Abstract
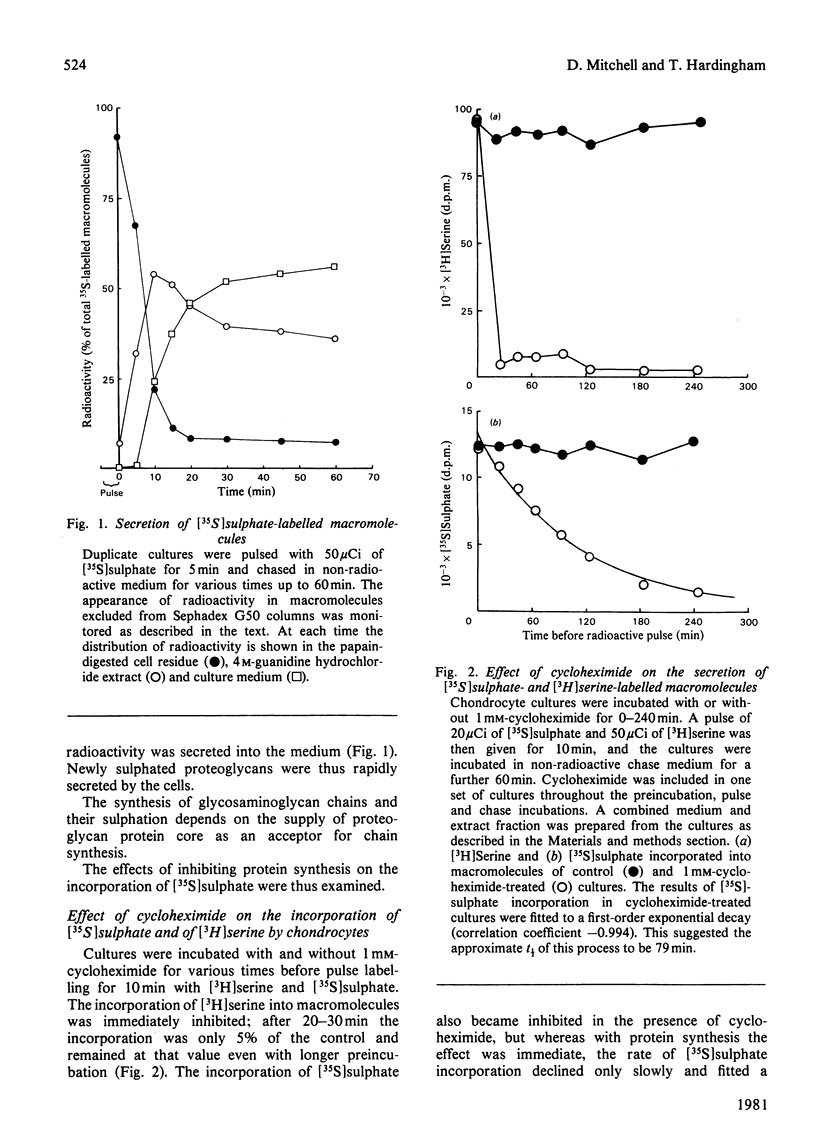
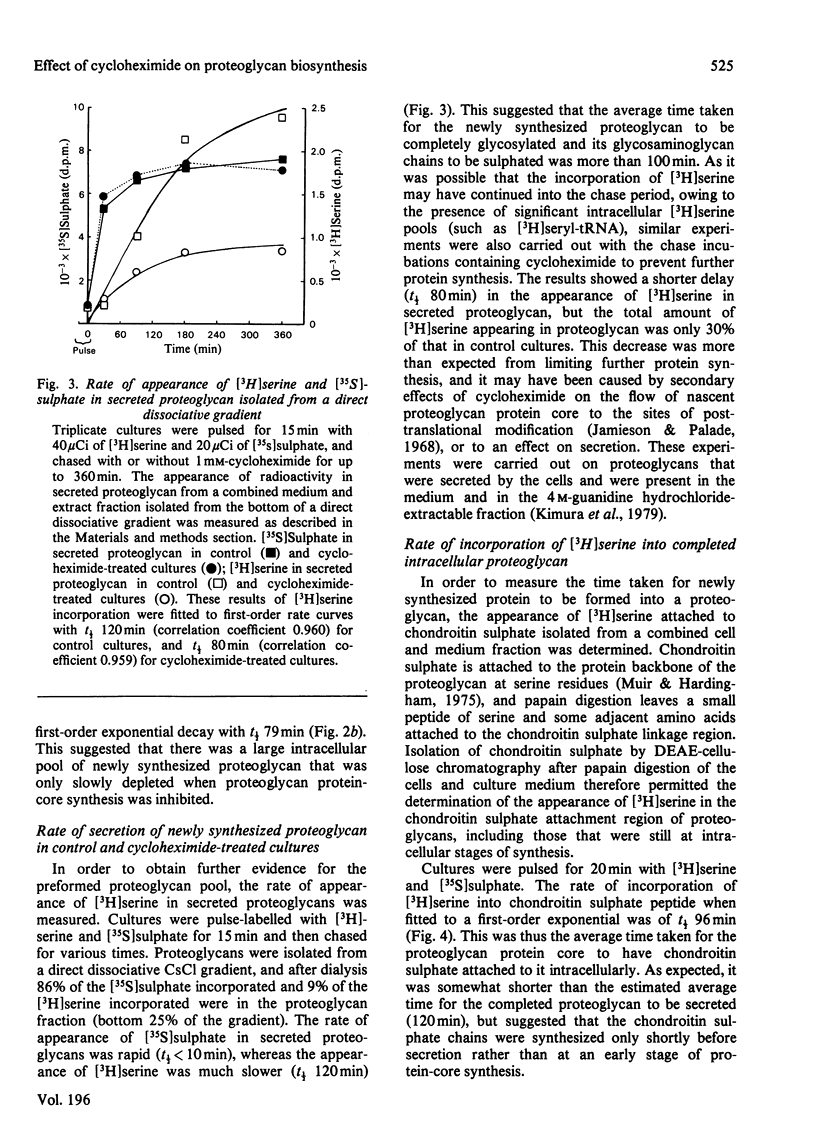
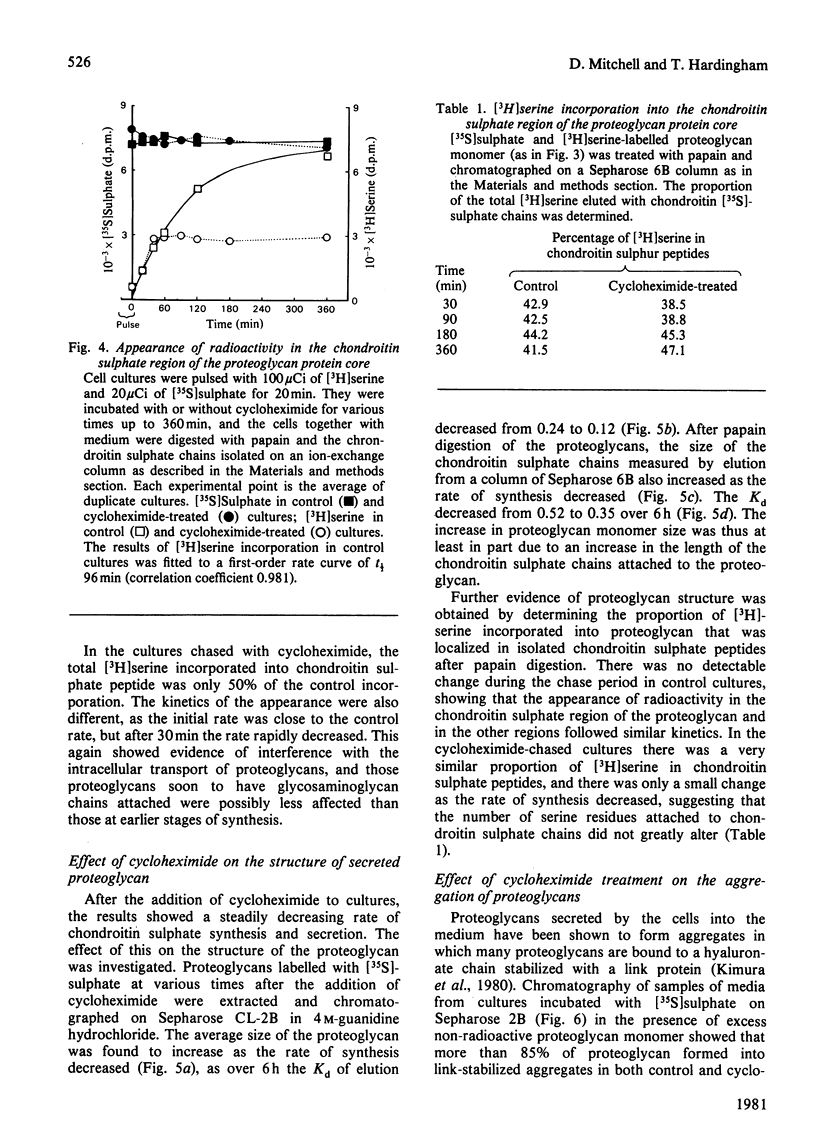
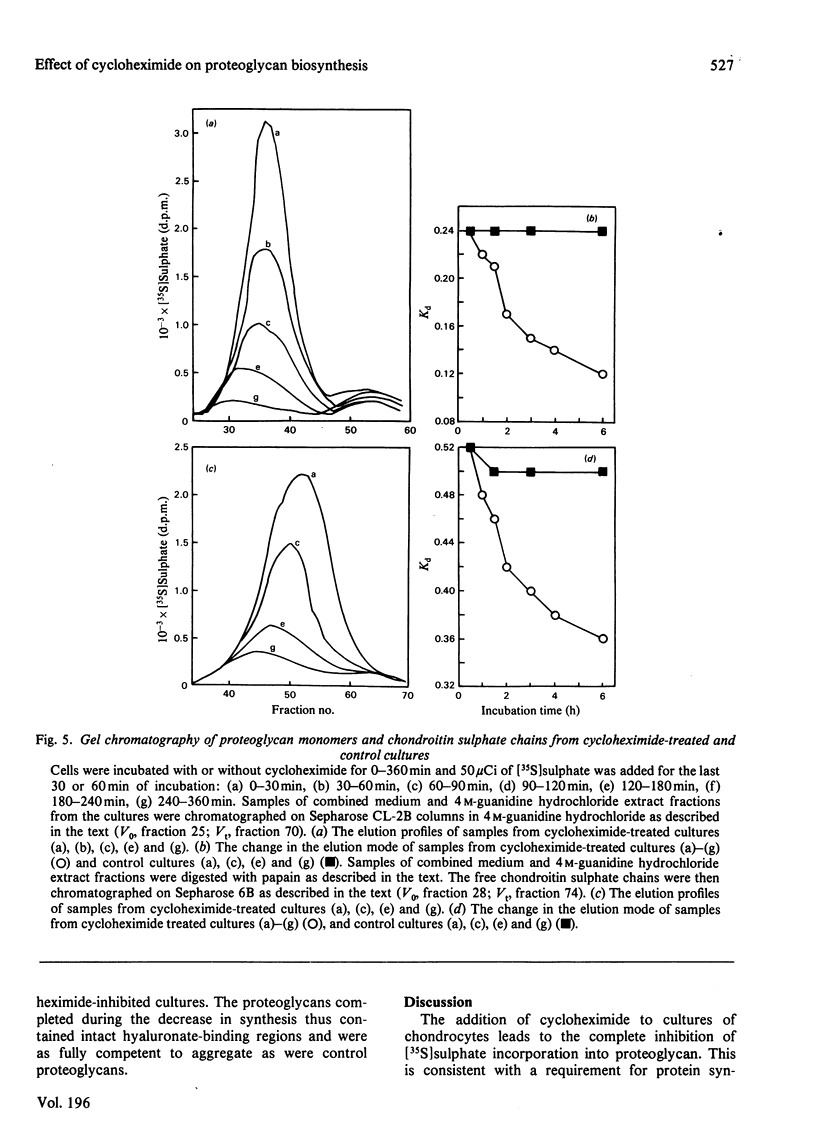
Proteoglycans synthesized by rat chondrosarcoma cells in culture are secreted into the culture medium through a pericellular matrix. The appearance of [35S]sulphate in secreted proteoglycan after a 5 min pulse was rapid (half-time, t 1/2 less than 10 min), but that of [3H]serine into proteoglycan measured after a 15 min pulse was much slower (t 1/2 120 min). The incorporation of [3H]serine into secreted protein was immediately inhibited by 1 mM-cycloheximide, but the incorporation of [35S]sulphate into proteoglycans was only inhibited gradually(t 1/2 79 min), suggesting the presence of a large intracellular pool of proteoglycan that did not carry sulphated glycosaminoglycans. Cultures were pulsed with [3H]serine and [35S]sulphate and chased for up to 6 h in the presence of 1 mM-cycloheximide. Analysis showed that cycloheximide-chased cells secreted less than 50% of the [3H]serine in proteoglycan of control cultures and the rate of incorporation into secreted proteoglycan was decreased (from t 1/2 120 min to t 1/2 80 min). Under these conditions cycloheximide interfered with the flow of proteoglycan protein core along the route of intracellular synthesis leading to secretion, as well as inhibiting further protein core synthesis. The results suggested that the newly synthesized protein core of proteoglycan passes through an intracellular pool for about 70-90 min before the chondroitin sulphate chains are synthesized on it, and it is then rapidly secreted from the cell. Proteoglycan produced by cultures incubated in the presence of cycloheximide and labelled with [35S]sulphate showed an increase with time of both the average proteoglycan size and the length of the constituent chondroitin sulphate chain. However, the proportion of synthesized proteoglycans able to form stable aggregates did not alter.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- De Luca S., Lohmander L. S., Nilsson B., Hascall V. C., Caplan A. I. Proteoglycans from chick limb bud chondrocyte cultures. Keratan sulfate and oligosaccharides which contain mannose and sialic acid. J Biol Chem. 1980 Jul 10;255(13):6077–6083. [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Inhibition of proteoglycan biosynthesis by hyaluronic acid in chondrocytes in cell culture. Biochim Biophys Acta. 1976 Aug 24;444(1):69–74. doi: 10.1016/0304-4165(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The effect of temperature on the biosynthesis of chondroitin 4-sulphate in cartilage slices in vitro. FEBS Lett. 1970 Sep 6;9(3):145–148. doi: 10.1016/0014-5793(70)80339-8. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The route of secretion of procollagen. The influence of alphaalpha'-bipyridyl, colchicine and antimycin A on the secretory process in embryonic-chick tendon and cartilage cells. Biochem J. 1976 Apr 15;156(1):81–90. doi: 10.1042/bj1560081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. 3. Dissociation of intracellular transport from protein synthesis. J Cell Biol. 1968 Dec;39(3):580–588. doi: 10.1083/jcb.39.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Kim J. J., Conrad H. E. Secretion of chondroitin SO4 by monolayer cultures of chick embryo chondrocytes. J Biol Chem. 1980 Feb 25;255(4):1586–1597. [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C. Assembly of newly synthesized proteoglycan and link protein into aggregates in cultures of chondrosarcoma chondrocytes. J Biol Chem. 1980 Aug 10;255(15):7134–7143. [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C., Solursh M. Biosynthesis of proteoglycans and their assembly into aggregates in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1979 Apr 25;254(8):2600–2609. [PubMed] [Google Scholar]
- Kimura J. H., Osdoby P., Caplan A. I., Hascall V. C. Electron microscopic and biochemical studies of proteoglycan polydispersity in chick limb bud chondrocyte cultures. J Biol Chem. 1978 Jul 10;253(13):4721–4729. [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]


