Abstract
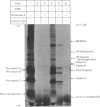
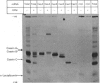
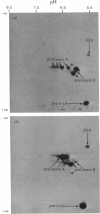
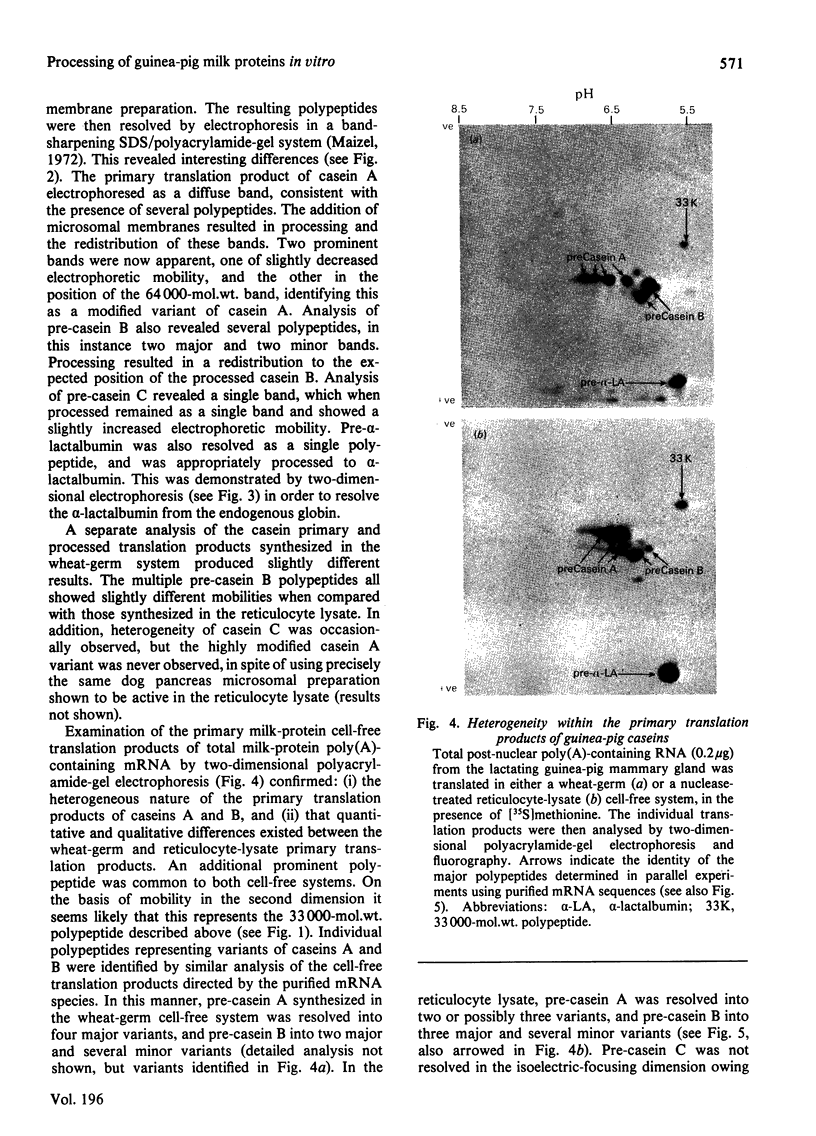
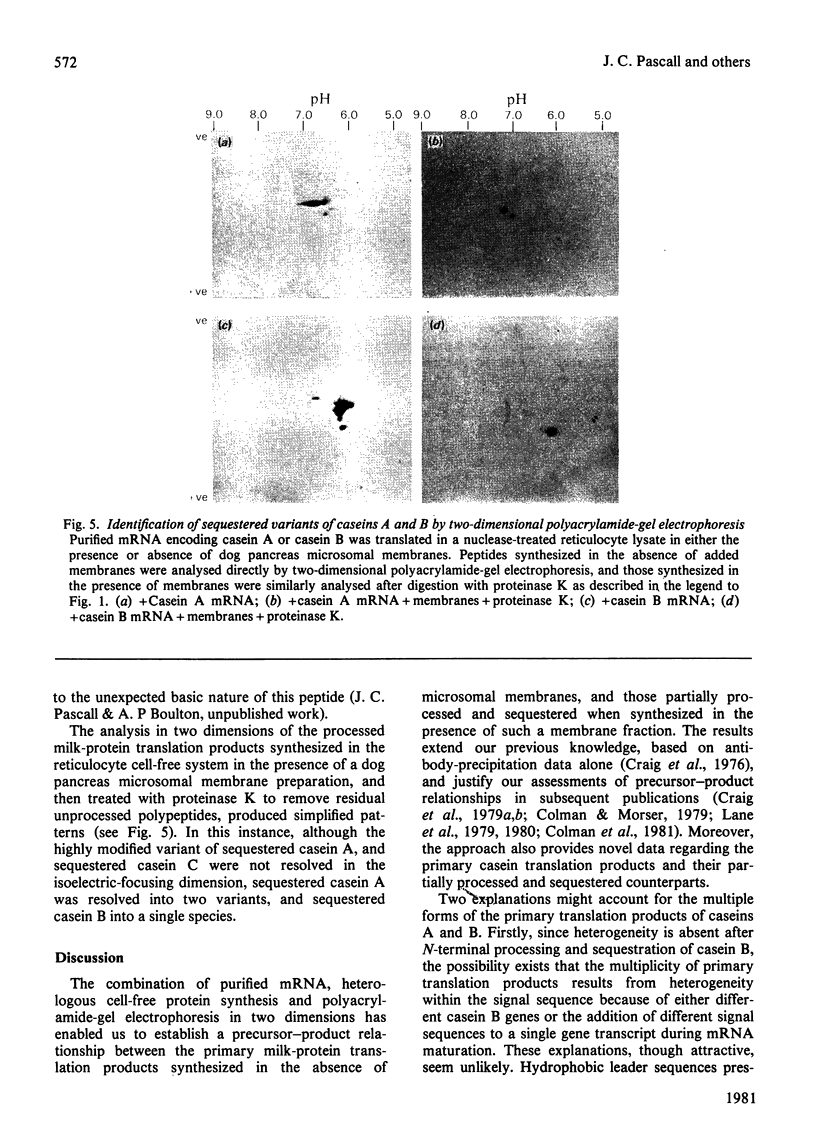
1. Individual mRNA species encoding guinea-pigs caseins A, B and C, and alpha-lactalbumin, were purified by hydridization to recombinant milk-protein plasmid DNA immobilized on diazobenzyloxymethyl-paper or diazobenzyloxymethyl-cellulose. Addition of the purified mRNA species to a reticulocyte-lysate cell-free system, in the presence or absence of a dog pancreas microsomal membrane fraction, established a precursor-product relationship between the primary translation products and those sequestered within microsomal vesicles, as determined by polyacrylamide-gel analysis in one and two dimensions. 2. Three sequestered variants of sequestered casein A were identified, but only single forms of sequestered casein B and alpha-lactalbumin. Sequestered variants of casein C proved to be unexpectedly basic, and did not focus on the pH gradient utilized. 3. Comparative analysis of milk proteins synthesized in the reticulocyte-lysate and wheat-germ cell-free systems by two-dimensional gel electrophoresis demonstrated both quantitative and qualitative differences. In particular, marked but variable heterogeneity was apparent within the primary translation products of casein A and casein B. Pre-casein C did not focus. Limited N-terminal processing of the primary translation products was also evident. These observations are discussed in relation to (i) unscheduled post-translational modifications by cell-free protein-synthesizing systems and (ii) multiplicity of signal sequences. 4. Overall we demonstrate that complex precursor-product relationships between primary translation products and their sequestered variants, programmed in vitro by a mixed mRNA population, may be readily analysed by using individual mRNA sequences purified by hybridization to immobilized cloned complementary-DNA sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M. R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burckhardt J., Telford J., Birnstiel M. L. Detection of labelled RNA species by contact hybridization. Nucleic Acids Res. 1979 Jul 11;6(9):2963–2971. doi: 10.1093/nar/6.9.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burditt L. J., Parker D., Craig R. K., Getova T., Campbell P. N. Differential expression of alpha-lactalbumin and casein genes during the onset of lactation in the guinea-pig mammary gland. Biochem J. 1981 Mar 15;194(3):999–1006. doi: 10.1042/bj1940999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Lane C. D., Craig R., Boulton A., Mohun T., Morser J. The influence of topology and glycosylation on the fate of heterologous secretory proteins made in Xenopus oocytes. Eur J Biochem. 1981 Jan;113(2):339–348. doi: 10.1111/j.1432-1033.1981.tb05072.x. [DOI] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Harrison O. S., Parker D., Campbell P. N. Studies on the intracellular segregation of polyribosome-associated messenger ribonucleic acid species in the lactating guinea-pig mammary gland. Biochem J. 1979 Sep 1;181(3):737–756. doi: 10.1042/bj1810737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Parker D., Campbell P. N. The construction, identification and partial characterization of plasmids containing guinea-pig milk protein complementary DNA sequences. Biochem J. 1981 Mar 15;194(3):989–998. doi: 10.1042/bj1940989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Perera P. A., Mellor A., Smith A. E. Initiation and processing in vitro of the primary translation products of guinea-pig caseins. Biochem J. 1979 Nov 15;184(2):261–267. doi: 10.1042/bj1840261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaye P., Gautron J. P., Mercier J. C., Hazé G. Amino terminal sequences of the precursors of ovine caseins. Biochem Biophys Res Commun. 1977 Dec 7;79(3):903–911. doi: 10.1016/0006-291x(77)91196-2. [DOI] [PubMed] [Google Scholar]
- Gaye P., Hue D., Mercier J. C., Haze G. Enzymatic processing of precursors of ovine lactoproteins by mammary microsomal membranes and a deoxycholate-soluble extract from rough microsomes. FEBS Lett. 1979 May 1;101(1):137–142. doi: 10.1016/0014-5793(79)81312-5. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. D., Colman A., Mohun T., Morser J., Champion J., Kourides I., Craig R., Higgins S., James T. C., Applebaum S. W. The Xenopus oocyte as a surrogate secretory system. The specificity of protein export. Eur J Biochem. 1980 Oct;111(1):225–235. doi: 10.1111/j.1432-1033.1980.tb06097.x. [DOI] [PubMed] [Google Scholar]
- Lane C., Shannon S., Craig R. Sequestration and turnover of guinea-pig milk proteins and chicken ovalbumin in Xenopus oocytes. Eur J Biochem. 1979 Nov;101(2):485–495. doi: 10.1111/j.1432-1033.1979.tb19743.x. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Haze G., Gaye P., Hue D. Amino terminal sequence of the precursor of ovine beta-lactoglobulin. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1236–1245. doi: 10.1016/0006-291x(78)90320-0. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Haze G., Gaye P., Petrissant G., Hue D., Boisnard M. Amino terminal sequence of the precursor of ovine alpha-lactalbumin. Biochem Biophys Res Commun. 1978 Nov 29;85(2):662–670. doi: 10.1016/0006-291x(78)91213-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peeters B., Mous J., van Bellegem H., Rombauts W. The wheat germ cell-free system possesses processing activity for the precursor of human placental lactogen. Biochim Biophys Acta. 1979 Feb 27;561(2):502–516. doi: 10.1016/0005-2787(79)90158-8. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]