Abstract
Background
Inflammation and insufficient physical inactivity contribute to individual-level risk of disease recurrence and death in stage III colon cancer. The extent to which increased inflammatory risk can be offset by sufficient physical activity remains unknown.
Methods
This cohort study was nested within the Cancer and Leukemia Group B (now part of the Alliance for Clinical Trials in Oncology) and Southwest Oncology Group randomized trial. Inflammatory burden was quantified by high-sensitivity C-reactive protein, interleukin-6, and soluble tumor necrosis factor-α receptor 2 after recovery from tumor resection. Physical activity was measured during and after postoperative chemotherapy. The primary endpoint was disease-free survival.
Results
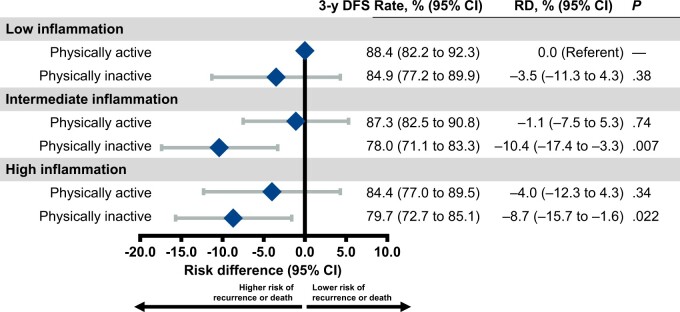
The 3-year disease-free survival rate was 88.4% among patients with low inflammation and sufficient physical activity (referent group for all comparisons), 84.9% with low inflammation and insufficient physical activity (absolute risk difference = −3.5 percentage points, 95% confidence interval [CI] = −11.3 to 4.3; P = .38), 78.0% with intermediate inflammation and insufficient physical activity (absolute risk difference = −10.4 percentage points, 95% CI = −17.4 to −3.3; P = .007), and 79.7% with high inflammation and insufficient physical activity (absolute risk difference = −8.7 percentage points, 95% CI = −15.7 to −1.6; P = .022). In contrast, the 3-year disease-free survival rate was 87.3% among patients with intermediate inflammation and sufficient physical activity (absolute risk difference = −1.1 percentage points, 95% CI = −7.5 to 5.3; P = .74) and 84.4% with high inflammation and sufficient physical activity (absolute risk difference = −4.0 percentage points, 95% CI = −12.3 to 4.3; P = .34).
Conclusion
In this observational study of stage III colon cancer patients, physical activity was associated with improved disease-free survival despite high inflammation. Patients with intermediate or high inflammation who were physically active had disease-free survival rates that were not statistically significantly different from those with low inflammation.
Inflammation is implicated in the progression of various solid tumors (1). Inflammation activates the Janus kinase/signal transducers and activators of transcription (JAK-STAT) and nuclear factor (NF)–κB signaling pathways to promote cell proliferation, migration, and invasion (2). Inflammation after recovery from colonic tumor resection is associated with shorter disease-free survival (3,4). In tumor-bearing preclinical models, inflammatory pathway blockade slows cancer cell growth and delays tumor progression (5).
Postoperative physical activity is associated with longer disease-free survival in patients with colon cancer (6,7). The biological mechanisms through which physical activity improves disease-free survival remain poorly understood; however, inflammation is postulated as a key mediator (8). Physical activity reduces inflammation in colon cancer survivors (9). However, it is uncertain if the negative prognostic effect of inflammation on disease-free survival in patients with colon cancer can be offset by sufficient physical activity.
We examined the association of inflammation and physical activity with disease-free survival in a prospectively nested cohort study of patients with stage III colon cancer enrolled in a National Cancer Institute–sponsored randomized multicenter trial of postoperative treatments (10). This study was uniquely designed to determine if a behavioral factor (physical activity) modifies the prognostic effect of a biological factor (inflammation) because inflammation was measured at baseline and physical activity was subsequently measured during and after chemotherapy. We hypothesized that physical activity would be associated with improved disease-free survival despite the presence of high inflammation.
Methods
Study design
The Cancer and Leukemia Group B (now part of the Alliance for Clinical Trials in Oncology) and Southwest Oncology Group trial 80702 was designed in collaboration with the National Cancer Institute. The trial used a 2 × 2 factorial design to test the primary hypothesis of the superiority of celecoxib compared with placebo and the secondary hypothesis of the noninferiority of 3 months compared with 6 months of chemotherapy as part of an international pooling project. The superiority of celecoxib vs placebo to improve disease-free survival was not proven (10), and results of the secondary hypothesis are reported (11).
All participants were allowed to consent to participate in several nested correlative science studies at the time of trial enrollment. One correlative study asked participants to provide a baseline blood sample for biospecimen assays. Another correlative study asked participants to complete standardized assessments of lifestyle factors during and after chemotherapy. Institutional review board approval was obtained at all participating centers, and participants provided written informed consent.
Study population
Participants were enrolled at community and academic centers across the National Cancer Trials Network in the United States and Canada. Eligible participants had resected, histologically documented colonic adenocarcinoma. Tumors had at least 1 pathologically confirmed metastatic lymph node or N1c designation, defined as tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic tissue without regional lymph node metastases. Participants were aged 18 years and older, with an Eastern Cooperative Oncology Group performance status of no more than 2, and had normal hepatic, renal, and hematologic values (10).
Inflammatory measures
Nonfasting EDTA-preserved plasma was collected 21-56 days after surgical tumor resection and before initiating chemotherapy and pharmacotherapy (ie, before random assignment). Inflammation measures of high-sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), and soluble tumor necrosis factor-alpha (TNFα) receptor 2 were assayed centrally (Clinical Chemistry Laboratory of the Children’s Hospital of Boston, Boston, MA, USA). These inflammatory measures were selected because of their associations with disease recurrence and death in studies of colon cancer (4). hs-CRP was measured as a marker of overall systemic inflammation (12). IL-6 was measured as an activator of the JAK-STAT pathway (13). Soluble TNFα receptor 2 was measured as an activator of the NF-kB pathway (14). Soluble TNFα receptor 2 is a surrogate marker for TNFα that is more stable in plasma and less sensitive to diurnal variation (15). The concentrations of these inflammatory measures do not differ by collection type (fasting vs nonfasting) (16), tumor resection procedure (open vs laparoscopic) (17), and return to baseline within 7 days after tumor resection (17). hs-CRP was quantified using an immunoturbidimetric assay (Roche Diagnostics). IL-6 and soluble TNFα receptor 2 were quantified using ultrasensitive sandwich enzyme immunoassays (R&D Systems). The laboratory characteristics of these assays are published (9).
Physical activity assessment
Physical activity was assessed midway through chemotherapy (4 months following surgical resection) and 6 months after completing chemotherapy (14 months after surgical resection) using the questionnaire implemented in the Nurses’ Health Study and Health Professionals Follow-Up Study [Supplementary Table 1, available online (18)]. Patients reported their average weekly time spent on various physical activities using a validated questionnaire during the preceding 2 months (18). Each physical activity was assigned a metabolic equivalent (MET) energy expenditure according to standardized criteria (19). We calculated the MET hours per week for each activity by multiplying the MET value by the patient’s reported number of hours of physical activity each week.
Study endpoints
The primary endpoint was disease-free survival, from random assignment to documented disease recurrence or death from any cause. Participants were assessed for disease recurrence by history, physical examination, and carcinoembryonic antigen measures every 3 months for the first 3 years and subsequently every 6 months until 6 years after enrollment or until disease recurrence, whichever came first. All participants had surveillance imaging of the chest, abdomen, and pelvis every 6 months for at least 3 years and then yearly for 3 years or until disease recurrence. Patients without an event were censored at their last disease evaluation date. The secondary endpoint was overall survival, defined as the time from random assignment to death from any cause.
Covariates
Potential confounding variables were identified based on expert knowledge as potentially causally related to inflammation, physical activity, or disease recurrence and death. Data for participant demographic factors, including age, sex, race, and ethnicity, were self-reported. Clinical factors including the extent of tumor invasion through the bowel wall (T-stage), the extent of lymph node metastases (N-stage), pathologic risk group (low [T1, T2, or T3, N1] or high [T4, N2, or both]), tumor location, performance status, and low-dose aspirin use were obtained from a combination of physician assessment and the medical record. Smoking history was self-reported. Body mass index was abstracted from a combination of the electronic medical record and self-report. Diet was assessed using a 131-item food frequency questionnaire; prudent and Western dietary patterns were defined using previously validated factor loadings in this population (20). Body mass index and diet were updated when physical activity was reassessed.
Statistical analysis
Physical activity was modeled using cumulative averaging, quantifying the time-weighted average of all reported physical activity. We updated physical activity based on the results of the second questionnaire, which was weighted proportional to the time between the first and second questionnaire and the time between the second questionnaire and the disease-free survival period. Repeatedly measured variables (eg, body mass index and dietary patterns) were considered time-varying confounders.
Flexible parametric proportional hazards survival models were used to quantify statistical associations. Parametric survival models allow for smooth predictions and the estimation of absolute and relative effects while permitting flexibility in the shape of the baseline hazard function. Absolute effects are presented as risk differences at 3 years for disease-free survival and 5 years for overall survival, as these time horizons are clinically meaningful (21). Relative effects are presented as the hazard ratio (HR) using all observed data in a time-to-event framework.
Each inflammatory biomarker was categorized into thirds to represent low (first tertile), intermediate (second tertile), and high (third tertile) inflammation. Tertiles of the 3 inflammatory biomarkers were then consolidated to generate a composite of the overall inflammatory burden. Participants in the lowest overall inflammatory burden category had no biomarker values in the third tertile, participants in the intermediate overall inflammatory burden category had 1 biomarker value in the third tertile, and participants in the high overall inflammatory burden category had 2 or 3 biomarker values in the third tertile. Participants who reported at least 9 MET hours per week were classified as sufficiently physically active (comparable with the energy expenditure of 150 min/wk of brisk walking or 75 min/wk of jogging, consistent with the current physical activity guidelines for cancer survivors) (22), and participants who reported less than 9 MET hours per week were classified as insufficiently physically active. A 6-level composite variable integrated the 3 inflammation categories with the 2 physical activity categories. The category of low inflammation and sufficient physical activity (best prognosis) was selected as a reference group because of a biological rationale to understand how physical activity may mitigate the known negative effect of inflammation on disease-free survival and the clinical rationale to inform patient counseling.
Multivariable (eg, confounder adjusted) models were fit to achieve parsimony based on stepwise selection, relative change in estimates, and minimization of the Akaike information criterion and Bayesian information criterion. All inferential models for the disease-free survival and overall survival endpoints are adjusted for sex, extent of invasion through the bowel wall, nodal stage, prudent dietary pattern (time varying), chemotherapy random assignment, and pharmacotherapy random assignment. All hypothesis tests were 2-sided with statistical significance established at a P value less than .05. Data were collected by the Alliance Statistics and Data Management Center. Data analysis was conducted by the Alliance Statistics and Data Management Center using SAS (Version 9.4) and R (Version 4.2.2) on a dataset locked on August 10, 2020. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies.
Results
From June 2010 to November 2015, a total of 2524 participants from 654 centers were eligible and randomly assigned. Data from both correlative studies were available for 1179 participants (46.7%) and were included in this analysis (Supplementary Figure 1, available online). Participants in this analysis were more likely to be White (82.5% vs 76.1%; P < .001), non-Hispanic (94.5% vs 90.6; P < .001), and to use aspirin (23.5% vs 19.7%; P = .021) compared with participants excluded from this analysis (Supplementary Table 2, available online).
The pharmacotherapy randomization group was not associated with physical activity (−0.0 MET-h/wk, 95% Confidence Interval (CI) = −1.4 to 1.2 MET-h/wk; P = .93). The median physical activity energy expenditure midway through chemotherapy was 5.0 MET-h/wk (interquartile range [IQR] = 0.7-15.9 MET-h/wk) and increased to 9.1 MET hours per week (IQR = 2.0-24.4) 6 months after completing chemotherapy (P < .001); physical activity between these 2 timepoints was correlated (ρ = 0.51, 95% CI = 0.44 to 0.58 MET-h/wk).
At baseline, the median hs-CRP was 2.6 mg/L (IQR = 1.2-5.7 mg/L), IL-6 was 3.7 pg/mL (IQR = 2.3-6.2 pg/mL), and soluble TNFα receptor 2 was 2870 pm/mL (IQR = 2338-3603 pm/mL), indicating low to moderate inflammation; 524 (44.4%) participants had a hs-CRP of at least 3.0 mg/L consistent with elevated overall systemic inflammation (Table 1). All 3 inflammatory biomarkers were statistically significantly correlated (Supplementary Table 3, available online). The overall inflammatory burden was classified as low, intermediate, and high in 295 (25.0%), 525 (44.5%), and 359 (30.5%) participants, respectively (inflammatory biomarker thresholds are presented in Supplementary Table 4, available online). As compared with participants with low inflammation at baseline, those with intermediate (relative risk [RR] = 0.92, 95% CI = 0.80 to 1.05) and high inflammation (RR = 0.58, 95% CI = 0.48 to 0.69) were less likely to have sufficient physical activity during and after postoperative chemotherapy (Ptrend < .001); participants with sufficient postoperative physical activity had lower baseline median hs-CRP (Δ = −0.66 mg/L, 95% CI = −0.96 to −0.39 mg/L; P < .001), IL-6 (Δ = −0.67 pg/mL, 95% CI = −0.96 to −0.40 mg/L; P < .001), and soluble TNFα receptor 2 (Δ = −361 pg/mL, 95% CI = −468 to −256 mg/L; P < .001) compared with participants with insufficient postoperative physical activity (Supplementary Table 5, available online).
Table 1.
Baseline patient characteristics
| Characteristics | Overall (n = 1179) |
|---|---|
| Demographic factors | |
| Age, mean (SD), y | 60.8 (10.5) |
| Sex, No. (%) | |
| Male | 658 (55.8) |
| Female | 521 (44.2) |
| Race, No. (%) | |
| Asian | 34 (2.9) |
| Black or African American | 131 (11.1) |
| Hispanic or Latino, No. (%) | 65 (5.5) |
| Others or not reported | 41 (3.5) |
| White | 973 (82.5) |
| Clinical factors | |
| Extent of invasion through the bowel wall, No. (%)a | |
| T1 or T2 | 230 (19.6) |
| T3 | 775 (66.1) |
| T4 | 167 (14.3) |
| Missing | 7 |
| Nodal stage, No. (%)b | |
| N1 | 871 (73.9) |
| N2 | 308 (26.1) |
| Risk group, No. (%) | |
| Low (T1, T2, or T3, N1) | 753 (64.2) |
| High (T4, N2, or both) | 419 (35.8) |
| Missing | 7 |
| Tumor location, No. (%) | |
| Left | 576 (49.3) |
| Right | 588 (50.3) |
| Multiple | 5 (0.4) |
| Missing | 10 |
| Eastern Cooperative Oncology Group performance status, No. (%)c | |
| 0 | 852 (72.3) |
| 1-2 | 327 (27.7) |
| Low dose aspirin use, No. (%) | 277 (23.5) |
| Behavioral factorsd | |
| Body mass index, mean (SD), kg/m2 | 28.4 (6.8) |
| Smoking history, No. (%) | |
| Never | 586 (49.7) |
| Former | 490 (41.6) |
| Current | 91 (7.7) |
| Not reported | 12 (1.0) |
| Western dietary pattern score, median (IQR) | −0.21 (−0.64-0.42) |
| Prudent dietary pattern score, median (IQR) | −0.07 (−0.56-0.41) |
| Physical activity, mean (SD), metabolic equivalent h/wk, | 15.6 (23.5) |
| Randomization groups | |
| Chemotherapy, No. (%) | |
| 3 mo | 603 (51.1) |
| 6 mo | 576 (48.9) |
| Pharmacotherapy, No. (%) | |
| Celecoxib | 598 (50.7) |
| Placebo | 581 (49.3) |
| Inflammatory biomarker concentrations | |
| High-sensitivity C-reactive protein, median (IQR), mg/L | 2.6 (1.2-5.7) |
| ≥3.0 mg/L, No. (%) | 524 (44.4) |
| Interleukin 6, median (IQR), pg/mL, | 3.7 (2.3-6.2) |
| Soluble tumor necrosis factor-α receptor 2, median (IQR) , pg/L | 2870 (2338-3603) |
T1 indicates tumor has grown into the submucosa; T2, growth into the muscularis propria; T3, growth through the muscularis propria and into the subserosa; T4, growth into the surface of the visceral peritoneum or into or has attached to other organs or structures. IQR = interquartile range.
N1 indicates 1-3 lymph nodes tested positive for cancer (or for this table, N1c: tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional lymph node metastases); N2, 4 or more lymph nodes tested positive for cancer.
Performance status: 0 indicates fully active; 1, restricted in physically strenuous activity but ambulatory and able to carry out light work; and 2, ambulatory and capable of all self-care but unable to carry out any work activities, up and about more than 50% of waking hours.
Body mass index, Western dietary pattern, prudent dietary pattern, and physical activity were calculated using the cumulative average method.
During a median follow-up of 6.0 years, 301 participants experienced disease recurrence or death; the 3-year disease-free survival rate for all patients was 83.7% (95% CI = 79.5% to 87.3%). The 3-year disease-free survival rate was 88.4% among patients with low inflammation and sufficient physical activity and 84.9% with low inflammation and insufficient physical activity (absolute risk difference = −3.5 percentage points, 95% CI = −11.3 to 4.3 percentage points; P = .38; HR = 1.33, 95% CI = 0.78 to 2.26; P = .29), 78.0% with intermediate inflammation and insufficient physical activity (absolute risk difference = −10.4 percentage points, 95% CI = −17.4 to −3.3 percentage points; P = .007; HR = 2.02, 95% CI = 1.30 to 3.16; P = .002), and 79.7% with high inflammation and insufficient physical activity (absolute risk difference = −8.7 percentage points, 95% CI = −15.7 to −1.6 percentage points; P = .022; HR = 1.85, 95% CI = 1.18 to 2.90; P = .008; Table 2, Figure 1). In contrast, the 3-year disease-free survival rate was 87.3% among patients with intermediate inflammation and sufficient physical activity (absolute risk difference = −1.1 percentage points, 95% CI = −7.5 to 5.3 percentage points; P = .74; HR = 1.11, 95% CI = 0.69 to 1.78; P = .67), and 84.4% with high inflammation and sufficient physical activity (absolute risk difference −4.0 percentage points, 95% CI = −12.3 to 4.3 percentage points; P = .34; HR = 1.38, 95% CI = 0.81 to 2.35; P = .23). Conclusions were comparable for individual inflammatory biomarkers (Supplementary Table 6, available online).
Table 2.
Association of disease-free survival endpoint with inflammatory burden and physical activity status
| Inflammatory burden and physical activity status | 3-y disease-free survival rate (95% CI)a,b | 3-y risk difference (95% CI)a,c | P c | Hazard ratio (95% CI)a | P |
|---|---|---|---|---|---|
| Low inflammation | |||||
| Sufficient physical activity | 88.4 (82.3 to 92.3) | 0.0 (Referent) | — | 1.00 (Referent) | — |
| Insufficient physical activity | 84.9 (77.2 to 89.9) | −3.5 (−11.3 to 4.3) | .38 | 1.33 (0.78 to 2.26) | .29 |
| Intermediate inflammation | |||||
| Sufficient physical activity | 87.3 (82.5 to 90.8) | −1.1 (−7.5 to 5.3) | .74 | 1.11 (0.69 to 1.78) | .67 |
| Insufficient physical activity | 78.0 (71.1 to 83.3) | −10.4 (−17.4 to −3.3) | .007 | 2.02 (1.30 to 3.16) | .002 |
| High inflammation | |||||
| Sufficient physical activity | 84.4 (77.0 to 89.5) | −4.0 (−12.3 to 4.3) | .34 | 1.38 (0.81 to 2.35) | .23 |
| Insufficient physical activity | 79.7 (72.7 to 85.1) | −8.7 (−15.7 to −1.6) | .022 | 1.85 (1.18 to 2.90) | .008 |
Adjusted for sex, extent of invasion through the bowel wall, nodal stage, prudent dietary pattern (time varying), chemotherapy random assignment, and pharmacotherapy random assignment. Continuous covariates were modeled linearly, and categorical covariates were modeled using the categories presented in Table 1. CI = confidence interval.
Covariates for predicting disease-free survival rates were set to the mean of the study population for continuous variables and most common category for categorical variables.
95% confidence intervals and P values were calculated via test of proportions.
Figure 1.
Association of disease-free survival endpoint with inflammatory burden and physical activity status. CI = confidence interval; DFS = disease-free survival; RD = absolute risk difference.
During follow-up, 185 participants experienced death from any cause; the 5-year overall survival rate was 89.4% (95% CI = 85.6% to 92.2%). The 5-year overall survival rate was 94.5% among patients with low inflammation and sufficient physical activity and 90.1% with low inflammation and insufficient physical activity (absolute risk difference = −4.4 percentage points, 95% CI = −10.6 to 1.8 percentage points; P = .15; HR = 1.85, 95% CI = 0.87 to 3.94; P = .11), 84.3% with intermediate inflammation and insufficient physical activity (absolute risk difference = −10.2 percentage points, 95% CI = −15.8 to −4.6 percentage points; P = .002; HR = 3.03, 95% CI = 1.58 to 5.81; P < .001), and 89.0% with high inflammation and insufficient physical activity (absolute risk difference = −5.5 percentage points, 95% CI = −10.8 to −0.1 percentage points; P = .056; HR = 2.08, 95% CI = 1.06 to 4.06; P = .030; Table 3). In contrast, the 5-year overall survival rate was 91.4% among patients with intermediate inflammation and sufficient physical activity (absolute risk difference = −3.1 percentage points, 95% CI = −8.0 to 1.8 percentage points; P = .24; HR = 1.61, 95% CI = 0.81 to 3.19; P = .17), and 91.2% with high inflammation and sufficient physical activity (absolute risk difference = −3.3 percentage points, 95% CI = −9.6 to 3.0 percentage points; P = .29; HR = 1.64, 95% CI = 0.77 to 3.51; P = .20). Conclusions were comparable for individual inflammatory biomarkers (Supplementary Table 7, available online).
Table 3.
Association of overall survival endpoint with inflammatory burden and physical activity status
| Inflammatory burden and physical activity status | 5-y overall survival rate (95% CI)a,b | 5-y risk difference (95% CI)a,c | P c | Hazard ratio (95% CI)a | P |
|---|---|---|---|---|---|
| Low inflammation | |||||
| Sufficient physical activity | 94.5 (89.6 to 97.0) | 0.0 (Referent) | — | 1.00 (Referent) | — |
| Insufficient physical activity | 90.1 (83.7 to 94.1) | −4.4 (−10.6 to 1.8) | .15 | 1.85 (0.87 to 3.94) | .11 |
| Intermediate inflammation | |||||
| Sufficient physical activity | 91.4 (87.4 to 94.2) | −3.1 (−8.0 to 1.8) | .24 | 1.61 (0.81 to 3.19) | .17 |
| Insufficient physical activity | 84.3 (77.9 to 89.2) | −10.2 (−15.8 to −4.6) | .002 | 3.03 (1.58 to 5.81) | <.001 |
| High inflammation | |||||
| Sufficient physical activity | 91.2 (85.0 to 94.7) | −3.3 (−9.6 to 3.0) | .29 | 1.64 (0.77 to 3.51) | .20 |
| Insufficient physical activity | 89.0 (83.8 to 92.8) | −5.5 (−10.8 to −0.1) | .056 | 2.08 (1.06 to 4.06) | .030 |
Adjusted for sex, extent of invasion through the bowel wall, nodal stage, prudent dietary pattern (time varying), chemotherapy random assignment, and pharmacotherapy random assignment. Continuous covariates were modeled linearly, and categorical covariates were modeled using the categories presented in Table 1. CI = confidence interval.
Covariates for predicting disease-free survival rates were set to the mean of the study population for continuous variables and most common category for categorical variables.
95% confidence intervals and P values were calculated via test of proportions.
Discussion
In this nested cohort study of 1179 patients with stage III colon cancer enrolled in a randomized multicenter trial of postoperative treatments, physical activity was associated with improved disease-free survival despite the presence of high inflammation. Compared with patients with low inflammation and sufficient physical activity, disease-free survival was not statistically significantly different in patients with intermediate inflammation and sufficient physical activity (88.4% vs 87.3% were alive and disease free at 3 years, an absolute difference of 1.1 percentage points) or high inflammation and sufficient physical activity (88.4% vs 84.4% were alive and disease free at 3 years, an absolute difference of 4.0 percentage points). In contrast, disease-free survival was statistically significantly lower in patients with intermediate inflammation and insufficient physical activity (88.4% vs 78.0% were alive and disease free at 3 years, an absolute difference of 11.3 percentage points) or high inflammation and insufficient physical activity (88.4% vs 79.7% were alive and disease free at 3 years, an absolute difference of 8.7 percentage points).
The results of this analysis support 4 noteworthy conclusions. First, the degree of inflammation after recovery from tumor resection is associated with shorter disease-free survival. Patients with intermediate or high inflammation and insufficient physical activity had a relative risk of disease recurrence or death that was approximately twofold higher than patients with low inflammation and sufficient physical activity. This finding is consistent with prior reports that high inflammation after surgical tumor resection is associated with shorter disease-free survival (3). Our analysis expands the evidence base on the negative prognostic effects of inflammation by demonstrating that both intermediate and high inflammation increase the risk of disease recurrence or death.
Second, patients with intermediate or high inflammation and sufficient physical activity had disease-free survival rates that were not statistically significantly different from patients with low inflammation and sufficient physical activity. Sufficient physical activity was defined as the energy expenditure equivalent of 150 minutes per week of moderate-intensity (eg, brisk walking) or 75 minutes per week of vigorous-intensity (eg, jogging) activity (22). In our analysis, approximately 10 patients with intermediate and high inflammation would need to be treated with physical activity to prevent 1 disease recurrence or death. This finding expands on prior observational studies that physical activity is associated with improved disease-free survival (6,7) and reinforces the clinical relevance of randomized studies in cancer survivors that report physical activity reduces hs-CRP by 30.2% and IL-6 by 30.9% compared with non-exercise control (9).
Third, patients with low inflammation and insufficient physical activity did not have statistically significantly worse disease-free survival than patients with low inflammation and sufficient physical activity. Although sufficient physical activity may confer a smaller disease-free survival benefit among patients with low inflammation, physical activity remains relevant in this population as it may improve chemotherapy relative dose intensity (7), decrease oxaliplatin-induced peripheral neuropathy severity (23), and improve quality of life (24).
Fourth, plasma inflammatory burden can be estimated with clinical assays and may be a useful method to identify patients likely to derive a disease-free survival benefit from physical activity. Before this study, identifying patient subgroups likely to benefit from physical activity was limited to molecular tumor features, including P21 or P27 expression (25), nuclear CTNNB1- (26), PTGS2 (COX-2)+ (27), and IRS1 low or negative status (28). Knowledge of inflammatory burden may motivate behavior change in patients and clarify the purpose of physical activity for patients and physicians (29). Inflammatory burden may be valuable as a predictive biomarker to enrich the study population of future randomized trials investigating the effects of physical activity in cancer survivors (30).
There are several limitations of this study. Because of the nonrandomized design, we cannot rule out the possibility of residual confounding. The generalizability of our findings requires consideration, as patients who participate in research differ from the underlying population. All participants were offered the opportunity to consent to participate in several nested correlative science studies, however, enrolled participants were more likely to be White, non-Hispanic, and to use aspirin. Although the inflammatory burden was limited to 3 biomarkers, these measures have been associated with prognosis in patients with colon cancer and represent distinct inflammatory signaling pathways (eg, JAK-STAT and NF-kB) and overall systemic inflammation. Although the physical activity questionnaire is valid and reproducible, the assessment was self-reported and restricted to specific recreational physical activities. Moreover, we did not assess physical activity before cancer diagnosis or surgery. However, the physical activity assessment was completed before knowledge of disease recurrence, reducing the likelihood of bias. Physical activity was not associated with pharmacotherapy random assignment (eg, celecoxib or placebo).
There are several strengths of this study. Conducting this nested cohort study within a randomized multicenter trial offers several advantages over other data sources. Because of enrollment in the trial, patients included in this analysis had baseline blood samples collected in a limited time frame after surgery. The treatment, follow-up care, and endpoint ascertainment were standardized in this trial. The prospective design permitted comprehensive ascertainment of confounding variables for multivariable modeling.
In this observational study of stage III colon cancer patients, physical activity was associated with improved disease-free survival despite the presence of high inflammation. Patients with intermediate or high inflammation who were physically active had disease-free survival rates that were not statistically significantly different from those with low inflammation. These findings support the mechanistic hypothesis that physical activity might normalize the host microenvironment possibly by interrupting the cross talk between inflammation and metastatic progression.
Supplementary Material
Acknowledgements
The funder did not play a role in the analysis and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Contributor Information
Justin C Brown, Pennington Biomedical Research Center, Baton Rouge, LA, USA; New Orleans School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA; Stanley S. Scott Cancer Center, Louisiana State University Health Sciences Center, New Orleans, LA, USA.
Chao Ma, Dana-Farber Cancer Institute, Boston, MA, USA.
Qian Shi, Alliance Statistics and Data Management Center, Mayo Clinic, Rochester, MN, USA.
Felix Couture, Hôtel-Dieu de Québec, Québec, Canada.
Philip Kuebler, Columbus National Cancer Institute Community Oncology Research Program, Columbus, OH, USA.
Pankaj Kumar, Heartland Cancer Research NCI Community Oncology Research Program, Illinois CancerCare PC, Peoria, IL, USA.
Benjamin Tan, Siteman Cancer Center, Washington University School of Medicine, Saint Louis, MO, USA.
Smitha Krishnamurthi, Cleveland Clinic, Cleveland, OH, USA.
Victor Chang, Veterans Administration New Jersey Health Care System, East Orange, NJ, USA.
Richard M Goldberg, West Virginia University Cancer Institute, Morgantown, WV, USA.
Eileen M O’Reilly, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical Center, New York, NY, USA.
Anthony F Shields, Karmanos Cancer Institute, Wayne State University, MI, USA.
Jeffrey A Meyerhardt, Dana-Farber Cancer Institute, Boston, MA, USA.
Data availability
Data described in the manuscript, code book, and analytic code will not be made available because study subjects did not consent for their data to be shared publicly. Our ability to preserve subject anonymity cannot be guaranteed.
Author contributions
Justin C. Brown, PhD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft), Chao Ma, MS (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing—review & editing), Qian Shi, PhD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—review & editing), Felix Couture, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing—review & editing), Philip Kuebler, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Pankaj Kumar, MD (Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Benjamin Tan, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Smitha Krishnamurthi, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Victor Chang, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Richard M. Goldberg, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Eileen M. O’Reilly, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), Anthony F. Shields, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing), and Jeffrey A. Meyerhardt, MD, MPH (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing).
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), https://acknowledgments.alliancefound.org; UG1CA233180, UG1CA233290, UG1CA233339, UG1CA189830, UG1CA189954; and U10CA180863 and CCS #707213 to the Canadian Cancer Trials Group; U10CA180820, and UG1CA233234 to the ECOG–ACRIN Cancer Research Group; U10CA180868, UG1CA189867 to NRG Oncology; U10CA180888 and UG1CA233163 to the SWOG Cancer Research Group. Also supported in part by Pfizer. Dr Brown is supported by R00CA218603, R01CA270274, U01CA271279, and OT2CA278684. Dr Meyerhardt is supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, the Project P fund, Anonymous Family Fund for Innovations in Colorectal Cancer, and the George Stone Family Foundation.
Conflicts of interest
Dr Brown reported receiving grants from the National Institutes of Health, the American Institute for Cancer Research, and Cancer Research UK paid to his institution. Dr Shi reported receiving institutional grant support from Celgene–Bristol Myers Squibb and Roche/Genentech; serving as a consultant to Yiviva Inc and Boehringer Ingelheim Pharmaceuticals; and owning stock in Johnson & Johnson, Merck, and Amgen. Dr Kuebler reported receiving grants from the Columbus National Community Oncology Research Program and the National Institutes of Cancer. Dr Goldberg reported serving as a paid consultant to Abbvie, Adaptimmune, Astra Zeneca, Bayer, Compass Therapeutics, Eisai, Focus Medical, Genentech, G1Therapeutics, GSK, Haystack Oncology, IQVIA, Inspirna, Merck, Sorrento, Taiho, and UpToDate. Dr O’Reilly reported receiving research funding paid to her institution from Genentech/Roche, BioNTech, AstraZeneca, Arcus, Elicio, Parker Institute, NIH/NCI, Pertzye; and serving as a consultant or data safety monitoring board member for Boehringer Ingelheim, BioNTech, Ipsen, Merck, Novartis, AstraZeneca, BioSapien, Astellas, Thetis, Autem, Novocure, Neogene, BMS, Tempus, Fibrogen, Merus, Agios (spouse), Genentech-Roche (spouse), Eisai (spouse). Dr Shields reported receiving grants from National Cancer Institute. Dr Meyerhardt reported receiving personal fees for serving on the advisory boards of Merck Pharmaceutical. All other authors disclosed no conflicts of interest.
References
- 1. Coussens LM, Werb Z.. Inflammation and cancer. Nature. 2002;420(6917):860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436-444. [DOI] [PubMed] [Google Scholar]
- 3. Yasui K, Shida D, Nakamura Y, et al. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br J Cancer. 2021;124(5):933-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng E, Shi Q, Shields AF, et al. Association of inflammatory biomarkers with survival among patients with stage III colon cancer. JAMA Oncol. 2023;9(3):404-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson DE, O’Keefe RA, Grandis JR.. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535-3541. [DOI] [PubMed] [Google Scholar]
- 7. Brown JC, Ma C, Shi Q, et al. Physical activity in stage III colon cancer: CALGB/SWOG 80702 (Alliance). J Clin Oncol. 2023;41(2):243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JC, Gilmore LA.. Physical activity reduces the risk of recurrence and mortality in cancer patients. Exerc Sport Sci Rev. 2020;48(2):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JC, Zhang S, Ligibel JA, et al. Effect of exercise or metformin on biomarkers of inflammation in breast and colorectal cancer: a randomized trial. Cancer Prev Res (Phila). 2020;13(12):1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyerhardt JA, Shi Q, Fuchs CS, et al. Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA. 2021;325(13):1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black S, Kushner I, Samols D.. C-reactive protein. J Biol Chem. 2004;279(47):48487-48490. [DOI] [PubMed] [Google Scholar]
- 13. Sansone P, Bromberg J.. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30(9):1005-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez M, Cabal-Hierro L, Carcedo MT, et al. NF-kappaB signal triggering and termination by tumor necrosis factor receptor 2. J Biol Chem. 2011;286(26):22814-22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan AT, Ogino S, Giovannucci EL, et al. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140(3):799-808, quiz e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herieka M, Erridge C.. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. 2014;58(1):136-146. [DOI] [PubMed] [Google Scholar]
- 17. Mehigan BJ, Hartley JE, Drew PJ, et al. Changes in T cell subsets, interleukin-6 and C-reactive protein after laparoscopic and open colorectal resection for malignancy. Surg Endosc. 2001;15(11):1289-1293. [DOI] [PubMed] [Google Scholar]
- 18. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991-999. [DOI] [PubMed] [Google Scholar]
- 19. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-S504. [DOI] [PubMed] [Google Scholar]
- 20. Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298(7):754-764. [DOI] [PubMed] [Google Scholar]
- 21. Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664-8670. [DOI] [PubMed] [Google Scholar]
- 22. Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245-271. [DOI] [PubMed] [Google Scholar]
- 23. Lee S, Ma C, Shi Q, et al. Potential mediators of oxaliplatin-induced peripheral neuropathy from adjuvant therapy in stage III colon cancer: findings from CALGB (Alliance)/SWOG 80702. J Clin Oncol. 2023;41(5):1079-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown JC, Damjanov N, Courneya KS, et al. A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology. 2018;27(4):1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyerhardt JA, Ogino S, Kirkner GJ, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15(18):5931-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305(16):1685-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamauchi M, Lochhead P, Imamura Y, et al. Physical activity, tumor PTGS2 expression, and survival in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1142-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanyuda A, Kim SA, Martinez-Fernandez A, et al. Survival benefit of exercise differs by tumor IRS1 expression status in colorectal cancer. Ann Surg Oncol. 2016;23(3):908-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrer R, Klein WM.. Risk perceptions and health behavior. Curr Opin Psychol. 2015;5:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Friedenreich CM, Neilson HK, Farris MS, et al. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22(19):4766-4775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available because study subjects did not consent for their data to be shared publicly. Our ability to preserve subject anonymity cannot be guaranteed.