Abstract
Purpose:
CD4/CD8 ratio <0.5 is associated with increased risk of advanced anal disease(AAD), but it is unknown if duration below 0.5 matters. The purpose of this study is to determine if duration CD4/CD8 ratio <0.5 is associated with increased risk of invasive anal cancer(IC) in people living with HIV and high-grade dysplasia(HSIL).
Methods:
This single institution, retrospective study uses the University of Wisconsin Hospital and Clinics Anal Dysplasia and Anal Cancer Database. Patients with IC vs HSIL alone were compared. Independent variables were mean and percent time CD4/CD8 ratio was <0.5. Multivariate logistic regression was performed to estimate adjusted odds of anal cancer.
Results:
We identified 107 patients with HIV infection and AAD(87 HSIL, 20 IC). History of smoking was significantly associated with development of IC(95% in IC vs 64% in HSIL;p=0.015). Mean time with CD4/CD8 < 0.5 was significantly longer in patients with IC compared to patients with HSIL(7.7 years vs 3.8 years respectively;p=0.002). Similarly, mean percent time CD4/CD8 ratio was <0.5 was higher in those with IC versus those with HSIL(80% vs 55% respectively;p=0.009). On multivariate analysis, duration CD4/CD8 was <0.5 was associated with increased odds of developing IC (OR: 1.25; 95% CI:1.02-1.53;p=0.034).
Conclusions:
In this retrospective, single institution study of a cohort of people living with HIV and HSIL, increasing duration that CD4/CD8 ratio was <0.5 was associated with increased odds of developing IC. Monitoring the number of years CD4/CD8 ratio is <0.5 could inform decision making in patients with HIV infection and HSIL.
Keywords: Anal cancer, high-grade dysplasia, CD4/CD8 ratio
Introduction:
Human Papilloma Virus (HPV)-associated cancers have a higher prevalence in people living with Human Immunodeficiency Virus (PLWH) than in the general population.1 PLWH have 40-80 fold increased risk of developing anal cancer compared to people without Human Immunodeficiency Virus (HIV) infection.1-4 Anal dysplasia (anal cancer precursor lesions) are often first identified in PLWH using screening anal Papanicolaou smears (Pap tests).5-7 Positive Pap test results are common and lead to anoscopy which also often requires biopsies, and/or excision and fulguration of lesions.8 Intervals for additional surveillance/follow-up exams are not well established and recurrences are common. Risk stratification of patients is needed to help determine those at highest risk and how frequently they should be monitored and treated.
High-grade dysplasia (HSIL) is considered a premalignant condition of invasive cancer (IC), and optimal management of HSIL can prevent progression to IC.8,9 The multi-institutional Anal Cancer/HSIL Outcomes Research (ANCHOR) study recently demonstrated that patients with HIV infection and HSIL treated with excision/fulguration or topical treatments had a 57% reduction in anal cancer incidence compared with those who received active monitoring alone.9 Despite treatment, some patients still progressed from HSIL to IC. While both rates of HSIL and IC are rising, rates of progression in the literature vary widely from 0.4% to 10% per year and are unpredictable.8,10 Immunologic laboratory values are a promising objective measure to aid in risk stratification of these patients. One such objective measure is the CD4/CD8 ratio.
CD4/CD8 values are routinely collected in PLWH to measure response to combination antiretroviral therapy (cART). CD4/CD8 ratios below 1 have been shown to be associated with immuosenescence, all-cause mortality, several non-AIDS defining events (NAE) and other malignancies such as lung cancer, cervical cancer, Kaposi sarcoma and non-Hodgkin’s lymphoma.11-15 The longer that a patient takes to recover their CD4/CD8 ratio, the higher their risk for development of NAE.14,16 In our previous work, we demonstrated a nadir CD4/CD8 ratio below 0.5 was a risk factor for development of high-grade anal dysplasia or invasive anal cancer in PLWH.17,18 This is a promising non-invasive method for tailoring anal cancer screening and surveillance protocols for PLWH.17,18 It remains unknown if duration of time CD4/CD8 ratio is low has an effect on development of anal cancer. In this study, we hypothesized that persistent CD4/CD8 ratio below 0.5 was associated with increased risk of development of IC in PLWH and HSIL.
Materials and Methods:
Patient population:
We performed a single center, retrospective study at the University of Wisconsin Hospital and Clinics. University of Wisconsin Institutional Review Board approval was obtained for this study (No. 2018-0557). We queried the University of Wisconsin Anal Dysplasia and Anal Cancer Database to identify all patients with a diagnosis of HIV and advanced anal disease (AAD), defined as history of HSIL (anal intraepithelial neoplasm (AIN) II or III) and/or IC from 2001 – 2019. Chart review was performed of pathology records, operative reports, and laboratory tests to confirm diagnosis and treatment. Patients with only a single immunologic panel of labs and those without at least two pathology results were excluded.
Variables:
The baseline patient characteristics collected included age, biological sex, race, history of smoking, non-HPV sexually transmitted infections (STIs), history of anal receptive intercourse, history of cardiovascular disease, and diabetes. We defined advanced anal disease as those with high-grade dysplasia or invasive cancer on at least one pathologic result. CD4/CD8 ratios were calculated from all resultant immunologic panels that were collected approximately every 6 months per standard institutional practice. We also calculated the time between the first resulted CD4/CD8 value to CD4/CD8 value nearest to diagnosis of AAD to determine total time under HIV care before AAD.
Treatment of patients:
We included all patients diagnosed with AAD whether diagnosed via screening or symptomatic disease. It is standard practice at our institution to treat all high-grade lesions with excision/ablation, with follow-up high resolution anoscopy (HRA) every 3-6 months. When no further high-grade disease detected on follow-up HRA, follow-up intervals are extended to 1 year.19 Chart review was performed to confirm that all patients in this study with HSIL were examined and any lesions noted were treated with excision/ablation.
Statistical Analysis:
Demographic and clinical characteristics, mean time CD4/CD8 ratio was below 0.5, and percent time CD4/CD8 ratio was below 0.5 were compared between patients with IC and HSIL alone. We also performed a subset analysis in which those who had IC as their first diagnosis were excluded to compare patients with HSIL who went on to develop IC versus those with HSIL and no diagnosis of IC. Pearson χ2 test was used for categorical variables, and Student’s t-test was used for continuous variables. Percent time CD4/CD8 ratio was below 0.5 and total time under HIV care before AAD were evaluated in a multivariate logistic regression model controlling for statistically significant patient characteristics. Time of CD4/CD8 below 0.5 was highly correlated total time under HIV care before AAD, so this was not used in the multivariate analysis. Adjusted ORs with 95% CIs were calculated. Finally, a time to event analysis was used to compare HSIL and IC. Statistical analysis was done using log rank test. Level of significance was determined at p=0.05. R or SAS statistical software was used for all analyses.
Results:
Invasive Cancer (IC) versus High-grade dysplasia (HSIL) alone:
A total of 107 patients met inclusion criteria, 20 with IC and 87 with HSIL only. Median follow-up time for the entire cohort was 11.6 years (IQR 7.5 - 16.5 years). There was no statistical difference in time under HIV care before AAD diagnosis for those with IC compared to those with HSIL alone (9.5 ± 7.0 years for IC and 7.1 ± 5.7 years for HSIL alone, p = 0.11). Using a time to event analysis, mean time to development of IC was longer compared to HSIL alone (10.9 ± 1.5 years for IC and 6.7 ± 0.6 years for HSIL, p = 0.013) (Figure 1). Ninety percent of patients in both groups were male. The two cohorts were similar with respect to race (p = 0.528). Patients with IC were older (59.3 ± 9.4 years for IC vs 52.4 ± 11.84 years for HSIL alone; p = 0.016). History of smoking was more common in those who developed IC (95% in IC vs 64% in HSIL alone; p = 0.015). On univariate analysis history of prior STIs was less common in those with IC (30% IC versus 58.6% HSIL alone; p = 0.039). History of cardiovascular disease (55% IC versus 18% HSIL alone; p=0.002), and history of diabetes mellitus (35% IC versus 2% HSIL alone; p<0.001) were associated with IC. Mean time with CD4/CD8 ratio below 0.5 was significantly longer in patients with IC compared with patients with HSIL alone (7.7 ± 5.9 years vs 3.8 ± 4.5 years respectively; p=0.002). Similarly, average percent of time of CD4/CD8 ratio below 0.5 was higher in those with IC versus those with HSIL alone (80% ± 30% vs 55% ± 40% respectively; p = 0.009) (Table 1).
Figure 1:
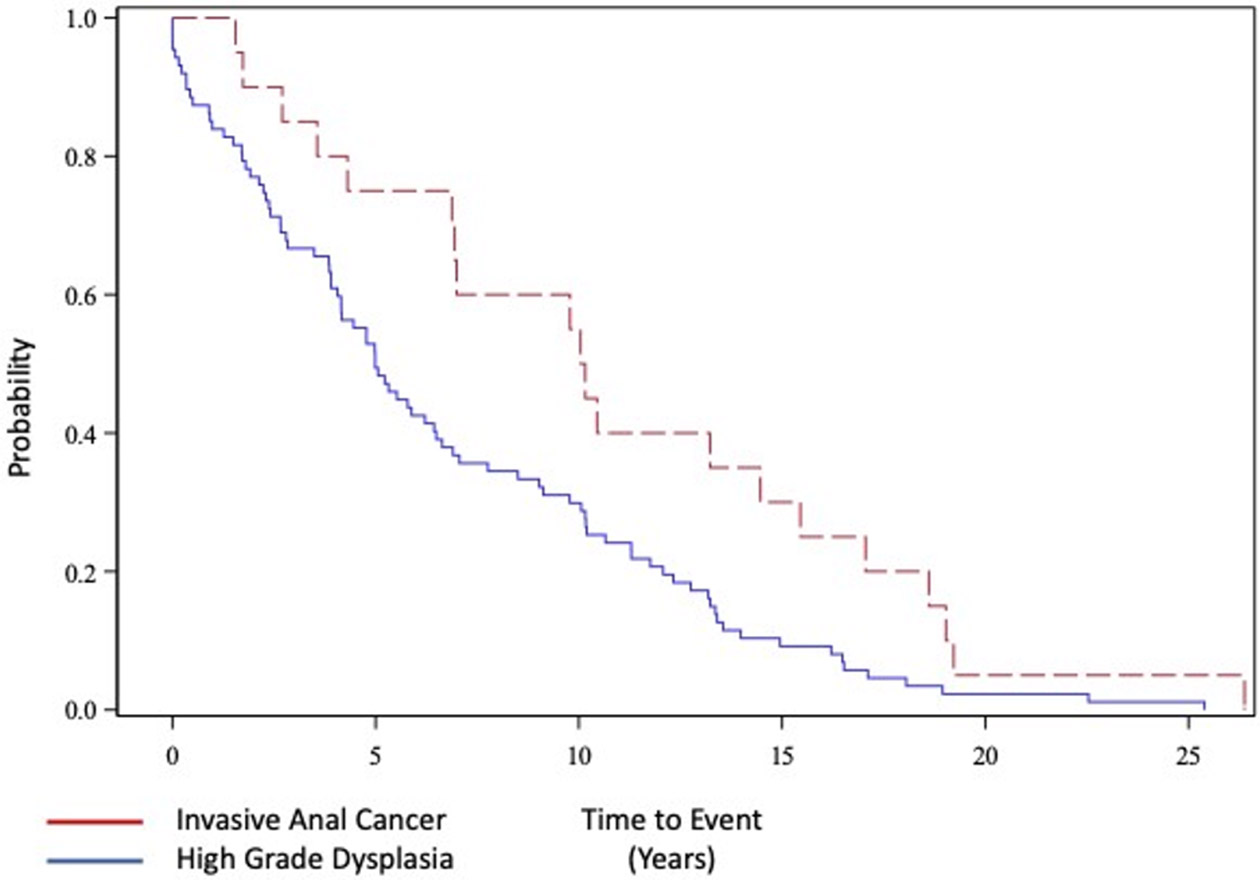
Time to event analysis of invasive anal cancer (red) and high-grade dysplasia (HSIL) (blue). Mean time to development of anal cancer 10.9 ± 1.5 years vs 6.7 ± 0.6 years for HSIL, p = 0.013).
Table 1:
Invasive anal cancer versus high-grade dysplasia alone:
| Anal Cancer |
HSIL | p-Value | |
|---|---|---|---|
| n | 20 | 87 | |
| Age (mean (SD)) | 59.30 (9.35) | 52.39 (11.84) | 0.016 |
| Male (%) | 18 (90.0) | 78 (89.7) | 1.000 |
| Race (%) | 0.528 | ||
| American Indian or Alaska | 0 ( 0.0) | 3 ( 3.4) | |
| Black | 3 (15.0) | 7 ( 8.0) | |
| Pacific islander | 0 ( 0.0) | 3 ( 3.4) | |
| White | 17 (85.0) | 74 (85.1) | |
| Smoking history (%) | 19 (95.0) | 56 (64.4) | 0.015 |
| History of STI (%) | 6 (30.0) | 51 (58.6) | 0.039 |
| History of Condyloma (%) | 14 (70.0) | 49 (56.3) | 0.385 |
| History of Anal Intercourse (%) | 3 (75.0) | 58 (84.1) | 1.0001 |
| Cardiovascular disease (%) | 11 (55.0) | 16 (18.4) | 0.002 |
| Diabetes mellitus (%) | 7 (35.0) | 2 ( 2.3) | <0.001 |
| Total time until first AAD diagnosis (Years; mean (SD)) | 9.45 (6.97) | 7.05 (5.65) | 0.1092 |
| Total time CD4/CD8 ratio less than 0.5 (Years; mean (SD)) | 7.68 (5.87) | 3.81 (4.49) | 0.0022 |
| Total percent time CD4/CD8 ratio less than 0.5 (mean (SD)) | 0.80 (0.30) | 0.55 (0.40) | 0.0092 |
STI: Sexually Transmitted Disease. HSIL: High-Grade Dysplasia. AAD: Advanced Anal Disease
Takes into account missing data on (n=34)
Takes into account missing data on (n=4)
IC developed from HSIL (IC-HSIL) versus HSIL alone:
Of the 20 patients with IC, 11 had no previous diagnosis of HSIL while 9 developed IC after previous HSIL diagnosis (IC-HSIL). All patients who developed IC-HSIL were previously treated with excision/fulguration. When comparing those patients with HSIL alone to those who developed IC-HSIL there was no statistical difference in follow-up time from first HIV diagnosis to first AAD diagnosis (8.3 ± 6.1 years for IC-HSIL and 7.1 ± 5.7 years for HSIL alone), p = 0.55 (Table 2). The two cohorts were similar with respect to sex, race, history of smoking, history of STIs and history of anal receptive intercourse (p = NS). Those with IC-HSIL were, on average, older than those with HSIL alone (59.3 ± 9.4 years vs 52.4 ± 11.8 years respectively, p = 0.016). History of cardiovascular disease and history of diabetes were significantly more prevalent in the IC-HSIL group versus the HSIL alone group (cardiovascular disease: 66.7% in HSIL-IC vs 18.4% in HSIL alone, p = 0.004; diabetes mellitus: 44% in HSIL-IC vs. 2.3% in HSIL alone, p < 0.001). Mean time CD4/CD8 was below 0.5 was longer in those who developed IC-HSIL than those who had HSIL alone (7.5 ± 6.2 years vs 3.8 ± 4.5 years respectively; p = 0.03). Average percent time CD4/CD8 was below 0.5 was also higher in those who developed IC-HSIL than those who had HSIL alone (82% ± 33% vs 55% ± 40%; p = 0.052).
Table 2:
Invasive anal cancer developed from high-grade dysplasia versus high-grade dysplasia alone:
| Cancer from HSIL | HSIL alone | p-Value | |
|---|---|---|---|
| n | 9 | 87 | |
| Age (mean (SD)) | 59.30 (9.35) | 52.39 (11.84) | 0.016 |
| Male (%) | 8 ( 88.9) | 78 (89.7) | 1.000 |
| Race (%) | 0.490 | ||
| American Indian or Alaska | 0 ( 0.0) | 3 ( 3.4) | |
| Black | 2 ( 22.2) | 7 ( 8.0) | |
| Pacific islander | 0 ( 0.0) | 3 ( 3.4) | |
| White | 7 ( 77.8) | 74 (85.1) | |
| Smoking history (%) | 9 (100.0) | 56 (64.4) | 0.072 |
| History of STI (%) | 3 ( 33.3) | 51 (58.6) | 0.270 |
| History of Condyloma (%) | 6 ( 66.7) | 49 (56.3) | 0.808 |
| History of Anal Intercourse (%) | 3 ( 75.0) | 58 (84.1) | 1.0001 |
| Cardiovascular disease (%) | 6 ( 66.7) | 16 (18.4) | 0.004 |
| Diabetes mellitus (%) | 4 ( 44.4) | 2 ( 2.3) | <0.001 |
| Total time to first AAD diagnosis (Years; mean (SD)) | 8.27 (6.06) | 7.05 (5.65) | 0.5482 |
| Total time CD4/CD8 ratio less than 0.5 (Years; mean (SD)) | 7.49 (6.24) | 3.81 (4.49) | 0.0282 |
| Total percent time CD4/CD8 ratio less than 0.5 (mean (SD)) | 0.82 (0.33) | 0.55 (0.40) | 0.0522 |
STI: Sexually Transmitted Disease. HSIL: High-Grade Dysplasia. AAD: Advanced Anal Disease
Takes into account missing data on (n=23)
Takes into account missing data on (n=4)
Multivariate logistic regression of IC versus HSIL alone:
On multivariate logistic regression, an increase in percent time CD4/CD8 ratio was below 0.5 of 0.1 units was associated with increased odds of developing IC compared with HSIL alone (OR 1.25; 95% CI:1.02-1.53; p = 0.034) (Table 3). Additional significant factors for development of IC included history of smoking (OR 18.74; 95% CI:1.45-242.13; p = 0.025) and diabetes (OR 13.74; 95% CI:1.15-164.66; p = 0.039). Total follow-up time of the two cohorts was not associated with cancer risk (OR 1, p=0.41).
Table 3:
Multivariate regression comparing invasive anal cancer versus high-grade dysplasia
| Odds ratio | 95% CI | p-Value | |
|---|---|---|---|
| Intercept | 0.00 | 0.00-0.05 | 0.003 |
| Age | 1.05 | 0.98-1.14 | 0.172 |
| History of Smoking | 18.74 | 1.45-242.13 | 0.025 |
| History of STI | 0.65 | 0.18-2.35 | 0.512 |
| History CVD | 1.52 | 0.34-6.79 | 0.585 |
| History DM | 13.74 | 1.15-164.66 | 0.039 |
| Percent CD4/CD8 below 0.5 (0.1 unit) | 1.25 | 1.02-1.53 | 0.034 |
| Total time to first AAD diagnosis | 1.00 | 1.00-1.00 | 0.409 |
DM: Diabetes Mellitus. STI: Sexually Transmitted Disease. CVD: Cardiovascular Disease. AAD: Advanced Anal Disease
Discussion:
With this single institution study of PLWH, advanced anal disease was first diagnosed on average 7.3 years into HIV disease follow-up. Longer mean and average percent time that CD4/CD8 ratio was below 0.5 were associated with progression from HSIL to invasive cancer. With the advent of cART therapy, PLWH can be expected to have near normal life-spans, and with longer life spans comes the risk of developing non-AIDs defining events (NAEs), such as anal cancer, which is driven by an immunocompromised state.4,12,14 Immune status can be complex to measure as PLWH on therapy typically have undetectable viral loads and CD4 values in the normal range. The CD4/CD8 ratio, however, has become a promising marker of immune status that correlates with NAEs. 8,14,18 We have demonstrated in this study of PLWH and advanced anal disease that time the CD4/CD8 ratio is low can correlate with progression from HSIL to invasive cancer and is a promising marker to risk stratify and inform surveillance/ treatment intervals for high-risk patients.
HSIL is a common diagnosis for PLWH.10 Rigorous surveillance protocols are followed for patients with HSIL in order to treat precancerous lesions and/or detect IC at its earliest treatable stages. Which PLWH and diagnosis of HSIL require the closest monitoring and treatment is currently unknown. With wide ranges of cancer progression reported in the literature, it is difficult to counsel patients on frequency of follow-up examinations/treatments with individualized risk stratification.10,20 Our previous work which identified the CD4/CD8 ratio as a marker of risk for advanced anal disease within PLWH didn’t examine how long the ratio was low.17,18 This current study demonstrates that for the subpopulation of PLWH and AAD, the mean length of time and average percent of time that the CD4/CD8 ratio is below 0.5 is correlated with their risk of developing IC. Progression to cancer occurs approximately 1-2% per year in PLWH with HSIL but has been variably reported from 0.4% to as high as 10% per year.10,20 Capturing patients with recurrent advanced lesions and treating them to prevent progression to invasion is critical.1,9 If invasive cancer does occur, earlier detection will improve survival.21 A normal CD4/CD8 ratio ranges from 1.5-2.5; however, up to 50% of PLWH never normalize their CD4/CD8 counts despite modern cART therapy. 14,16,22 Our study suggests that a subset of patients with prior diagnosis of HSIL are at an increased risk of developing IC, despite treatment of their HSIL lesions, and can be identified by monitoring how long the CD4/CD8 ratio is below 0.5. In particular, PLWH with an average of 7.7 years and/or 80% of their HIV monitoring period with CD4/CD8 ratio below 0.5 are at the highest risk for progression to anal cancer.
In this study mean time from the start of HIV monitoring to development of IC is 10.9 years and for HSIL alone it is 6.7 years. This is compatible with results seen in a systematic review of HIV patients who develop HSIL.23 Some authors recommend yearly testing with anal Pap tests for PLWH older than 35 to diagnose HSIL.5 Surveillance and treatment intervals of PLWH once HSIL has been diagnosed are less clear. Our work identifies the CD4/CD8 ratio as an objective laboratory value that can be utilized to help clinicians determine surveillance intervals. In addition to CD4/CD8, we found other risk predictors to be highly associated with development of IC. Smokers had an 18-fold increased odds of developing IC when compared to remaining with HSIL only. Smoking is a known risk factor for the development of anal cancer in PLWH as it is thought to cause re-activation of replication.24,25 When controlling for confounders, we also found diabetes to be a significant risk predictor with 13-fold increase in risk of IC. This is an interesting finding and may be related to the immunomodulation that occurs in those with diabetes.26
There are several limitations to our study. This is a single institution, retrospective study representing a fairly homogenous population (majority are white males). Time of HIV diagnosis and monitoring is variable as it relates to timing of HIV infection, which cannot be determined and remains a limitation of any study of PLWH. This study included patients followed over an 18 year period. In more recent years practice protocols have evolved and screening has become more rigorous. The limited number of patients in the IC-HSIL group may limit generalizability. Since over 95% of anal cancers are HPV-related, our institution does not routinely look for HPV on histopathology when cancer is diagnosed which limits interpretation of pathogenesis. Finally, we excluded those patients who did not follow-up with surveillance exams. Natural history of disease in patients that are noncompliant with follow-up may be different than our study population.
Conclusions:
In conclusion, higher mean and average percent time CD4/CD8 ratio is below 0.5 correlates with higher odds of progression to anal cancer in patients with HIV and HSIL. Those with anal cancer on average had a CD4/CD8 ratio below 0.5 for more than 7.7 years and/or longer than 80% of their follow-up time. Monitoring length and percent time CD4/CD8 ratio is below 0.5 could help inform decision-making and frequency of anal cancer surveillance exams in PLWH.
Synopsis.
With this single institution study of people living with HIV (PLWH) and high-grade anal dysplasia (HSIL), longer mean and average percent time that CD4/CD8 ratio was below 0.5 was associated with progression from HSIL to invasive cancer. Immune status can be complex to measure as PLWH on therapy typically have undetectable viral loads and CD4 values in the normal range. The CD4/CD8 ratio, however, is a promising marker of immune status that correlates with anal cancer risk and can be used to stratify patients.
Acknowledgements:
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number T32AI055397. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Funding:
Funding for this project was provided through institutional funds from the University of Wisconsin School of Medicine and Public Health
Footnotes
Disclaimer:
Matthew Freeman’s spouse is Systems Engineer employed by GE Healthcare - Anesthesia and Respiratory Care Division.
Presentation:
This manuscript was presented as a podium presentation at the American Society of Colorectal Surgeons annual meeting 2022 in Tampa, Florida
References:
- 1.Lee JY et al. Design of the ANal Cancer/HSIL Outcomes Research study (ANCHOR study): A randomized study to prevent anal cancer among persons living with HIV. Contemp Clin Trials 113, 106679, doi: 10.1016/j.cct.2022.106679 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaisa M, Sigel K, Hand J & Goldstone S High rates of anal dysplasia in HIV-infected men who have sex with men, women, and heterosexual men. AIDS 28, 215–222, doi: 10.1097/QAD.0000000000000062 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Sigel K & Gaisa MM Human Papillomavirus Genotypes Predict Progression of Anal Low-Grade Squamous Intraepithelial Lesions. J Infect Dis 218, 1746–1752, doi: 10.1093/infdis/jiy463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dandapani SV, Eaton M, Thomas CR Jr. & Pagnini PG HIV- positive anal cancer: an update for the clinician. J Gastrointest Oncol 1, 34–44, doi: 10.3978/j.issn.2078-6891.2010.005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown G. in Screening for Anal Dysplasia and Cancer in Patients With HIV New York State Department of Health AIDS Institute Clinical Guidelines (2020). [Google Scholar]
- 6.Massad LS et al. Long-term cumulative detection of human papillomavirus among HIV seropositive women. AIDS 28, 2601–2608, doi: 10.1097/QAD.0000000000000455 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry JM et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer 134, 1147–1155, doi: 10.1002/ijc.28431 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Lee GC et al. What Is the Risk of Anal Carcinoma in Patients With Anal Intraepithelial Neoplasia III? Dis Colon Rectum 61, 1350–1356, doi: 10.1097/DCR.0000000000001219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palefsky JM et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N Engl J Med 386, 2273–2282, doi: 10.1056/NEJMoa2201048 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cajas-Monson LC, Ramamoorthy SL & Cosman BC Expectant Management of High-Grade Anal Dysplasia in People with HIV: Long-term Data. Dis Colon Rectum 61, 1357–1363, doi: 10.1097/DCR.0000000000001180 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Caby F. et al. CD4/CD8 Ratio and the Risk of Kaposi Sarcoma or Non-Hodgkin Lymphoma in the Context of Efficiently Treated Human Immunodeficiency Virus (HIV) Infection: A Collaborative Analysis of 20 European Cohort Studies. Clin Infect Dis 73, 50–59, doi: 10.1093/cid/ciaa1137 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Hema MN et al. Low CD4/CD8 Ratio Is Associated with Non AIDS-Defining Cancers in Patients on Antiretroviral Therapy: ANRS CO8 (Aproco/Copilote) Prospective Cohort Study. PLoS One 11, e0161594, doi: 10.1371/journal.pone.0161594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano-Villar S. et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 9, e85798, doi: 10.1371/journal.pone.0085798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han WM et al. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res Ther 15, 13, doi: 10.1186/s12981-018-0200-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brito MJ et al. CD4(+) and CD8(+) cell populations in HIV-positive women with cervical squamous intra-epithelial lesions and squamous cell carcinoma. Int J Infect Dis 103, 370–377, doi: 10.1016/j.ijid.2020.10.083 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Mussini C. et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2, e98–106, doi: 10.1016/S2352-3018(15)00006-5 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Sanger CB et al. Prevalence of High-Grade Anal Dysplasia and Anal Cancer in Veterans Living With HIV and CD4/CD8 Ratio as a Marker For Increased Risk: A Regional Retrospective Cohort Study. Dis Colon Rectum 64, 805–811, doi: 10.1097/DCR.0000000000002009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geltzeiler CB et al. CD4/CD8 Ratio as a Novel Marker for Increased Risk of High-Grade Anal Dysplasia and Anal Cancer in HIV+ Patients: A Retrospective Cohort Study. Dis Colon Rectum 63, 1585–1592, doi: 10.1097/DCR.0000000000001763 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Geltzeiler CB et al. Anal Intraepithelial Neoplasia Screening With Anal Pap Tests: Follow-up and Corresponding Histology. J Surg Res 244, 117–121, doi: 10.1016/j.jss.2019.06.029 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Tomassi MJ, Abbas MA & Klaristenfeld DD Expectant management surveillance for patients at risk for invasive squamous cell carcinoma of the anus: a large US healthcare system experience. Int J Colorectal Dis 34, 47–54, doi: 10.1007/s00384-018-3167-7 (2019). [DOI] [PubMed] [Google Scholar]
- 21.SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute, <Available from https://seer.cancer.gov/explorer/.> (2022). [Google Scholar]
- 22.McBride JA & Striker R Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog 13, e1006624, doi: 10.1371/journal.ppat.1006624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machalek DA et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 13, 487–500, doi: 10.1016/S1470-2045(12)70080-3 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Wieland U. et al. Smoking and anal high-risk human papillomavirus DNA loads in HIV-positive men who have sex with men. Int J Med Microbiol 305, 689–696, doi: 10.1016/j.ijmm.2015.08.019 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Daling JR et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 101, 270–280, doi: 10.1002/cncr.20365 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues Mantuano N. et al. Hyperglycemia Enhances Cancer Immune Evasion by Inducing Alternative Macrophage Polarization through Increased O-GlcNAcylation. Cancer Immunol Res 8, 1262–1272, doi: 10.1158/2326-6066.CIR-19-0904 (2020). [DOI] [PubMed] [Google Scholar]


