectious diseases established that PO antibiotics can be an effective treatment option for complicated intra-abdominal infections. A randomized control trial demonstrated that in patients able to tolerate oral intake, a sequential IV-to-PO antibiotic regimen of ciprofloxacin to metronidazole was at least as effective as an IV-alone regimen of imipenem and cilastatin.18 Another study compared sequential IV ciprofloxacin to PO metronidazole to an IV regimen of piperacillin/tazobactam; finding patients able to tolerate PO medications in fact had better clinical responses compared with the piperacillin/tazobactam group.19 For pyogenic liver abscesses, patients who were transitioned to PO antibiotic treatment showed similar relapse rates, decreased length of stay, and lower costs compared with an IV antibiotic regimen alone.20 From the pediatric literature, discharge PO antibiotics for complicated appendicitis have been shown to have noninferior or even superior outcomes compared with IV antibiotics.21–23 Those discharged on IV antibiotics were more likely to have growth of antibiotic-resistant organisms, which is relatively unsurprising given antibiotic resistance may preclude transition to oral antibiotics. After controlling for antibiotic resistance along with other relevant variables, our study demonstrates that patients receiving IV antibiotics were less likely to be readmitted and had longer intervals from discharge to nonsurgical readmission, though analysis of reasons for nonsurgical readmission was inconclusive. We additionally found no difference in surgical requirement within 12 months or time to surgery.
The finding of decreased 12-month nonsurgical readmissions in the IV group may be driven by decreased readmissions for an additional course of antibiotics compared with the PO group, though this trend did not meet statistical significance. This is unsurprising given that patients on PO antibiotics could be escalated to IV therapy, while the IV group likely had more limited options for escalation of antibiotics. Of note, all patients readmitted for antibiotics received IV antibiotics. Known risk factors for readmission in CD include inadequate pain control, opiate use disorder, requirement for total parenteral nutrition, lack of adequate follow-up, depression, anxiety, and tobacco abuse.24 Prior radiographic models have determined patients with penetrating disease are at a higher risk for undergoing surgery and subsequent readmission.25 Intra-abdominal fistulas and abscesses, complications of penetrating disease, have also been associated with increased 30-day readmission rates.26,27
The majority of patients in both groups required surgery within 1 year (PO = 61%, IV = 76%; P = .18). Similar rates of surgery at one year have been described in the pediatric population.28 On the other hand, the requirement for surgery in our cohort overall was higher than other prior studies, where only 40% to 50% of Crohn’s patients with intra-abdominal abscesses treated with medical therapy required surgery.6,7,29 Referral bias is possible, given that the 3 participating sites are large, academic inflammatory bowel disease (IBD) referral centers. Also, this study focused on patients with an index admission for abscess treatment. Nonhospitalized patients who were excluded from this study may have lower rates of surgery and readmission. Of note, abscess size >6 cm has previously been associated with increased risk for surgery, and while there was a trend towards this finding in our study, it did not meet statistical significance.30
Our findings have important clinical implications. Administration of antibiotics orally is preferred by most patients. Long-term IV antibiotics generally require placement of a PICC. Coordination of care for IV antibiotics and PICC lines is onerous and expensive. Parenteral outpatient antibiotics are less expensive than inpatient treatment but still maintain higher costs than outpatient oral regimens.16,17 Moreover, PICC lines can lead to complications including line infection, bacteremia, venous thrombosis, and mechanical complications (withdrawal, occlusion).14 Requirement for a PICC line can have a significant impact on a patient’s quality of life. PICC placement has been associated with increased limitations and discomfort with daily activities such as dressing or personal hygiene.31 Our data suggest that patients discharged on PO antibiotics may be more likely to be readmitted for IV antibiotics and potentially long-term PICC placement, but it is important to note that the majority of patients discharged on PO (>75%) were not readmitted for antibiotics, potentially avoiding the requirement for PICC lines and related potential complications.
Our study has several strengths. First is novelty, being the first study to assess outcomes between PO and IV postdischarge antibiotic regimens in patients with CD hospitalized with an intra-abdominal abscess. Additionally, the patients in the study come from multiple institutions with varying practice patterns, improving the generalizability of our findings. The study design allowed for inclusion of abscess- and disease-related details, which cannot be ascertained in larger claims-based studies.
The major limitations of this study are the retrospective nature along with limitations in sample size. However, our cohort size is similar to or greater than the couple of prior retrospective studies assessing outcomes between surgical and medical outcomes in intra-abdominal abscesses related to CD.5,6 Other potential limitations include the inability to collect granular data such as subspecialty consults (ie, IBD subspecialist or infectious disease specialist) that may have affected choice of discharge antibiotic regimen. We additionally lack data regarding the duration of antibiotics postdischarge, and thus it is unclear what duration of antibiotic therapy is optimal. Additionally, only 15% of patients had repeat imaging prior to discharge, limiting assessment of abscess improvement as a factor that may determine the route of antibiotics. Those who did undergo repeat imaging did have significant improvement in abscess size, though the progression to surgery remained high. To bolster our retrospective findings, additional prospective observational or clinical trials would be of benefit.
Conclusion
The optimal route of antibiotic administration after discharge for Crohn’s-related intra-abdominal abscesses is poorly studied. In this multicenter, retrospective study, we provide novel insights comparing IV and PO discharge antibiotic regimens, demonstrating that overall risk of surgery is similar between the 2 groups; although those who receive IV antibiotics are less likely to be readmitted and have longer time intervals to nonsurgical readmission. Our findings build on an existing foundation of infectious disease literature that suggests transitioning to PO antibiotics in intra-abdominal abscesses is feasible and can reduce the risk of complications from long-term IV antibiotics. For those who did require readmission on PO antibiotics, further research is required to identify potential risk factors to guide clinical decision-making for providers. Moreover, the route of antibiotic administration has strong implications for patient satisfaction, quality of life, and health care costs.
Supplementary Data
Supplementary data is available at Inflammatory Bowel Diseases online.
Glossary
Abbreviations:
- CD
Crohn’s disease
- PO
oral
- IV
intravenous
- MGH
Massachusetts General Hospital
- IR
interventional radiology
- PICC
peripherally inserted central venous catheter; IQR interquartile range
- CRP
C-reactive protein
- OR
odds ratio
- CI
confidence interval
- HR
hazard ratio
- TNF
tumor necrosis factor
Contributor Information
Kush Fansiwala, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Alison Rusher, Division of Gastroenterology, Massachusetts General Hospital, Boston, MA, USA.
Brandon Shore, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Hans H Herfarth, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Edward Barnes, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Bharati Kochar, Division of Gastroenterology, Massachusetts General Hospital, Boston, MA, USA.
Shannon Chang, Inflammatory Bowel Disease Center, Division of Gastroenterology, NYU Grossman School of Medicine, New York, NY, USA.
Funding
This research was supported by the National Institutes of Health (K23DK127157-01, E.L.B.). All authors have approved the final version of this article.
Conflicts of Interest
K.F., B.K., A.R., B.S.: None
H.H.H.: Scientific advisory Board participation for AMAG, BMS, Fresenius Kabi, Janssen, Merck, Pfizer, Seres. Consultant for, Alivio, ExeGI Finch, Gilead, Lycera, Otsuka, PureTech Research support from Artizan Biosciences, Allakos, NovoNordisk, and Pfizer.
E.L.B.: consultant for AbbVie, Lilly, Target RWE.
S.C.: consultant for AbbVie, Pfizer, and Bristol Myers Squibb.
References
- 1. Greenstein AJ, Sachar DB, Greenstein RJ, Janowitz HD, AufsesAH, Jr. Intraabdominal abscess in Crohn’s (ileo) colitis. Am J Surg. 1982;143(6):727-730. [DOI] [PubMed] [Google Scholar]
- 2. Feagins LA, Holubar SD, Kane SV, Spechler SJ.. Current strategies in the management of intra-abdominal abscesses in Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9(10):842-850. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg DM, Cooke WT, Alexander-Williams J.. Abscess and fistulae in Crohn’s disease. Gut. 1973;14(11):865-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bossuyt P, Debeuckelaere C, Ferrante M, et al. The operative risk and natural history after the diagnosis of ileal penetrating Crohn’s disease. Eur J Gastroenterol Hepatol. 2018;30(5):539-545. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen DL, Sandborn WJ, LoftusEV, Jr, et al. Similar outcomes of surgical and medical treatment of intra-abdominal abscesses in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2012;10(4):400-404. [DOI] [PubMed] [Google Scholar]
- 6. Garcia JC, Persky SE, Bonis PA, Topazian M.. Abscesses in Crohn’s disease: outcome of medical versus surgical treatment. J Clin Gastroenterol. 2001;32(5):409-412. [DOI] [PubMed] [Google Scholar]
- 7. Bermejo F, Garrido E, Chaparro M, et al. Efficacy of different therapeutic options for spontaneous abdominal abscesses in Crohn’s disease: are antibiotics enough? Inflamm Bowel Dis. 2012;18(8):1509-1514. [DOI] [PubMed] [Google Scholar]
- 8. Wacha H, Warren B, Bassaris H, Nikolaidis P, Intra-Abdominal Infections Study Group. Comparison of sequential intravenous/oral ciprofloxacin plus metronidazole with intravenous ceftriaxone plus metronidazole for treatment of complicated intra-abdominal infections. Surg Infect (Larchmt). 2006;7(4):341-354. [DOI] [PubMed] [Google Scholar]
- 9. Weiss G, Reimnitz P, Hampel B, et al. Moxifloxacin for the treatment of patients with complicated intra-abdominal infections (the AIDA Study). J Chemother. 2009;21(2):170-180. [DOI] [PubMed] [Google Scholar]
- 10. Fraser JD, Aguayo P, Leys CM, et al. A complete course of intravenous antibiotics vs a combination of intravenous and oral antibiotics for perforated appendicitis in children: a prospective, randomized trial. J Pediatr Surg. 2010;45(6):1198-1202. [DOI] [PubMed] [Google Scholar]
- 11. Kleif J, Rasmussen L, Fonnes S, et al. Enteral antibiotics are non-inferior to intravenous antibiotics after complicated appendicitis in adults: a retrospective multicentre non-inferiority study. World J Surg. 2017;41(11):2706-2714. [DOI] [PubMed] [Google Scholar]
- 12. Etzioni DA, Chiu VY, Cannom RR, et al. Outpatient treatment of acute diverticulitis: rates and predictors of failure. Dis Colon Rectum. 2010;53(6):861-865. [DOI] [PubMed] [Google Scholar]
- 13. Siewert B, Tye G, Kruskal J, et al. Impact of CT-guided drainage in the treatment of diverticular abscesses: size matters. AJR Am J Roentgenol. 2006;186(3):680-686. [DOI] [PubMed] [Google Scholar]
- 14. Grau D, Clarivet B, Lotthe A, Bommart S, Parer S.. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;6(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH.. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886-892. [DOI] [PubMed] [Google Scholar]
- 16. McMeekin N, Geue C, Briggs A, et al. ; OVIVA collaborators. Cost-effectiveness of oral versus intravenous antibiotics (OVIVA) in patients with bone and joint infection: evidence from a non-inferiority trial. Wellcome Open Res. 2019;4(108). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solomkin JS, Reinhart HH, Dellinger EP, et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections The Intra-Abdominal Infection Study Group. Ann Surg. 1996;223(3):303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohn SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg. 2000;232(2):254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng FH, Wong WM, Wong BC, et al. Sequential intravenous/oral antibiotic vs continuous intravenous antibiotic in the treatment of pyogenic liver abscess. Aliment Pharmacol Ther. 2002;16(6):1083-1090. [DOI] [PubMed] [Google Scholar]
- 21. Rangel SJ, Anderson BR, Srivastava R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. [DOI] [PubMed] [Google Scholar]
- 22. Acker SN, Hurst AL, Bensard DD, et al. Pediatric appendicitis and need for antibiotics at time of discharge: does route of administration matter? J Pediatr Surg. 2016;51(7):1170-1173. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Li Y, Ji Y.. Intravenous versus intravenous/oral antibiotics for perforated appendicitis in pediatric patients: a systematic review and meta-analysis. BMC Pediatr. 2019;19(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes EL, Kochar B, Long MD, et al. Modifiable risk factors for hospital readmission among patients with inflammatory bowel disease in a nationwide database. Inflamm Bowel Dis. 2017;23(6):875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lowe SC, Ream J, Hudesman D, et al. A clinical and radiographic model to predict surgery for acute small bowel obstruction in Crohn’s disease. Abdom Radiol (NY). 2020;45(9):2663-2668. [DOI] [PubMed] [Google Scholar]
- 26. Micic D, Gaetano JN, Rubin JN, et al. Factors associated with readmission to the hospital within 30 days in patients with inflammatory bowel disease. PLoS One. 2017;12(8):e0182900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hazratjee N, Agito M, Lopez R, Lashner B, Rizk MK.. Hospital readmissions in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108(7):1024-1032. [DOI] [PubMed] [Google Scholar]
- 28. Dotson JL, Bashaw H, Nwomeh B, Crandall WV.. Management of intra-abdominal abscesses in children with Crohn’s disease: a 12-year, retrospective single-center review. Inflamm Bowel Dis. 2015;21(5):1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee H, Kim YH, Kim JH, et al. Nonsurgical treatment of abdominal or pelvic abscess in consecutive patients with Crohn’s disease. Dig Liver Dis. 2006;38(9):659-664. [DOI] [PubMed] [Google Scholar]
- 30. Perl D, Waljee AK, Bishu S, et al. Imaging features associated with failure of nonoperative management of intraabdominal abscesses in Crohn disease. Inflamm Bowel Dis. 2019;25(12):1939-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johansson E, Engervall P, Bjorvell H, Hast R, Bjorkholm M.. Patients’ perceptions of having a central venous catheter or a totally implantable subcutaneous port system-results from a randomised study in acute leukaemia. Support Care Cancer. 2009;17(2):137-143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.