Abstract
Gastroesophageal reflux disease (GERD) is one of the most common problems encountered in outpatient general medicine and gastroenterology clinics. GERD may present with classic esophageal symptoms, extraesophageal symptoms, or mixed symptoms. The diagnosis and treatment of GERD are challenging due to the variety of symptoms and multifactorial pathophysiology. Since there is no consensus on the diagnosis and treatment of GERD in Saudi Arabia, the Saudi Gastroenterology Association established an expert group to formulate a consensus on the clinical care pathway for the diagnosis and treatment of GERD to update health-care providers in Saudi Arabia. The expert group reviewed the literature including recently published international guidelines, clinical trials, and expert opinion and conducted virtual and in-person meetings. A total of 22 statements on the definition, diagnosis, and treatment of GERD were formulated, and three algorithms for the clinical care of GERD were developed with a detailed description for each step. The expert group endorsed the new definition of GERD, the practical principles of interpretation of the diagnostic GERD evaluation, and the practical guidance for GERD treatment including medical, surgical, and endoscopic therapy. The expert group recommends further studies to investigate local data on the diagnosis and treatment of GERD.
Keywords: Acid exposure time, ambulatory pH monitoring, esophagitis, gastroesophageal reflux disease
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a chronic disorder of the upper gastrointestinal (GI) tract in which the stomach contents pass effortlessly into the esophagus, causing troublesome symptoms or complications.[1] GERD is a common condition affecting 13%–20% of the population worldwide, with a clear evidence of increasing prevalence in many countries.[2,3,4,5] It has significant medical consequences, negatively affecting work productivity and the quality of life, and imposes a considerable economic burden in terms of direct and indirect costs[9,10,11] with an estimated cost of around $20 billion per year in the USA, the majority of which is attributable to acid inhibitor therapy.[12,13,14] The etiology of GERD is usually multifactorial and includes poor dietary habits, obesity, smoking, gastroparesis, abnormal esophageal clearance through ineffective peristalsis, disruption of the antireflux barrier associated with a hiatus hernia, and a hypotensive lower esophageal sphincter (LES).[1,6,7,8,9,10,15]
As there is no previous clinical guideline for the diagnosis of GERD in Saudi Arabia, and the perception of proton pump inhibitor (PPI) overuse/misuse, the Saudi Gastroenterology Association (SGA) has developed this consensus on the clinical care pathway to provide health-care providers with the latest evidence on the definition, clinical presentation, diagnosis, and treatment of GERD.
METHODS
The SGA assigned a group of experts from different regions of Saudi Arabia to develop a consensus on the clinical care pathway for patients with GERD based on available published evidence, including population-based studies, randomized clinical trials, current clinical guidelines, and expert opinion. The first virtual meeting took place in early November 2023. The group reviewed the literature and combined its opinion with clinical data to develop an updated consensus statement on the clinical care pathway, which takes into account the context of the health-care system in Saudi Arabia. Subsequently, all members of the working group met and discussed the combined clinical care pathway algorithms and consensus statements in a closed door meeting at the SGA conference (Saudi Digestive Disease Forum, December 2023, Jeddah). The final consensus statements and algorithms for the clinical care pathway were formulated in the final draft and approved by all members.
Three clinical care pathways for GERD were suggested [Figures 1–3]. The first step pertains to the clinical assessment of GERD [Figure 1]. The diagnostic workup for GERD is depicted in a separate pathway [Figure 2]. Treatment of the proven GERD, including medical, endoscopic, and surgical treatment, is shown in Figure 3. The consensus statements are summarized in Table 1, and the evidence and rationale for each consensus statement of the clinical care pathway are described below.
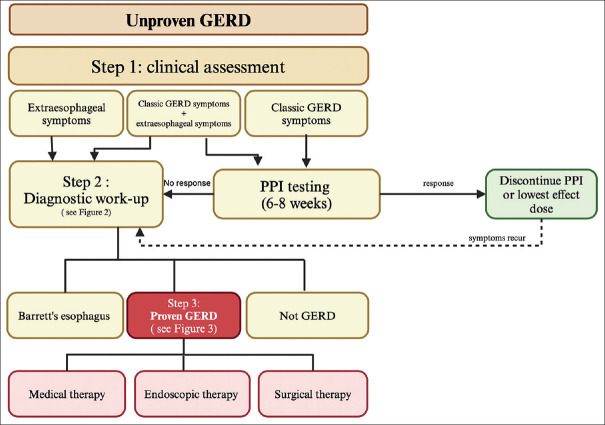
Figure 1.
The algorithm for the clinical care pathway for GERD includes all three steps. Step 1 shows the clinical assessment of patients with GERD symptoms. In patients with classic GERD symptoms, the clinician should administer a standard dose of PPIs for 6–8 weeks. If there is a sufficient response, discontinue the PPI or reduce the dose to the lowest effective dose. In patients with persistent GERD despite PPI therapy, patients with predominant extraesophageal symptoms, patients with alarming features such as dysphagia, weight loss, and anemia, or in patients with multiple risk factors for Barrett’s esophagus (age >50 years, male gender, white race, hiatal hernia, obesity), the clinician should proceed to step 2 for diagnostic workup. GERD = gastroesophageal reflux disease, PPI = proton pump inhibitor
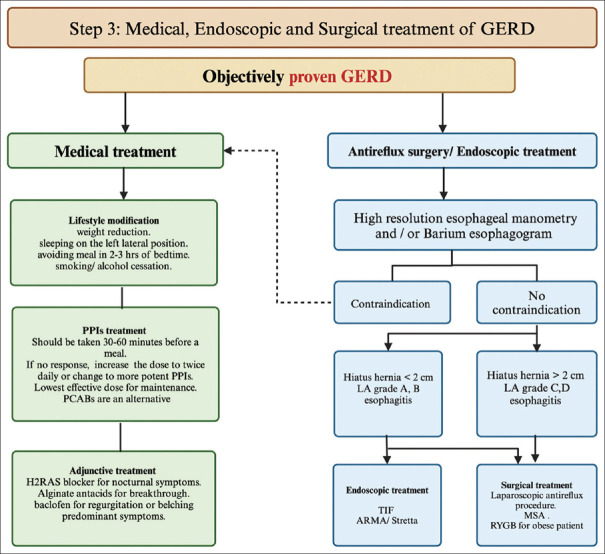
Figure 3.
Algorithm for proven GERD treatment, which includes medical, surgical, and endoscopic treatment. Medical treatment includes lifestyle modification, optimization of PPI treatment, and adjunctive treatment, which should be tailored to the GERD phenotype. If antireflux surgery or endoscopic treatment is planned, the first step is high-resolution esophageal manometry and/or a barium esophagram to assess the esophageal function and rule out contraindications such as achalasia. Endoscopic treatments such as TIF, ARMA, and Stretta should not be offered to patients with hiatus hernia >2 cm or severe esophagitis LA grade C and D. Surgical treatments include laparoscopic fundoplication and MSA. RYGB may be considered for obese patients with proven GERD. ARMA = antireflux mucosal ablation, GERD = gastroesophageal reflux disease, H2RA = histamine type-2 receptor antagonist, LA = Los Angeles, MSA = magnetic sphincter augmentation, P-CAB = potassium-competitive acid blocker, PPI = proton pump inhibitor, RYGB = Roux-en-Y gastric bypass, TIF = transoral incisionless fundoplication
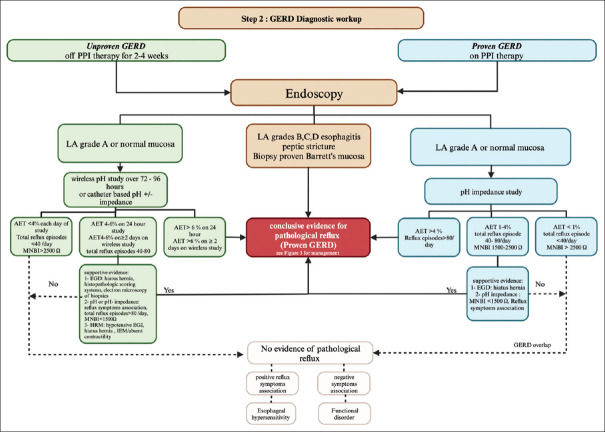
Figure 2.
Algorithm for GERD diagnosis. If GERD is unproven, endoscopy and ambulatory pH monitoring should be performed off PPI therapy. Based on the endoscopic findings, LA esophagitis grades B, C, and D, peptic stricture, and biopsy-proven Barrett’s mucosa are conclusive evidence for pathologic reflux. For patients with endoscopic findings of LA grade A or normal mucosa, either a wireless pH study or catheter-based pH impedance study can be performed off PPI therapy. Reflux monitoring results: if AET >6% on 24-h pH monitoring or AET >6% in ≥2 days on the wireless pH study, this is considered conclusive evidence for pathologic reflux. If AET <4% on each day of the study and total reflux episodes <40/day, it is considered physiologic. If AET is 4%–6% on a 24-h study, AET is 4%–6% in ≥2 days on a wireless pH study, or total reflux episodes are 40–80 per day, further supportive evidence by endoscopy, ambulatory pH monitoring, and HREM is needed. In patients with proven GERD and persistent symptoms despite optimal PPI therapy, endoscopy and 24-h pH impedance monitoring should be investigated on PPI. The presence of LA grades B, C, and D or peptic stricture is a conclusive evidence of refractory pathologic GERD. The pH impedance result: if AET >4% on a 24-h study or reflux episodes >80/day, it is considered conclusive evidence for pathologic reflux. If AET 1%–4% on a 24-h study, total reflux episodes 40–80/day, or mean nocturnal baseline impedance (MNBI) 1500–2500 Ω, it needs more supportive evidence. If AET <1% on a 24-h study, total reflux episode <40/day, or MNBI >2500 Ω, it does not indicate ongoing evidence of GERD and may indicate esophageal hypersensitivity if there are positively associated reflux episodes with symptoms or a functional disorder if negatively associated reflux episodes with symptoms. AET = acid exposure time, GERD = gastroesophageal reflux disease, HREM = high-resolution esophageal manometry, LA = Los Angeles, PPI = proton pump inhibitor
Table 1.
Consensus statements summary
| Definition of GERD: |
|---|
| Statement 1: GERD is defined as objective evidence of GERD by mucosal damage on endoscopy and/or abnormal pH monitoring in the presence of compatible troublesome symptoms |
| Clinical assessment and role of PPI: |
| Statement 2: Two terms are used in the evaluation of symptomatic patients with GERD: unproven and proven. The term “unproven GERD” refers to patients in whom endoscopy and/or ambulatory pH monitoring have not revealed conclusive evidence of GERD. In contrast, the term “proven GERD” is used when there is conclusive evidence for GERD on endoscopy and/or ambulatory pH monitoring |
| Statement 3: Classic GERD symptoms include heartburn, regurgitation, and noncardiac chest pain, while extraesophageal manifestations include globus sensation, asthma, chronic cough, laryngitis, and dental erosions |
| Statement 4: In patients with classic GERD symptoms who have no alarm features, the physician should start 6–8 weeks of treatment with a standard dose of PPI or potassium-competitive acid blocker (P-CAB). If there is an adequate response, PPIs should be discontinued or reduced to the lowest effective dose |
| Statement 5: In patients with extraesophageal GERD manifestations without classic GERD symptoms, the physician should consider a diagnostic GERD workup before initiating PPI therapy |
| Upper GI endoscopy: |
| Statement 6: Endoscopy is recommended as a first step in the evaluation of patients with alarm symptoms (dysphagia, loss of weight, anemia) and in patients with multiple risk factors for Barrett’s esophagus |
| Statement 7: For unproven GERD, discontinuation of PPI for a minimum of 2 weeks before upper GI endoscopy is recommended |
| Statement 8: For upper GI endoscopic examination, the physician should use the LA classification for grading esophagitis, measure the length of the axial hiatal hernia, and use the AFS endoscopic classification of esophagogastric junction integrity and the Prague classification for grading Barrett’s esophagus |
| Statement 9: Mucosal damage on upper GI endoscopy (LA grade B, C, and D esophagitis, peptic stricture, and Barrett’s esophagus) is a conclusive evidence of GERD ( proven GERD) |
| Ambulatory pH monitoring: |
| Statement 10: For unproven GERD, ambulatory pH monitoring should be performed off PPI therapy. If available, wireless pH monitoring for 72–92 hours is preferred |
| Statement 11: In proven GERD with persistent symptoms despite the standard dose of PPI therapy, ambulatory pH impedance monitoring should be performed on PPI therapy to identify the mechanism of persistent GERD symptoms |
| HREM: |
| Statement 12: HREM is recommended in the evaluation of patients with proven GERD and persistent symptoms to identify the mechanism of refractoriness, in patients with persistent GERD symptoms and negative testing to rule out the alternative diagnosis, in patients with regurgitation and negative EGD and pH monitoring testing to rule out achalasia, rumination/belching disorders, and before antireflux surgical and endoscopic treatment to rule out achalasia and absent contractility |
| Barium esophagram: |
| Statement 13: Barium esophagogram is not recommended for the diagnosis of GERD, but can be used to assess structural abnormalities of the esophagus before endoscopic treatment and antireflux surgery |
| Medical treatment: |
| Statement 14: In symptomatic GERD patients, lifestyle changes should be recommended, including weight reduction, avoiding meals within 2–3 hours of bedtime, elevating the head of the bed during sleep, and smoking/alcohol cessation |
| Statement 15: PPIs are more effective in the treatment of proven GERD and healing esophagitis. P-CABs are an alternative when available. Other adjunctive pharmacotherapy should be tailored to the GERD phenotype. Adjunctive medications include H2RAs, which can be used for nocturnal symptoms, alginate antacids, which can be used for breakthrough symptoms, and baclofen, which can be used for regurgitation or belching |
| Endoscopic treatment: |
| Statement 16: TIF may be considered an effective minimal-invasive option in selected patients with proven GERD or who have refused antireflux surgery |
| Statement 17: TIF should not be recommended for patients with severe esophagitis LA grades C and D, hiatal hernias >2 cm in length, a peptic stricture, and Barrett’s esophagus |
| Statement 18: Due to limited data, ARMS, ARMA, and Stretta may be considered as alternative options in patients with proven GERD who are not candidates for antireflux surgery or who refuse antireflux surgery and TIF |
| Surgical treatment: |
| Statement 19: Antireflux surgery (laparoscopic fundoplication and MSA) is recommended as an effective treatment for objectively proven GERD |
| GERD and bariatric surgery: |
| Statement 20 : RYGB is considered as a treatment option for proven GERD in obese patients |
| GERD treatment in pregnancy: |
| Statement 21: Lifestyle changes are recommended for pregnant women with GERD symptoms. If lifestyle changes fail, antacids and sucralfate are the first choice of treatment |
| Statement 22: PPIs (except omeprazole) are considered safe during pregnancy |
AFS=American Foregut Society, ARMA=antireflux mucosal ablation, ARMS=antireflux mucosectomy, GERD=gastroesophageal reflux disease, GI=gastrointestinal, H2RA=histamine type-2 receptor antagonist, HREM=high-resolution esophageal manometry, MSA=magnetic sphincter augmentation, P-CAB=potassium-competitive acid blocker, PPI=proton pump inhibitor, RYGB=Roux-en-Y gastric bypass, TIF=transoral incisionless fundoplication
THE NEW DEFINITION OF GERD
Statement 1: GERD is defined as objective evidence of GERD by mucosal damage on endoscopy and/or abnormal pH monitoring in the presence of compatible troublesome symptoms.
According to the Montreal Consensus, GERD is defined as the reflux of gastric contents into the esophagus, causing bothersome symptoms and possible complications.[1] The development of GERD requires either increased esophageal exposure to gastric juice or a reduced threshold for epithelial injury and perception of symptoms.[15] The American College of Gastroenterology guideline objectively defines GERD by the presence of mucosal damage on endoscopy and/or abnormal ambulatory reflux monitoring.[16] Recently, the Lyon Consensus 2.0 updated its criterion for the modern diagnosis of GERD and reaffirmed the concepts of unproven and proven GERD to guide the evaluation of symptomatic patients. According to this consensus, patients with no prior conclusive evidence of GERD are best evaluated with prolonged wireless pH monitoring or catheter-based pH monitoring off antisecretory medication, whereas patients with conclusive evidence of GERD and persistent symptoms are evaluated using pH impedance monitoring with optimizing antisecretory therapy.[17]
PATHOPHYSIOLOGY
The pathogenesis of GERD is multifactorial, including LES pressure dysfunction, esophageal motility disorders, gastric acid production (in a small proportion of patients), as well as other emerging theories.[15,18] LES plays a role in preventing the reflux of stomach contents into the esophagus. When LES is not functioning properly; decreased LES pressure and/or transient relaxation contributes to the development of GERD. In addition, a hiatal hernia can weaken LES, making individuals more susceptible to reflux.[19,20] Esophageal motility disorders, including ineffective esophageal motility and impaired clearance of refluxed material from the esophagus, contribute to persistent symptoms and increased severity of GERD.[21,22,23] These motility abnormalities can result from neurogenic dysfunction, altered esophageal anatomy, or connective tissue disorders. Increased acid production is often due to lifestyle choices. Prolonged exposure to gastric acid can result in inflammation and erosion, potentially leading to Barrett’s esophagus.[24,25]
Recent studies have shown how genetic factors, inflammation, and the esophageal microbiome can influence GERD.[25] In addition, improvements in imaging techniques and ambulatory pH monitoring have given us insights into how esophageal function and reflux patterns change over time.
Step 1: Clinical assessment and response to PPI therapy [Figure 1]
Statement 2: Two terms are used in the evaluation of symptomatic patients with GERD: unproven and proven. The term “unproven GERD” refers to patients in whom endoscopy and/or ambulatory pH monitoring have not revealed conclusive evidence of GERD. In contrast, the term “proven GERD” is used when there is conclusive evidence for GERD on endoscopy and/or ambulatory pH monitoring.
Statement 3: Classic GERD symptoms include heartburn, regurgitation, and noncardiac chest pain, while extraesophageal manifestations include globus sensation, asthma, chronic cough, laryngitis, and dental erosions.
Statement 4: In patients with classic GERD symptoms who have no alarm features, the physician should start 6–8 weeks of treatment with a standard dose of PPI or potassium-competitive acid blocker (P-CAB). If there is an adequate response, PPIs should be discontinued or reduced to the lowest effective dose.
Statement 5: In patients with extraesophageal GERD manifestations without classic GERD symptoms, the physician should consider a diagnostic GERD workup before initiating PPI therapy.
Obtaining a good history is critical in the evaluation of GERD. This step focuses on presenting symptoms: alarm symptoms, such as dysphagia, loss of weight, anemia, and GI bleeding; response to a PPI trial; and other factors that may influence the severity of reflux, such as obesity, scleroderma, or diabetes mellitus.[16,17] Risks for Barrett’s esophagus include age (above 50 years), white race, male gender, hiatal hernia, and obesity.[16,17] In addition, assessment of visceral sensitivity, anxiety, and hypervigilance is an important part of evaluating a patient with GERD and its treatment strategies.
Step 1.1: Classic GERD symptoms
Typical GERD symptoms include heartburn, esophageal chest pain, and regurgitation. Heartburn is a burning sensation in the retrosternal area that originates in the epigastric region and spreads toward the throat. However, regurgitation is the effortless reflux of stomach contents into the mouth and can be associated with a bitter, sour taste. Chest pain may be a consequence of GERD and may be associated with heartburn or regurgitation or occurs independently of both.[16,17] Notably, other etiologies can have similar symptoms and should be addressed in those at risk, including cardiac and pulmonary diseases, eosinophilic esophagitis, achalasia, rumination, and functional disorders.[16,17] When the presentation is chest pain, an evaluation for a cardiac cause should precede esophageal evaluation, especially in the presence of risk factors for cardiovascular disease and in middle-aged and older individuals.[16,17]
Step 1.2: Extraesophageal GERD symptoms
Extraesophageal symptoms that can be attributed to GERD include globus sensation, laryngitis, chronic cough, frequent throat clearing, wheezing, dental erosion, and pulmonary fibrosis.[16,17,26] However, it is difficult to attribute these symptoms to GERD unless they occur concurrently with typical symptoms or evidence of mucosal damage on endoscopy or abnormal pH monitoring. Differential diagnoses for typical GERD symptoms include eosinophilic esophagitis, achalasia, rumination syndrome, and functional disorders, while the differential diagnoses for atypical symptoms are much broader and difficult to prove, especially when these symptoms are not accompanied by typical symptoms.
Step 1.3: Role of PPI testing
The latest guidelines recommend a 4–8 weeks standard dose of a PPI or P-CAB trial.[16,17,27,28] A suboptimal response to therapy or recurrence of symptoms despite response to therapy is a reason for further investigation with endoscopy and reflux pH monitoring.[16,27,28] Although response to PPI in patients with classic GERD symptoms has been used as a diagnostic test for GERD (with the reference standard being endoscopic evaluation and pH reflux testing), according to a meta-analysis, this test has a low sensitivity (78%) and specificity (54%) and is less useful in patients with chest pain.[16,29] It has been argued that recurrence of symptoms is inherently common in GERD and a large proportion of patients would eventually require endoscopy. In addition, objective signs of reflux disease (i.e., esophagitis) may be detected at the initial endoscopy, which may reduce the need for reflux monitoring and detect Los Angeles (LA) class C and D esophagitis, thereby avoiding long-term complications in these patients.[29,30]
Step 2: Diagnostic workup for GERD [Figure 2]
Current guidelines emphasize the importance of an objective, evidence-based diagnosis to confirm the diagnosis, assess the severity of GERD, and exclude other conditions. Upper GI endoscopy, ambulatory pH monitoring, and high-resolution esophageal manometry (HREM) are valuable diagnostic tools that allow direct visualization of esophageal abnormalities, assess esophageal acid exposure, and evaluate esophageal motility, respectively. This step focuses on these examination tools and their interpretation. By combining these objective measurements with clinical information, health-care providers can make a more informed decision regarding GERD diagnosis and treatment plans and ensure that patients receive appropriate care for their complaints.
Step 2.1: Upper GI endoscopy
Indication for endoscopy:
Statement 6: Endoscopy is recommended as a first step in the evaluation of patients with alarm symptoms (dysphagia, loss of weight, anemia) and in patients with multiple risk factors for Barrett’s esophagus.
Statement 7: For unproven GERD, discontinuation of PPI for a minimum of 2 weeks before upper GI endoscopy is recommended.
Statement 8: For upper GI endoscopic examination, the physician should use the LA classification for grading esophagitis, measure the length of the axial hiatal hernia, and use the American Foregut Society (AFS) endoscopic classification of esophagogastric junction (EGJ) integrity and the Prague classification for grading Barrett’s esophagus.
Statement 9: Mucosal damage on upper GI endoscopy (LA grade B, C, and D esophagitis, peptic stricture, and Barrett’s esophagus) is conclusive evidence of GERD (proven GERD).
Upper GI endoscopy is considered an important tool for the diagnosis of GERD and its associated complications. It allows visual inspection of the distal esophagus and the EGJ, hiatal hernia measurement, and dynamic assessment of the Hill flap valve. Endoscopy is usually recommended in patients with GERD who have experienced persistent symptoms despite optimal PPI therapy; those who have alarm symptoms such as dysphagia, loss of weight, and anemia; those who have complications of GERD, such as an esophageal stricture, Barrett’s esophagus, and/or esophageal adenocarcinoma; and in patients with extraesophageal symptoms, such as cough, or asthma, suspected to be caused by GERD.[31,32,33]
Discontinuation of PPIs before endoscopy:
Discontinuation of PPIs before endoscopy is important for several reasons: (1) it prevents masking of esophageal abnormalities[32] including eosinophilic esophagitis (EoE), since eosinophils may be suppressed by PPIs;[34] (2) it enables accurate assessment of esophageal acid exposure in the case of esophageal pH monitoring for accurate esophageal acid exposure, a key factor in GERD diagnosis;[16,35] and (3) it facilitates the assessment of esophageal motility as PPIs can influence the assessment of esophageal motility by manometry.[36] The specific duration of PPI discontinuation before endoscopy may depend on several factors, including the type of procedure, patient’s overall health status, and the severity of GERD symptoms. Therefore, in patients with severe GERD symptoms, it may be decided to discontinue PPIs for a short period to avoid rebound symptoms. However, this should be balanced with the need for an accurate endoscopic assessment.[37]
Grading esophagitis severity and EGJ integrity assessment:
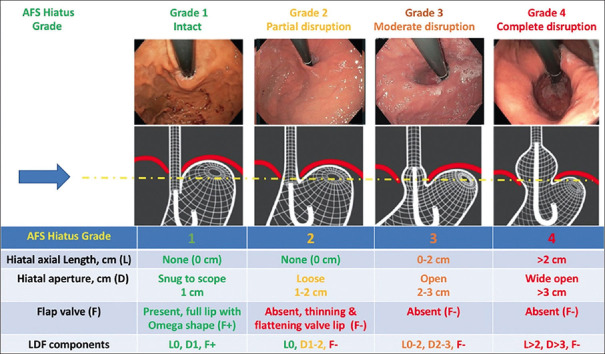
Recent guidelines recommend the use of LA classification to grade the severity of esophagitis [Table 2] and the AFS endoscopic classification to assess EGJ integrity for accurate diagnosis and treatment of GERD [Figure 4].[16,17,38,39] Grading esophagitis severity provides several important advantages: (1) distinguishing among mild, moderate, and severe GERD; (2) informing treatment planning to ensure appropriate treatment intensity based on the disease severity; (3) monitoring the response to assess treatment efficacy and for making appropriate adjustments; and (4) prognosis and risk stratification to predict the likelihood of complications and provide preventive measures.[40] The recent guidelines endorse LA grades B, C, and D, biopsy-proven Barrett’s esophagus, and peptic strictures as conclusive evidence of GERD.[17] The Hill classification is a simple grading system for the relationship between gastroesophageal flap valve (GEFV) competence and GERD symptoms. Patients with a Hill grade III or IV GEFV incompetence are more likely to experience GERD symptoms than patients with a grade of I or II.[41,42] The new AFS endoscopic classification is more comprehensive than the Hill classification and contains three components called LDF components: the length of the axial hiatal hernia measured in centimeters, the hiatal aperture diameter measured in centimeters, and the presence or absence function of the flap valve. The AFS grade has four grades: grade 1 represents an intact EGJ valve, while grades 2–4 represent EGJ disruption with progressive degrees.[39]
Table 2.
The LA classification of esophagitis
| Grade | Endoscopic description |
|---|---|
| LA grade A | One or more mucosal break <5 mm that does not extend between the top of two mucosal folds |
| LA grade B | One or more mucosal break >5 mm that does not extend between the top of two folds |
| LA grade C | One or more mucosal break that is continuous between the top of two or more mucosal break, but which involves <75% of the circumference |
| LA grade D | One or more mucosal break which involves at least 75% of the esophageal circumference |
LA=Los Angeles
Figure 4.
The AFS endoscopic classification for EGJ integrity by using LDF. AFS = American Foregut Society. LDF = length/diameter/flap valve
Tissue sampling and histology:
Tissue sampling can be used as a supportive measure for GERD diagnosis by revealing characteristic microscopic changes in the esophageal lining and detecting Barrett’s esophagus. Current guidelines do not require routine esophageal biopsies unless there is a history of dysphagia, chest pain during eating, endoscopic suspension for eosinophilic esophagitis, or Barrett’s esophagus.[16,17,43]
Step 2.2: Ambulatory pH monitoring
Statement 10: For unproven GERD, ambulatory pH monitoring should be performed off PPI therapy. If available, wireless pH monitoring for 72–92 hours is preferred.
Statement 11: In proven GERD with persistent symptoms despite the standard dose of PPI therapy, ambulatory pH impedance monitoring should be performed on PPI therapy to identify the mechanism of persistent GERD symptoms.
Since 70% of symptomatic GERD patients have normal esophageal mucosa on endoscopic examination, the next step is ambulatory pH monitoring, which is the gold standard for quantification of esophageal acid burden, reflux events, and correlation between symptoms and reflux events. Ambulatory pH monitoring is available through wireless pH monitoring and 24-h transnasal catheter with or without impedance. In patients with unproven GERD, ambulatory pH monitoring off PPI therapy should be performed to determine the presence of GERD at baseline. However, if GERD is objectively proven by mucosal damage on endoscopy or previous abnormal pH monitoring, ambulatory pH impedance monitoring on PPI therapy should be performed to study the mechanism for refractory GERD.
Wireless pH monitoring off PPI therapy:
In patients with unproven GERD, prolonged wireless pH monitoring is the preferred diagnostic tool. It can be placed during an upper endoscopy and can monitor acid reflux over an extended period, up to 96 h. The 72–96 h of pH monitoring has a better diagnostic performance and prediction of PPI discontinuation than 48 h of pH monitoring.[37,43,44,45] Due to the cost and availability of wireless pH monitoring, 24-h transnasal pH monitoring with or without impedance remains an alternative test.
Ambulatory pH impedance monitoring off PPI therapy:
Ambulatory pH impedance monitoring off PPI therapy is the preferred diagnostic tool for unproven GERD when regurgitation is a predominant GERD symptom, belching, rumination syndrome is suspected, or extraesophageal GERD symptoms are present. Ambulatory pH impedance monitoring off a PPI is a more accurate test than pH monitoring alone and can help rule out other conditions that may mimic GERD.[44,46,47]
Ambulatory pH impedance monitoring on PPI therapy:
Ambulatory pH impedance monitoring on PPI therapy is a valuable aid in patients with previous conclusive evidence of GERD who do not respond to twice-daily PPI therapy. Ambulatory pH impedance provides a comprehensive assessment of esophageal reflux and provides valuable insights into the frequency and severity of both weakly acidic and nonacidic reflux events. In addition, it can help determine the phenotype of patients who would respond to antireflux surgery by determining the reflux burden.[45,46,47,48]
Interpretation of ambulatory pH monitoring:
Based on the recent Lyon consensus 2.0, in wireless pH monitoring off PPI therapy, an acid exposure time (AET) >6.0% for ≥2 days is consistent with conclusive evidence of GERD, an AET <4.0% on all days with negative symptom association rules out GERD, and an AET between 4.0% and 6.0% is an inconclusive evidence of GERD and requires further testing.[17] On pH impedance monitoring off PPI therapy, an AET >6.0% is a conclusive evidence for GERD, an AET <4.0% is physiologic, and an AET between 4.0% and 6% is inconclusive.[17] Furthermore, according to the Lyon consensus, more than 80 reflux episodes per day is an adjunctive evidence for objective GERD, a total reflux episode of less than 40 events/day is physiologic, and between 40 and 80 events/day is inconclusive.[17] For pH impedance mentoring on PPI therapy, an AET >4% and more than 80 reflux episodes per day on PPI therapy are indicative evidence of refractory GERD.[17] In addition, the Lyon consensus endorses baseline impedance as an adjunctive test of value for GERD diagnosis, especially when AET is inconclusive and can be used to measure esophageal mucosal integrity.[17] A baseline impedance of <1500 Ω is additional evidence for GERD, while a baseline impedance of >2500 Ω rules out pathologic GERD.
Ambulatory reflux monitoring can also be used to evaluate the relationship between reflux episodes and GERD symptoms. A symptom index (SI) is considered positive if ≥50% of symptoms are associated with reflux events. A symptom–reflux association (Symptom-reflux association probability [SAP]) is considered positive if the probability of a true association between reflux and symptom is >95%. If no mucosal damage is detected at endoscopy, the physiological AET, and reflux episodes are within normal limits and a positive SI and/or SAP is present, this is compatible with reflux hypersensitivity, while a negative SI and SAP is compatible with a functional disorder.[17]
Step 2.3: High-resolution esophageal manometry
Statement 12: HREM is recommended in the evaluation of patients with proven GERD and persistent symptoms to identify the mechanism of refractoriness, in patients with persistent GERD symptoms and negative testing to rule out the alternative diagnosis, in patients with regurgitation and negative esophagogastroduodenoscopy (EGD) and pH-monitoring testing to rule out achalasia, rumination/belching disorders, and before antireflux surgical and endoscopic treatment to rule out achalasia and absent contractility.
HREM is a tool for evaluating patients with suspected esophageal motility disorders, including those with reflux symptoms, and provides an assessment of esophageal contractility and coordination.[49,50,51] It should be used in cases with proven GERD and persistent symptoms and in those with GERD symptoms with negative testing to rule out alternative diagnoses. Patients with regurgitation and a negative EGD and ambulatory pH monitoring need to have HREM to rule out achalasia and rumination/belching disorders. HREM can be used to diagnose motility disorders such as achalasia, which may contribute to GERD symptoms. In addition to identifying motility disorders, HREM can also assess esophageal function in patients with conditions such as scleroderma and systemic lupus erythematosus.[49,50,52] It can also be used to detect and measure hiatal hernias and assess the function of LES. In addition, HREM plays an important role in the assessment of esophageal function before antireflux surgery, especially fundoplication.[53,54]
Step 2.4: Barium esophagram
Statement 13: Barium esophagogram is not recommended for the diagnosis of GERD, but can be used to assess structural abnormalities of the esophagus before endoscopic treatment and antireflux surgery.
Barium esophagogram is used to assess structural abnormalities of the upper GI tract, particularly in patients with dysphagia associated with GERD.[55] Studies have shown its sensitivity and specificity for the diagnosis of GERD to be 50% and 64%, respectively.[56,57] Current guidelines do not recommend this as the sole test for diagnosing GERD.[16]
Step 2.5: Emerging tests for GERD
The field of GI diagnostics is constantly evolving, and innovative tests are emerging that allow a more accurate and comprehensive evaluation of GERD. These emerging tests have the potential to improve diagnostic accuracy, identify patients with hidden GERD, and develop personalized treatment strategies. Novel impedance markers, such as bile acid and transmucosal impedance, are being investigated for their potential to detect and characterize reflux events. In particular, transmucosal impedance, which measures the resistance of the esophageal mucosa, provides information about the mucosal integrity and the function of the reflux barrier.[58,59] Esophageal impedance spectroscopy is a noninvasive test that measures the electrical impedance of the esophagus at different frequencies. This test can provide information about the composition of the esophageal mucosa, inflammation, and the presence of reflux material.[54] Salivary pepsin measurement is a noninvasive test that detects the presence of pepsin, a digestive enzyme produced by the stomach, in saliva. Elevated levels of pepsin in saliva may indicate the presence of hidden GERD, particularly in patients with extraesophageal symptoms or in patients who respond poorly to PPI therapy.[58,60] Advanced methods for studying esophageal motility, such as artificial intelligence (AI), have been used to analyze data from various sources such as endoscopic images, ambulatory reflux monitoring results, and patient symptoms to aid in the diagnosis of GERD through algorithms that may be able to identify patterns and correlations for early detection and accurate GERD diagnosis.[61]
Step 2.6: Refractory GERD workup
Two groups of patients could have persistent GERD symptoms despite optimizing PPI therapy. The first group consists of patients with persistent symptoms and no previous objective evidence of GERD (unproven GERD) based on endoscopy or ambulatory pH monitoring. In these patients, there is a need to review the patient’s history and response to PPI therapy, and confirm the diagnosis of GERD by endoscopy and/or ambulatory pH monitoring off PPI therapy.[16,17] The second group includes patients with symptoms and previous conclusive evidence of GERD (proven GERD). In this context, there is a need to reassess the refractoriness of reflux burden by reviewing the patient’s history, compliance with PPI therapy, and performing endoscopy and/or ambulatory pH monitoring on PPI therapy. HREM is warranted to investigate the mechanism of reflux and rule out non-reflux causes.[16,17]
Step 3: GERD treatment [Figure 3]
GERD is not experienced in the same way by all patients. The information gained from the diagnostic evaluation for GERD will help the clinician develop therapeutic strategies for the treatment of GERD. Treatment of classic and extraesophageal GERD aims to achieve and maintain symptom relief, heal mucosal damage, and prevent complications. Therapeutic strategies include medical, endoscopic, and surgical treatment. HREM is recommended for any patient being considered for antireflux surgery or endoscopic therapy to assess the esophageal function, rule out contraindications, such as achalasia, and to investigate the mechanism of GERD for better therapeutic strategies. If HREM is not available, a barium esophagogram would be an option before antireflux surgery and endoscopic therapy to exclude achalasia.
Step 3.1: Medical management
Statement 14: In symptomatic GERD patients, lifestyle changes should be recommended, including weight reduction, avoiding meals within 2–3 hours of bedtime, elevating the head of the bed during sleep, and smoking/alcohol cessation.
Statement 15: PPIs are more effective in the treatment of proven GERD and healing esophagitis. P-CABs are an alternative when available. Other adjunctive pharmacotherapy should be tailored to the GERD phenotype. Adjunctive medications include histamine type-2 receptor antagonists (H2RAs), which can be used for nocturnal symptoms, alginate antacids, which can be used for breakthrough symptoms, and baclofen, which can be used for regurgitation or belching.
Lifestyle modifications:
Many lifestyle and dietary changes are considered the first step and are recommended for patients with GERD. Current guidelines recommend weight loss, avoiding meals 3–4 h before bedtime, and elevating the head of the bed when sleeping.[62] A prospective study has shown that sleeping in the left decubitus position significantly shortens the time of acid exposure and the time of acid clearance.[63] There are conflicting data on the effect of avoiding carbonated drinks and spicy foods and reducing certain ingredients such as onion, peppermint, tomatoes, and chocolate.[64] Therefore, a personalized approach is suggested by reducing trigger foods, rather than avoiding a long list of foods. Smoking and alcohol consumption are also discouraged.[16]
Pharmacologic treatment:
Medications are the mainstay of GERD treatment. They work by neutralizing or suppressing stomach acid. They come in the form of antacids, H2RAs, and PPIs, which can be administered daily or as needed, depending on the severity of symptoms. Antacids are often used to treat heartburn. They have a short and rapid effect on symptoms.[65] H2RAs are used to treat GERD symptoms and have been considered for uncontrolled nocturnal reflux symptoms, in addition to PPI therapy. The use of H2RAs has been limited due to their lower overall effect on the healing of erosive esophagitis, compared to PPIs and tachyphylaxis.[66] PPIs are the treatment of choice for symptomatic GERD owing to their efficacy in relieving symptoms, healing erosive esophagitis, and preventing complications.[67,68] Approximately seven PPIs are available with similar efficacy and safety profiles and should be taken 30–60 min before a meal, except dexalasoprazole, which can be taken at any time. In low-risk patients who do not have erosive esophagitis or Barrett’s esophagus, PPIs can be administered as needed and discontinued when symptoms have resolved. In patients who require a PPI for maintenance, the lowest possible dose should be used. The use of PPIs is strongly recommended in patients with Barrett’s esophagus and grade C and D erosive esophagitis.[16] P-CABs are a new additional class of acid blockers that have shown promising results in the treatment of GERD and erosive esophagitis. Due to their longer half-life, it is not necessary to restrict the timing of intake. A recent study showed that vonoprazan is noninferior to lansoprazole and has a greater effect on the healing of severe erosive esophagitis.[69] Sucralfate is a mucosal-protective agent as well as alginate. It relieves the symptoms of GERD, similar to H2RAs, and has been used in pregnancy due to its local effect.[70] Prokinetics play a limited role in GERD treatment due to their side effects and limited data on their efficacy. They increase LES pressure, improve esophageal peristalsis, and assist gastric emptying. Current guidelines do not support the use of prokinetics for GERD. Baclofen is a gamma-aminobutyric acid agonist with limited use in refractory GERD. It has a minimal effect in reducing postprandial reflux and nocturnal symptoms, in addition to episodes of belching and regurgitation.[71]
Step 3.2: Endoscopic treatment
Statement 16: TIF may be considered an effective minimal-invasive option in selected patients with proven GERD or who have refused antireflux surgery.
Statement 17: TIF should not be recommended for patients with severe esophagitis LA grades C and D, hiatal hernias >2 cm in length, a peptic stricture, and Barrett’s esophagus.
Statement 18: Due to limited data, antireflux mucosectomy (ARMS), antireflux mucosal ablation (ARMA), and Stretta may be considered as alternative options in patients with proven GERD who are not candidates for antireflux surgery or who refuse antireflux surgery and TIF.
Over the past two decades, several endoscopic devices have been introduced for the treatment of GERD, most of which have been withdrawn from the market due to safety and efficacy concerns. Currently available endoscopic GERD treatments are radiofrequency antireflux treatment (Stretta; Restech, Houston, TX, USA), GERDX (G-Surg), and EsophyX (endogastric solutions). Studies on endoscopic procedures have generally excluded patients with hiatal hernias >2 cm, severe esophagitis (LA grades C and D), peptic strictures, and long-segment Barrett’s esophagus.
GERD-X system – TIF:
GERD-X enables the endoscopic fundoplication technique, also known as the endoscopic full-thickness fundoplication technique. This procedure aims to narrow the cardia of the stomach by including part of the proximal stomach using a system of two sutures. In a randomized, sham-controlled trial study of 70 patients, the technique resulted in a significant improvement in health-related quality of life (GERD-HRLQ) at 3 months, with 62.8% of patients no longer taking PPIs at 12 months, compared to 11.4% in the sham group.[72] The procedure is considered safe, effective, and improves the short- and long-term quality of life of PPI-dependent patients, mostly nonerosive patients with reflux disease. No serious procedure-related adverse events were observed. Moderate adverse events included left-sided chest pain and pleural effusion with fever. Satisfaction with GERD-X at 12 months was 71%.[72]
EsophyX system – TIF:
This technique mimics surgical fundoplication 270°–320° at the EGJ by plicating a portion of the proximal stomach with T-fasteners. Several studies have demonstrated the efficacy of TIF in significantly improving classic and extraesophageal GERD-related symptoms. A meta-analysis of eight studies (418 patients, males 55.5%) examined the long-term outcomes of TIF, with a mean follow-up of 5.3 years. The analysis showed a pooled proportion of patient-reported satisfaction before and after TIF of 12.3% and 70.6%, respectively. The pooled rates of patients discontinuing PPIs completely and taking PPIs occasionally were 53.8% and 75.8%, respectively. The overall pooled rates of normalization of heartburn and regurgitation scores were 73% and 86%, respectively.[73] A systematic review of 10 studies (564 patients) at the 6- and 12-month follow-up time points showed a mean reduction in reflux symptom index (RSI) score of 15.72 points and 14.73 points, respectively, after TIF, with a technical success rate of 99.5% and a pooled adverse event rate of 1%. At both time points, more than two-thirds of patients were satisfied with their health status and about three-quarters were able to discontinue their daily PPIs.[74]
ARMS and ARMA procedures:
ARMS involves resection of the gastric cardia mucosa to reduce the opening of the gastroesophageal junction by healing and resulting scar formation. ARMA is based on the same principle and uses argon plasma coagulation to induce scar formation. Reports on the results of both techniques are encouraging. A meta-analysis of 25 studies, 15 of which were nonrandomized (12 ARMS, n = 331; three ARMA, n = 130), was performed in patients with refractory GERD. The technical success rate was 100%. The pooled short-term (within the first 6 months), 1-year, and 3-year clinical success rates were 78%, 72%, and 73%, respectively.[75] Similar to other endoscopic interventions, clinical trials play a critical role in understanding the outcomes and safety profiles of ARMS and ARMA. Rigorous research protocols, including randomized controlled trials (RCTs) and systematic reviews, are essential to fully evaluate their impact on GERD symptoms, quality of life, and the use of PPIs after these interventions. These studies will help to refine patient selection criteria and optimize the long-term efficacy of the procedure.
Stretta procedure:
Evaluation of the mechanism of the Stretta procedure as an antireflux therapy is challenging. Originally, the Stretta procedure was thought to control reflux by inducing swelling and mechanical change at the EGJ. In an early study, Stretta was shown to improve GERD symptoms and quality of life 6 months after the procedure, but had no effect on reducing esophageal acid exposure. Other systematic reviews and meta-analyses showed conflicting results regarding Stretta’s efficacy. While a meta-analysis limited to RCTs showed no improvement in esophageal acid exposure, quality of life, or the ability to discontinue PPIs,[76] in another meta-analysis including both controlled and open-label cohort studies, Stretta showed an improvement in esophageal acid exposure, quality of life, and the ability to discontinue PPIs.[77] Stretta appears to be a safe procedure with mild side effects such as chest pain, gastroparesis, and fever. Due to the discrepancy between the current guideline recommendations, Stretta may be considered for patients who refuse antireflux surgery and TIF.[16,78]
Step 3.3: Surgical management
Statement 19: Antireflux surgery (laparoscopic fundoplication and magnetic sphincter augmentation [MSA]) is recommended as an effective treatment for objectively proven GERD
Laparoscopic fundoplication:
Laparoscopic fundoplication was introduced in the early 1990s and is the standard method for fundoplication. There are two types: complete fundoplication (Nissen fundoplication- 360°) and partial fundoplication (Toupet fundoplication- 270° and Dor fundoplication-180°).[79] Recent studies have shown that 70%–90% of patients who have undergone fundoplication no longer take PPI therapy during a follow-up period of 3–5 years. A recent meta-analysis showed that heartburn and regurgitation occur less frequently in the surgical group compared to the drug therapy group.[80] In addition, both types of fundoplication were equally good in relieving GERD symptoms; however, postoperative dysphagia and difficulty belching were more common in the Nissen fundoplication group.[81] However, partial fundoplication is the preferred antireflux surgery in patients with HREM results which show ineffective esophageal motility disorder or impaired peristaltic reserve.[82]
Magnetic sphincter augmentation:
MSA is a minimally invasive and reversible procedure. A chain of magnetic cores and titanium beads is used. This encircles the esophagus distal to LES and prevents reflux.[83] There were no differences in GERD symptom control rates and PPI use after the procedure between the MSA and fundoplication groups.[83] The most common postoperative adverse event was dysphagia in 11% after 1 year and 4% after 3 years, and the risk of erosion was 3% at 4 years.[83,84,85] The contraindications for MSA are large hiatal hernias and severe esophagitis at endoscopy.[86] However, MSA is one of the options for the treatment of GERD that develops after bariatric surgery.[87]
GERD and bariatric surgery:
Statement 20: Roux-en-Y gastric bypass (RYGB) is considered a treatment option for proven GERD in obese patients.
The prevalence of obesity has increased, as has its associated complications on the health of individuals and health-care systems.[88] GERD is one of the common conditions associated with obesity, and the association between GERD and obesity has been established, including hiatal hernias and high transdiaphragmatic pressure gradient due to the increase in abdominal pressure.[88,89] Obstructive sleep apnea is also closely related to GERD.[89]
Bariatric procedures and endoluminal bariatric interventions are effective methods for managing obesity, and the association between these procedures and increased GERD symptoms is established.[89,90] The association between intragastric balloons and GERD reported in the literature is approximately 7%.[90] Patients usually respond well to treatment with PPIs.[90]
GERD in endoluminal vertical gastroplasty is rare[91] and could develop in 1.9% of patients.[92,93,94] Endoscopic GI bypass (Endobarrier) has been withdrawn from the market due to the development of liver abscesses.[95] The relationship between GERD and duodenal mucosal resurfacing is unclear and unknown.[96]
Most studies have demonstrated a close association between laparoscopic sleeve gastrectomy and GERD.[97] The prevalence of GERD ranges from 33.0% to as high as 84%.[98,99,100,101] The role of upper endoscopy and hiatal hernia repair is important in reducing the prevalence of GERD. A study reported that de novo erosive esophagitis occurred in 15.0% of patients with adjustable bands[102] and it was associated with complications like band erosion, migration, and achalasia-like obstructive symptoms.
RYGB showed improvement in GERD symptoms and weight reduction in obese patients. The improvement in GERD symptoms is due to several mechanisms and is independent of weight loss.[103,104,105,106] The current guidelines recommend RYGB as an effective treatment for obese patients with proven GERD.[16]
GERD treatment in pregnancy:
Statement 21: Lifestyle changes are recommended for pregnant women with GERD symptoms. If lifestyle changes fail, antacids and sucralfate are the first choice of treatment.
Statement 22: PPIs (except omeprazole) are considered safe during pregnancy.
Pregnant women often suffer from GERD. The prevalence of GERD in pregnancy is high, and can begin as early as the first trimester and worsen in the third trimester if left untreated.[107] Heartburn is the most common GERD symptom in pregnancy.[108,109] Medical treatment based on guidelines is the first step.[110] The diagnosis of GERD in pregnancy depends on classic GERD symptoms. Endoscopy and pH monitoring are not recommended in pregnancy.[111] Management of GERD in pregnancy differs from that in other patients and takes into account the safety and efficacy of medications in the first trimester. In general, lifestyle modifications are helpful in controlling some GERD symptoms, but might not completely relieve symptoms. If lifestyle changes are not effective, medications can be administered. Antacids are helpful in treating GERD during pregnancy as the initial therapy. These are nonsystemic medications that do not cause harm to the fetus. Sucralfate is a mucosal protective agent with a very good safety profile and is considered the first choice for treatment.[112] H2RAs are the most commonly used systemic drug for the treatment of GERD in pregnant women and are considered category (B) drugs. PPIs can be used for severe GERD symptoms in pregnancy, and all PPIS are category B drugs, except omeprazole, which is a category C drug. Promotility drugs (metoclopramide) are often used to improve gastric emptying and increase LES pressure.[112,113]
CONCLUSION
This consensus on the clinical care pathways has reviewed the diagnostic workup and treatment of GERD. We have proposed a three-step algorithm for the approach to diagnosis and treatment of the major presentations of GERD symptoms and have provided our discussion in the body of this consensus. We expect that new diagnostic and treatment methods for GERD in the future, together with further research in endoscopic treatment and advances in AI will change the future of diagnosis and treatment of GERD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors extend their appreciation to Dr. John Pandolfino and Dr. C Prakash Gyawali for reviewing the manuscript.
The authors extend their appreciation to SAGE publications for reprint permission from the paper (Nguyen NT, Thosani NC, Canto MI, et al. The American Foregut Society White Paper on the Endoscopic Classification of Esophagogastric Junction Integrity. Foregut. 2022;2(4):339-348).
REFERENCES
- 1.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus G. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almadi MA, Almousa MA, Althwainy AF, Altamimi AM, Alamoudi HO, Alshamrani HS, et al. Prevalence of symptoms of gastroesopahgeal reflux in a cohort of Saudi Arabians: A study of 1265 subjects. Saudi J Gastroenterol. 2014;20:248–54. doi: 10.4103/1319-3767.136982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsaleem MA, Awadalla NJ, Shehata SF, Saeed Alsamghan A, AlFlan MA, et al. Prevalence and factors associated with gastroesophageal reflux disease among primary health care attendants at Abha city, southwestern Saudi Arabia. Saudi Pharm J. 2021;29:597–602. doi: 10.1016/j.jsps.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosli M, Alkhathlan B, Abumohssin A, Merdad M, Alherabi A, Marglani O, et al. Prevalence and clinical predictors of LPR among patients diagnosed with GERD according to the reflux symptom index questionnaire. Saudi J Gastroenterol. 2018;24:236–41. doi: 10.4103/sjg.SJG_518_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fass R. Gastroesophageal reflux disease. N Engl J Med. 2022;387:1207–16. doi: 10.1056/NEJMcp2114026. [DOI] [PubMed] [Google Scholar]
- 7.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: A meta-analysis. Gut. 2018;67:430–40. doi: 10.1136/gutjnl-2016-313589. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2014;63:871–80. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamasaki T, Hemond C, Eisa M, Ganocy S, Fass R. The changing epidemiology of gastroesophageal reflux disease: Are patients getting younger? J Neurogastroenterol Motil. 2018;24:559–69. doi: 10.5056/jnm18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreasson A, Talley NJ, Walker MM, Jones MP, Platts LG, Wallner B, et al. An increasing incidence of upper gastrointestinal disorders over 23 years: A prospective population-based study in Sweden. Am J Gastroenterol. 2021;116:210–3. doi: 10.14309/ajg.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 11.Alzahrani MA, Alamri FS, Alshahrani HM, Alshahrani AM, Mohammed AM, Saif RA, et al. Factors influencing the quality of life of GERD patients in the Aseer Region, Saudi Arabia. J Family Med Prim Care. 2023;12:3217–21. doi: 10.4103/jfmpc.jfmpc_620_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henke CJ, Levin TR, Henning JM, Potter LP. Work loss costs due to peptic ulcer disease and gastroesophageal reflux disease in a health maintenance organization. Am J Gastroenterol. 2000;95:788–92. doi: 10.1111/j.1572-0241.2000.01861.x. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–38. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 14.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16:e228–34. [PubMed] [Google Scholar]
- 15.Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 2018;154:277–88. doi: 10.1053/j.gastro.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 16.Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117:27–56. doi: 10.14309/ajg.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyawali CP, Yadlapati R, Fass R, Katzka D, Pandolfino J, Savarino E, et al. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut. 2024;73:361–71. doi: 10.1136/gutjnl-2023-330616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaezi MF, Singh S, Richter JE. Role of acid and duodenogastric reflux in esophageal mucosal injury: A review of animal and human studies. Gastroenterology. 1995;108:1897–907. doi: 10.1016/0016-5085(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 19.Mittal RK, Rochester DF, McCallum RW. Effect of the diaphragmatic contraction on lower oesophageal sphincter pressure in man. Gut. 1987;28:1564–8. doi: 10.1136/gut.28.12.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut. 1999;44:476–82. doi: 10.1136/gut.44.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh P, Adamopoulos A, Taylor RH, Colin-Jones DG. Oesophageal motor function before and after healing of oesophagitis. Gut. 1992;33:1590–6. doi: 10.1136/gut.33.12.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leite LP, Johnston BT, Barrett J, Castell JA, Castell DO. Ineffective esophageal motility (IEM): The primary finding in patients with nonspecific esophageal motility disorder. Dig Dis Sci. 1997;42:1859–65. doi: 10.1023/a:1018802908358. [DOI] [PubMed] [Google Scholar]
- 23.Ribolsi M, Balestrieri P, Emerenziani S, Guarino MP, Cicala M. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46–51. doi: 10.1038/ajg.2013.373. [DOI] [PubMed] [Google Scholar]
- 24.Kahrilas PJ. The role of hiatus hernia in GERD. Yale J Biol Med. 1999;72:101–11. [PMC free article] [PubMed] [Google Scholar]
- 25.Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology. 2018;154:302–18. doi: 10.1053/j.gastro.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Chen JW, Vela MF, Peterson KA, Carlson DA. AGA clinical practice update on the diagnosis and management of extraesophageal gastroesophageal reflux disease: Expert review. Clin Gastroenterol Hepatol. 2023;21:1414–21.e3. doi: 10.1016/j.cgh.2023.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Yadlapati R, Gyawali CP, Pandolfino JE, Participants CGCC. AGA clinical practice update on the personalized approach to the evaluation and management of GERD: Expert review. Clin Gastroenterol Hepatol. 2022;20:984–94.e1. doi: 10.1016/j.cgh.2022.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung HK, Tae CH, Song KH, Kang SJ, Park JK, Gong EJ, et al. 2020 Seoul consensus on the diagnosis and management of gastroesophageal reflux disease. J Neurogastroenterol Motil. 2021;27:453–81. doi: 10.5056/jnm21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: A meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518–27. doi: 10.7326/0003-4819-140-7-200404060-00011. [DOI] [PubMed] [Google Scholar]
- 30.Pierce CW. A stitch in time: Rethinking the rationale for empiric PPIs in the ACG GERD guideline. Am J Gastroenterol. 2022;117:1883–4. doi: 10.14309/ajg.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 31.Badillo R, Francis D. Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:105–12. doi: 10.4292/wjgpt.v5.i3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahrilas PJ, Boeckxstaens G, Smout AJ. Management of the patient with incomplete response to PPI therapy. Best Pract Res Clin Gastroenterol. 2013;27:401–14. doi: 10.1016/j.bpg.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zerbib F, Bredenoord AJ, Fass R, Kahrilas PJ, Roman S, Savarino E, et al. ESNM/ANMS consensus paper: Diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol Motil. 2021;33:e14075. doi: 10.1111/nmo.14075. doi: 10.1111/nmo.14075. [DOI] [PubMed] [Google Scholar]
- 34.Odiase E, Schwartz A, Souza RF, Martin J, Konda V, Spechler SJ. New eosinophilic esophagitis concepts call for change in proton pump inhibitor management before diagnostic endoscopy. Gastroenterology. 2018;154:1217–21.e3. doi: 10.1053/j.gastro.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunbar KB, Agoston AT, Odze RD, Huo X, Pham TH, Cipher DJ, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–12. doi: 10.1001/jama.2016.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jandee S, Geeraerts A, Geysen H, Rommel N, Tack J, Vanuytsel T. Management of ineffective esophageal hypomotility. Front Pharmacol. 2021;12:638915. doi: 10.3389/fphar.2021.638915. doi: 10.3389/fphar.2021.638915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hojo M, Asaoka D, Shimada Y, Nojiri S, Nagahara A. Strategies for discontinuation of proton pump inhibitors (PPIs) in patients with long-term PPI administration: A randomized controlled trial. BMC Gastroenterol. 2022;22:21. doi: 10.1186/s12876-021-02086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen NT, Thosani NC, Canto MI, Chang K, Lipham J, Abu Dayyeh B, et al. The American Foregut Society white paper on the endoscopic classification of esophagogastric junction integrity. Foregut. 2022;2:339–48. [Google Scholar]
- 40.Nguyen AD, Spechler SJ, Shuler MN, Souza RF, Dunbar KB. Unique clinical features of los angeles grade d esophagitis suggest that factors other than gastroesophageal reflux contribute to its pathogenesis. J Clin Gastroenterol. 2019;53:9–14. doi: 10.1097/MCG.0000000000000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brannstrom L, Werner M, Wallner B, Franklin KA, Karling P. What is the significance of the Hill classification? Dis Esophagus. 2023;36 doi: 10.1093/dote/doad004. doad004. doi: 10.1093/dote/doad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansdotter I, Bjor O, Andreasson A, Agreus L, Hellström P, Forsberg A, et al. Hill classification is superior to the axial length of a hiatal hernia for assessment of the mechanical anti-reflux barrier at the gastroesophageal junction. Endosc Int Open. 2016;4:E311–7. doi: 10.1055/s-0042-101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olmos JA, Pandolfino JE, Piskorz MM, Zamora N, Valdovinos Díaz MA, Remes Troche JM, et al. Latin American consensus on diagnosis of gastroesophageal reflux disease. Neurogastroenterol Motil. 2024;36:e14735. doi: 10.1111/nmo.14735. [DOI] [PubMed] [Google Scholar]
- 44.Chae S, Richter JE. Wireless 24, 48, and 96 hour or impedance or oropharyngeal prolonged pH monitoring: Which test, when, and why for GERD? Curr Gastroenterol Rep. 2018;20:52. doi: 10.1007/s11894-018-0659-0. [DOI] [PubMed] [Google Scholar]
- 45.Kessels SJM, Newton SS, Morona JK, Merlin TL. Safety and efficacy of wireless pH monitoring in patients suspected of gastroesophageal reflux disease: A systematic review. J Clin Gastroenterol. 2017;51:777–88. doi: 10.1097/MCG.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 46.Hemmink GJ, Bredenoord AJ, Weusten BL, Monkelbaan JF, Timmer R, Smout AJ. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: ‘On’ or ‘off’ proton pump inhibitor? Am J Gastroenterol. 2008;103:2446–53. doi: 10.1111/j.1572-0241.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 47.Cho YK. How to interpret esophageal impedance pH monitoring. J Neurogastroenterol Motil. 2010;16:327–30. doi: 10.5056/jnm.2010.16.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson MK, Venkatesh M, Liu N, Breuer CR, Shada AL, Greenberg JA, et al. pH impedance parameters associated with improvement in GERD health-related quality of life following anti-reflux surgery. J Gastrointest Surg. 2021;25:28–35. doi: 10.1007/s11605-020-04831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yadlapati R. High-resolution esophageal manometry: Interpretation in clinical practice. Curr Opin Gastroenterol. 2017;33:301–9. doi: 10.1097/MOG.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)) Neurogastroenterol Motil. 2021;33:e14058. doi: 10.1111/nmo.14058. doi: 10.1111/nmo.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alzahrani MA, Alfahadi MA, Alshehri MA, Alamri AH, Almahjani EA, Alahmari AM, et al. Association of esophageal motility disorder symptoms with Chicago classification versions 3.0 and 4.0 using high-resolution esophageal manometry: A single-center experience from Saudi Arabia. Saudi J Gastroenterol. 2024;30:96–102. doi: 10.4103/sjg.sjg_243_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peedikayil M. Esophageal manometry in patients with gas-troesophageal reflux disease; A study from Saudi Arabia. J Gstro Hepato. 2022;9:1–4. [Google Scholar]
- 53.Tucker E, Knowles K, Wright J, Fox MR. Rumination variations: Aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Aliment Pharmacol Ther. 2013;37:263–74. doi: 10.1111/apt.12148. [DOI] [PubMed] [Google Scholar]
- 54.Carlson DA, Pandolfino JE. High-resolution manometry in clinical practice. Gastroenterol Hepatol (N Y) 2015;11:374–84. [PMC free article] [PubMed] [Google Scholar]
- 55.Debi U, Sharma M, Singh L, Sinha A. Barium esophagogram in various esophageal diseases: A pictorial essay. Indian J Radiol Imaging. 2019;29:141–54. doi: 10.4103/ijri.IJRI_465_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnston BT, Troshinsky MB, Castell JA, Castell DO. Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease. Am J Gastroenterol. 1996;91:1181–85. [PubMed] [Google Scholar]
- 57.Serna-Gallegos D, Basseri B, Bairamian V, Pimentel M, Soukiasian HJ. Gastroesophageal reflux reported on esophagram does not correlate with pH monitoring and high-resolution esophageal manometry. Am Surg. 2014;80:1026–9. [PubMed] [Google Scholar]
- 58.Guo Z, Wu H, Jiang J, Zhang C. Pepsin in saliva as a diagnostic marker for gastroesophageal reflux disease: A meta-analysis. Med Sci Monit. 2018;24:9509–16. doi: 10.12659/MSM.913978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel DA, Higginbotham T, Slaughter JC, Aslam M, Yuksel E, Katzka D, et al. Development and Validation of a Mucosal Impedance Contour Analysis System to Distinguish Esophageal Disorders. Gastroenterology. 2019;156:1617–26.e1. doi: 10.1053/j.gastro.2019.01.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarrett DM, Hachem C. Gastroesophageal Reflux Disease (GERD) Mo Med. 2018;115:214–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Wong MW, Rogers BD, Liu MX, Lei WY, Liu TT, Yi CH, et al. Application of artificial intelligence in measuring novel pH-impedance metrics for optimal diagnosis of GERD. Diagnostics (Basel) 2023;13:960. doi: 10.3390/diagnostics13050960. doi: 10.3390/diagnostics13050960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965–71. doi: 10.1001/archinte.166.9.965. [DOI] [PubMed] [Google Scholar]
- 63.Schuitenmaker JM, van Dijk M, Oude Nijhuis RAB, Smout A, Bredenoord AJ. Associations between sleep position and nocturnal gastroesophageal reflux: A study using concurrent monitoring of sleep position and esophageal ph and impedance. Am J Gastroenterol. 2022;117:346–51. doi: 10.14309/ajg.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 64.Ness-Jensen E, Hveem K, El-Serag H, Lagergren J. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2016;14:175–82.e1-3. doi: 10.1016/j.cgh.2015.04.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilkinson J, Wade A, Thomas SJ, Jenner B, Hodgkinson V, Coyle C. Randomized clinical trial: A double-blind, placebo-controlled study to assess the clinical efficacy and safety of alginate-antacid (Gaviscon Double Action) chewable tablets in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2019;31:86–93. doi: 10.1097/MEG.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 66.McRorie JW, Kirby JA, Miner PB. Histamine2-receptor antagonists: Rapid development of tachyphylaxis with repeat dosing. World J Gastrointest Pharmacol Ther. 2014;5:57–62. doi: 10.4292/wjgpt.v5.i2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: Expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–15. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 68.Wang WH, Huang JQ, Zheng GF, Xia HH, Wong WM, Lam SK, et al. Head-to-head comparison of H2-receptor antagonists and proton pump inhibitors in the treatment of erosive esophagitis: A meta-analysis. World J Gastroenterol. 2005;11:4067–77. doi: 10.3748/wjg.v11.i26.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laine L, DeVault K, Katz P, Mitev S, Lowe J, Hunt B, et al. Vonoprazan versus lansoprazole for healing and maintenance of healing of erosive esophagitis: A randomized trial. Gastroenterology. 2023;164:61–71. doi: 10.1053/j.gastro.2022.09.041. [DOI] [PubMed] [Google Scholar]
- 70.Simon B, Ravelli GP, Goffin H. Sucralfate gel versus placebo in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1996;10:441–6. doi: 10.1111/j.0953-0673.1996.00441.x. [DOI] [PubMed] [Google Scholar]
- 71.Grossi L, Spezzaferro M, Sacco LF, Marzio L. Effect of baclofen on oesophageal motility and transient lower oesophageal sphincter relaxations in GORD patients: A 48-h manometric study. Neurogastroenterol Motil. 2008;20:760–6. doi: 10.1111/j.1365-2982.2008.01115.x. [DOI] [PubMed] [Google Scholar]
- 72.Kalapala R, Karyampudi A, Nabi Z, Darisetty S, Jagtap N, Ramchandani M, et al. Endoscopic full-thickness plication for the treatment of PPI-dependent GERD: Results from a randomised, sham controlled trial. Gut. 2022;71:686–94. doi: 10.1136/gutjnl-2020-321811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Testoni S, Hassan C, Mazzoleni G, Antonelli G, Fanti L, Passaretti S, et al. Long-term outcomes of transoral incisionless fundoplication for gastro-esophageal reflux disease: Systematic-review and meta-analysis. Endosc Int Open. 2021;9:E239–46. doi: 10.1055/a-1322-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haseeb M, Brown JRG, Hayat U, Bay C, Bain PA, Jirapinyo P, et al. Impact of second-generation transoral incisionless fundoplication on atypical GERD symptoms: A systematic review and meta-analysis. Gastrointest Endosc. 2023;97:394–406.e2. doi: 10.1016/j.gie.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimamura Y, Inoue H, Tanabe M, Ushikubo K, Yamamoto K, Kimoto Y, et al. Clinical outcomes of anti-reflux mucosal ablation for gastroesophageal reflux disease: An international bi-institutional study. J Gastroenterol Hepatol. 2024;39:149–56. doi: 10.1111/jgh.16370. [DOI] [PubMed] [Google Scholar]
- 76.Corley DA, Katz P, Wo JM, Stefan A, Patti M, Rothstein R, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: A randomized, sham-controlled trial. Gastroenterology. 2003;125:668–76. doi: 10.1016/s0016-5085(03)01052-7. [DOI] [PubMed] [Google Scholar]
- 77.Fass R, Cahn F, Scotti DJ, Gregory DA. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc. 2017;31:4865–82. doi: 10.1007/s00464-017-5431-2. [DOI] [PubMed] [Google Scholar]
- 78.Auyang ED, Carter P, Rauth T, Fanelli RD, Committee SG. SAGES clinical spotlight review: Endoluminal treatments for gastroesophageal reflux disease (GERD) Surg Endosc. 2013;27:2658–72. doi: 10.1007/s00464-013-3010-8. [DOI] [PubMed] [Google Scholar]
- 79.Spechler SJ. Surgery for gastroesophageal reflux disease: Esophageal impedance to progress? Clin Gastroenterol Hepatol. 2009;7:1264–5. doi: 10.1016/j.cgh.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rickenbacher N, Kotter T, Kochen MM, Scherer M, Blozik E. Fundoplication versus medical management of gastroesophageal reflux disease: Systematic review and meta-analysis. Surg Endosc. 2014;28:143–55. doi: 10.1007/s00464-013-3140-z. [DOI] [PubMed] [Google Scholar]
- 81.Du X, Wu JM, Hu ZW, Wang F, Wang ZG, Zhang C, et al. Laparoscopic Nissen (total) versus anterior 180 degrees fundoplication for gastro-esophageal reflux disease: A meta-analysis and systematic review. Medicine (Baltimore) 2017;96:e8085. doi: 10.1097/MD.0000000000008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108:1706–12. doi: 10.1038/ajg.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganz RA, Peters JH, Horgan S. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:2039–40. doi: 10.1056/NEJMc1303656. [DOI] [PubMed] [Google Scholar]
- 84.Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR, et al. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol. 2016;14:671–7. doi: 10.1016/j.cgh.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 85.Alicuben ET, Bell RCW, Jobe BA, Buckley FP, 3rd, Daniel Smith C, Graybeal CJ, et al. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22:1442–7. doi: 10.1007/s11605-018-3775-0. [DOI] [PubMed] [Google Scholar]
- 86.Buckley FP, 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc. 2018;32:1762–8. doi: 10.1007/s00464-017-5859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riva CG, Asti E, Lazzari V, Aquilino K, Siboni S, Bonavina L. Magnetic sphincter augmentation after gastric surgery. JSLS. 2019;23 doi: 10.4293/JSLS.2019.00035. e2019.00035. doi: 10.4293/JSLS.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delshad SD, Almario CV, Chey WD, Spiegel BMR. Prevalence of gastroesophageal reflux disease and proton pump inhibitor-refractory symptoms. Gastroenterology. 2020;158:1250–61.2. doi: 10.1053/j.gastro.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Momen A, El-Mogy I. Intragastric balloon for obesity: A retrospective evaluation of tolerance and efficacy. Obes Surg. 2005;15:101–5. doi: 10.1381/0960892052993558. [DOI] [PubMed] [Google Scholar]
- 91.Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, Adam A, Neto MG, Badurdeen D, et al. Efficacy and safety of endoscopic sleeve gastroplasty: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:1043–53.e4. doi: 10.1016/j.cgh.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 92.Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51–8. doi: 10.1016/j.gie.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 93.Brethauer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis. 2012;8:296–303. doi: 10.1016/j.soard.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Fayad L, Adam A, Schweitzer M, Cheskin LJ, Ajayi T, Dunlap M, et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: A case-matched study. Gastrointest Endosc. 2019;89:782–8. doi: 10.1016/j.gie.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 95.Ruban A, Ashrafian H, Teare JP. The EndoBarrier: Duodenal-jejunal bypass liner for diabetes and weight loss. Gastroenterol Res Pract 2018. 2018 doi: 10.1155/2018/7823182. 7823182. doi: 10.1155/2018/7823182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Baar ACG, Holleman F, Crenier L, Haidry R, Magee C, Hopkins D, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: One year results from the first international, open-label, prospective, multicentre study. Gut. 2020;69:295–303. doi: 10.1136/gutjnl-2019-318349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiu S, Birch DW, Shi X, Sharma AM, Karmali S. Effect of sleeve gastrectomy on gastroesophageal reflux disease: A systematic review. Surg Obes Relat Dis. 2011;7:510–5. doi: 10.1016/j.soard.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 98.DuPree CE, Blair K, Steele SR, Martin MJ. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: A national analysis. JAMA Surg. 2014;149:328–34. doi: 10.1001/jamasurg.2013.4323. [DOI] [PubMed] [Google Scholar]
- 99.Raj PP, Bhattacharya S, Misra S, Kumar SS, Khan MJ, Gunasekaran SC, et al. Gastroesophageal reflux-related physiologic changes after sleeve gastrectomy and Roux-en-Y gastric bypass: A prospective comparative study. Surg Obes Relat Dis. 2019;15:1261–9. doi: 10.1016/j.soard.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 100.Borbely Y, Schaffner E, Zimmermann L, Huguenin M, Plitzko G, Nett P, et al. De novo gastroesophageal reflux disease after sleeve gastrectomy: Role of preoperative silent reflux. Surg Endosc. 2019;33:789–93. doi: 10.1007/s00464-018-6344-4. [DOI] [PubMed] [Google Scholar]
- 101.Pilone V, Tramontano S, Renzulli M, Zulli C, Schiavo L. Gastroesophageal reflux after sleeve gastrectomy: New onset and effect on symptoms on a prospective evaluation. Obes Surg. 2019;29:3638–45. doi: 10.1007/s11695-019-04046-5. [DOI] [PubMed] [Google Scholar]
- 102.Woodman G, Cywes R, Billy H, Montgomery K, Cornell C, Okerson T, et al. Effect of adjustable gastric banding on changes in gastroesophageal reflux disease (GERD) and quality of life. Curr Med Res Opin. 2012;28:581–9. doi: 10.1185/03007995.2012.666962. [DOI] [PubMed] [Google Scholar]
- 103.Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA. 2018;319:255–65. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madhok BM, Carr WR, McCormack C, Boyle M, Jennings N, Schroeder N, et al. Preoperative endoscopy may reduce the need for revisional surgery for gastro-oesophageal reflux disease following laparoscopic sleeve gastrectomy. Clin Obes. 2016;6:268–72. doi: 10.1111/cob.12153. [DOI] [PubMed] [Google Scholar]
- 105.Sebastianelli L, Benois M, Vanbiervliet G, Bailly L, Robert M, Turrin N, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of barrett's esophagus: Results of a multicenter study. Obes Surg. 2019;29:1462–9. doi: 10.1007/s11695-019-03704-y. [DOI] [PubMed] [Google Scholar]
- 106.Merrouche M, Sabate JM, Jouet P, Harnois F, Scaringi S, Coffin B, et al. Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg. 2007;17:894–900. doi: 10.1007/s11695-007-9166-3. [DOI] [PubMed] [Google Scholar]
- 107.Ramu B, Mohan P, Rajasekaran MS, Jayanthi V. Prevalence and risk factors for gastroesophageal reflux in pregnancy. Indian J Gastroenterol. 2011;30:144–7. doi: 10.1007/s12664-010-0067-3. [DOI] [PubMed] [Google Scholar]
- 108.Malfertheiner M, Malfertheiner P, Costa SD, Pfeifer M, Ernst W, Seelbach-Göbel B, et al. Extraesophageal symptoms of gastroesophageal reflux disease during pregnancy. Z Gastroenterol. 2015;53:1080–3. doi: 10.1055/s-0034-1399453. [DOI] [PubMed] [Google Scholar]
- 109.Dall'alba V, Callegari-Jacques SM, Krahe C, Bruch JP, Alves BC, Barros SG. Health-related quality of life of pregnant women with heartburn and regurgitation. Arq Gastroenterol. 2015;52:100–4. doi: 10.1590/S0004-28032015000200005. [DOI] [PubMed] [Google Scholar]
- 110.MacFarlane B. Management of gastroesophageal reflux disease in adults: A pharmacist's perspective. Integr Pharm Res Pract. 2018;7:41–52. doi: 10.2147/IPRP.S142932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gomes CF, Sousa M, Lourenco I, Martins D, Torres J. Gastrointestinal diseases during pregnancy: What does the gastroenterologist need to know? Ann Gastroenterol. 2018;31:385–94. doi: 10.20524/aog.2018.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thelin CS, Richter JE. Review article: The management of heartburn during pregnancy and lactation. Aliment Pharmacol Ther. 2020;51:421–34. doi: 10.1111/apt.15611. [DOI] [PubMed] [Google Scholar]
- 113.Ali RAR, Hassan J, Egan LJ. Review of recent evidence on the management of heartburn in pregnant and breastfeeding women. BMC Gastroenterol. 2022;22:219. doi: 10.1186/s12876-022-02287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]