Abstract
Background
The cutoff of <1% positive cells to define estrogen receptor (ER) negativity by immunohistochemistry (IHC) in breast cancer (BC) is debated. We explored the tumor immune microenvironment and gene-expression profile of patients with early-stage HER2-negative ER-low (ER 1%-9%) BC, comparing them to ER-negative (ER <1%) and ER-intermediate (ER 10%-50%) tumors.
Methods
Among 921 patients with early-stage I-III, ER ≤50%, HER2-negative BCs, tumors were classified as ER-negative (n = 712), ER-low (n = 128), or ER-intermediate (n = 81). Tumor-infiltrating lymphocytes (TILs) were evaluated. CD8+, FOXP3+ cells, and PD-L1 status were assessed by IHC and quantified by digital pathology. We analyzed 776 BC-related genes in 116 samples. All tests were 2-sided at a <.05 significance level.
Results
ER-low and ER-negative tumors exhibited similar median TILs, statistically significantly higher than ER-intermediate tumors. CD8/FOXP3 ratio and PD-L1 positivity rates were comparable between ER-low and ER-negative groups. These groups showed similar enrichment in basal-like intrinsic subtypes and comparable expression of immune-related genes. ER-low and ER-intermediate tumors showed significant transcriptomic differences. High TILs (≥30%) were associated with improved relapse-free survival (RFS) in ER-low (5-year RFS 78.6% vs 66.2%, log-rank P = .033, hazard ratio [HR] 0.37 [95% CI = 0.15 to 0.96]) and ER-negative patients (5-year RFS 85.2% vs 69.8%, log-rank P < .001, HR 0.41 [95% CI = 0.27 to 0.60]).
Conclusions
ER-low and ER-negative tumors are similar biological and molecular entities, supporting their comparable clinical outcomes and treatment responses, including to immunotherapy. Our findings contribute to the growing evidence calling for a reevaluation of ER-positive BC classification and management, aligning ER-low and ER-negative tumors more closely.
Estrogen receptor (ER) expression serves as the main predictive biomarker for endocrine therapy (ET) responsiveness in breast cancer (BC). The current threshold for ER positivity, defined by immunohistochemistry (IHC) as ≥1% of positively stained cancer cells (1), is debated. Patients with low ER levels (1% to 9%, ER-low) derive limited benefit from adjuvant ET (2-9), and yet share similar clinicopathological characteristics (3,4,10), prognosis (11,12), response rates to neoadjuvant chemotherapy (NACT) (11,13), and prognostic effect of pathological complete response (pCR) (14) as ER <1%/HER2-negative (ER-neg) BC. Biological data substantiate these clinical similarities, because ER-low and ER-neg BC show similar gene-expression profiles (GEP) such as intrinsic molecular subtyping (15-18) and prognostic genomic assays (18), and comparable germline BRCA mutation (19,20).
The immunological features of ER-low BC remain largely underexplored. ER-neg BC typically exhibits a “hot” tumor microenvironment (TME), which contrasts the immune-suppressive features of ER-positive tumors (21). Although tumor-infiltrating lymphocytes (TILs) have a positive prognostic significance in ER-neg BC (22,23), their impact in ER-positive BC patients remains ambiguous (24), with some studies suggesting a detrimental effect (23,25). Preliminary data indicate no significant differences in TME between ER-neg and ER-low tumors (4,16), but the prognostic value of TILs in ER-low BC has yet to be defined.
Immunotherapy has become a standard treatment for ER-neg BC (26,27), but its efficacy in ER-positive BC is less pronounced (28-35), benefiting only a few patients (32-35). Regarding the subset of ER-low BCs, studies suggest a similar antitumor activity of immune checkpoint inhibitors (ICIs) to that observed in ER-neg BC (36), and higher than seen in ER-positive patients (ER ≥1%) (34). However, ER-low patients were excluded from pivotal trials leading to the approval of ICIs for ER-neg BC (26,27), leading to a lack of access to promising immunotherapy-based treatments.
Given the uncertainties surrounding the impact of varying ER-expression levels on immune dynamics, paralleled by the potential to modulate them with immune-modulatory strategies such ICIs (34-36), there is an urgent, unmet need for the poor-prognosis subset of ER-low patients.
This multicentric study aims to address these gaps by comparing the TME and GEP in early-stage (I-III) HER2-negative BC by ER status and investigate TILs’ prognostic significance in ER-low tumors.
Methods
Population
This study includes 921 patients with early-stage (I-III), HER2-negative BC from 4 institutions: Istituto Oncologico Veneto (IOV) Padova, Italy (n = 451); Montpellier Cancer Institute (MCI), Montpellier, France (n = 223); Istituto Nazionale Tumori (INT), Milano, Italy (n = 178); and Istituto Europeo di Oncologia (IEO), Milano, Italy (n = 69). Patients were selected based on an expression of ER between 0% and 50% of cancer cells by IHC, according to local review. Tumors were classified as ER-neg (ER 0%, n = 712), ER-low (ER 1%-9%, n = 128), or ER-intermediate (ER-int) (ER 10%-50%, n = 81, included as a control cohort). Allowed progesterone (PgR) levels were up to 10% for ER-neg and ER-low cases. ER-neg and ER-low cases from IOV, MCI, and INT were consecutively treated (March 2000 to December 2021, June 2002 to November 2012, and December 2005 to May 2022, respectively). Supplementary Figure 1 (available online) shows patient disposition.
Patients with ER-int and all patients from IEO were derived from nonconsecutive cohorts enriched in patients who experienced disease relapse; these patients were excluded from survival analyses.
Clinicopathological, treatment, and follow-up data were collected.
Pathology
Treatment-naïve formalin-fixed paraffin-embedded (FFPE) tumor samples were collected: surgery specimens for patients treated with primary surgery and pretreatment core-biopsies for patients treated with neoadjuvant treatment.
All IHC protocols relevant to this study are reported as Supplementary Material (available online).
ER status was locally reviewed on previously stained IHC slides by dedicated breast pathologists.
HER2 status was scored according to ASCO/CAP recommendations in place at the time of diagnosis.
Blinded histopathological assessment of stromal TILs density on hematoxylin-eosin stained whole-slides (WS) was conducted locally by dedicated pathologists, following standardized guidelines (37). TILs were evaluated both as continuous and as categorical variables at the ≥30% cutoff validated in triple-negative BC (22,38).
To investigate the existence of more granular differences in TILs’ composition across two cohorts of ER-low and ER-neg tumors, we evaluated the density of CD8+ cells, the primary mediators of tumor killing, FOXP3+ T regulatory cells, which tamper antitumor immune responses by exerting strong immunosuppressive functions, and the immune-checkpoint PD-L1. Since an enhanced FOXP3+ cell infiltrate may contrast the antitumor activity of CD8+ cells (39), we used the ratio of CD8/FOXP3 positive cells to infer the polarization of the TME toward an immune-active or an immune-suppressive state (40). CD8/FOXP3 and PD-L1 IHC staining was evaluated only in ER-neg and ER-low samples (n = 477), sourced from IOV and MCI. At IOV, samples were handled as WS, whereas MCI employed tissue-microarray (TMA). For each case, consecutive slides were locally stained for CD8, FOXP3, and PD-L1 and then scanned using a NanoZoomer C12740 digital scanner. All digital slides were centrally evaluated at IOV for CD8, FOXP3, and PD-L1 metrics using Visiopharm software applications, following a previously described digital pathology workflow (41). Scanned slides from IOV were aligned with a MNF116 stained slide from the same sample to define the stromal compartment of the tumor. The densities of CD8+ and FOXP3+ cells were measured as number of positive cells/mm2. At IOV, this measurement was performed in the stromal area of the tumor. For MCI cases, the intratumoral area of TMA foci was considered. To account for outliers, the CD8/FOXP3 density ratio was log-transformed. PD-L1 expression was evaluated on tumor-infiltrating immune cells (IC score) with the SP142 clone (Ventana), and cases with immunoreactive immune cells covering ≥1% of the tumor area were considered positive.
Gene expression
Gene-expression analyses were performed locally at IOV and INT. Pathologists reviewed FFPE samples for tumor tissue quality and quantity. From samples with adequate material (>40% of tumor cells), a cohort of ER-low and ER-neg cases matched for age (<50, 50-65, or >65 years old), histotype (ductal, lobular, or other), and stage (I, II, or III) were identified. A control cohort of unmatched ER-int cases was included.
RNA extracted from FFPE was used to measure gene expression using the Breast Cancer 360 Panel on the nCounter platform (NanoString Technologies, Inc, Seattle, WA, USA) covering 776 genes from different independent signatures, including the PAM50 signature (Supplementary Material, available online). Gene-expression data were normalized using a ratio of the expression value to the geometric mean of the housekeeper genes of the PAM50 signature. Data were then log2 transformed. Intrinsic molecular subtyping was determined using the previously reported PAM50 subtype predictor (42). An unpaired 2-class SAM analysis with a 5% false discovery rate (FDR) was used to identify genes differentially expressed in different subgroups.
Statistical analysis
Statistical analyses were performed using IBM software SPSS v.29.0 and R (version 4.2.1); all tests were 2-sided, and an alpha < 0.05 significance level was used.
The association between variables was evaluated using the Mann-Whitney or Kruskal-Wallis nonparametric tests for continuous variables, and the χ2 test or Fisher exact test for categorical variables, as appropriate.
Relapse-free survival (RFS) was defined as the time from diagnosis to relapse or death from any cause, and overall survival (OS) as the time from diagnosis to death from any cause. Patients without events were censored at the time of the last follow-up.
The Kaplan-Meier method was used to estimate survival curves, the log-rank test to compare survival curves, and the Cox regression model to calculate hazard ratios (HR) and 95% confidence intervals (95% CI).
Ethical considerations
Tumor samples were collected after approval from the Institutional Review Board of each participating center and in accordance with the Declaration of Helsinki. Written consent was obtained from each participant who was alive at the time of study entry.
Results
Patients’ characteristics
We included a total of 921 patients: 712 patients with ER-neg, 128 with ER-low, and 81 with ER-int BC (Supplementary Figure 1, available online). Table 1 presents the clinicopathological data of the two primary patient groups: ER-low and ER-neg.
Table 1.
Clinicopathological data of patients with estrogen receptor (ER)-low (ER 1%-9%) and ER-negative (ER-neg, <1%) tumors
| Clinicopathological characteristics | ER-neg (n = 712) | ER-low (n = 128) | P | |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age, years | Median (IQR) | 54 (45-64) | 53 (44-67) | .713 |
| Range | 22-98 | 29-90 | ||
| Histology | Ductal/NOS | 614 (88.1%) | 113 (89.0%) | .022 |
| Lobular | 22 (3.2%) | 11 (8.7%) | ||
| Apocrine | 17 (2.4%) | 0 | ||
| Metaplastic | 9 (1.3%) | 0 | ||
| Medullary | 4 (0.6%) | 0 | ||
| Other | 31 (4.4%) | 3 (2.3%) | ||
| Grade | 1 | 4 (0.6%) | 0 | .243 |
| 2 | 78 (11.3%) | 20 (16.4%) | ||
| 3 | 607 (88.1%) | 102 (83.6%) | ||
| PgR, % | Median (IQR) | 0 (0-0) | 0 (0-1) | <.001 |
| Range | 0-5 | 0-9 | ||
| HER2 status | 0 | 459 (64.5%) | 57 (44.9%) | <.001 |
| 1+ | 182 (25.5%) | 55 (43.3%) | ||
| 2+/ISH unamplified | 71 (10.0%) | 15 (11.8%) | ||
| Ki67, % | Median (IQR) | 60 (35-70) | 60 (35-75) | .659 |
| Range | 1-95 | 5-95 | ||
| Stage | I | 212 (29.9%) | 43 (33.9%) | .230 |
| II | 402 (56.7%) | 62 (48.8%) | ||
| III | 95 (13.4%) | 22 (17.3%) | ||
| Nodal status | Negative | 386 (60.8%) | 68 (54.8%) | .217 |
| Positive | 249 (39.2%) | 56 (45.2%) | ||
| Neoadjuvant CT | No | 411 (57.7%) | 94 (73.4%) | <.001 |
| Yes | 301 (42.3%) | 34 (26.6%) | ||
| Neoadjuvant carboplatin | No | 117 (48.8%) | 18 (62.1%) | .151 |
| Yes | 127 (52.0%) | 11 (37.9%) | ||
| Neoadjuvant anthracyclines | No | 4 (1.6%) | 2 (6.9%) | .125 |
| Yes | 240 (98.4%) | 27 (93.1%) | ||
| Neoadjuvant taxanes | No | 1 (0.4%) | 0 | >.999 |
| Yes | 244 (99.6%) | 29 (100%) | ||
| Response to neoadjuvant treatment | Residual disease | 177 (58.8%) | 20 (58.8%) | .998 |
| pCR | 124 (41.2%) | 14 (41.2%) | ||
| Adjuvant CT | No | 280 (39.3%) | 44 (34.4%) | .289 |
| Yes | 432 (60.7%) | 84 (65.6%) | ||
| Adjuvant CT after NACT (residual disease) | No | 115 (65.0%) | 13 (65.0%) | .998 |
| Yes | 62 (35.0%) | 7 (35.0%) | ||
| CT exposure | No | 43 (6.0%) | 17 (13.3%) | .003 |
| Yes | 669 (94.0%) | 111 (86.7%) | ||
| Endocrine therapy | No | 476 (94.6%) | 71 (67.6%) | <.001 |
| Yes | 27 (5.4%)a | 34 (32.4%) | ||
| Adjuvant radiotherapy | No | 117(31.5%) | 16 (38.1%) | .389 |
| Yes | 254 (68.5%) | 26 (61.9%) | ||
A limited number of ER-neg patients received endocrine therapy, probably due to some degree of ER positivity on residual disease after NACT. ER-neg = ER-negative; IQR = interquartile range; NOS = not otherwise specified; ER = estrogen receptor; PgR = progesterone receptor; ISH = in situ hybridization; pCR = pathological complete response (ypT0/is ypN0); CT = chemotherapy.
Statistics: χ2, or Fisher exact test when appropriate, was employed to test the distribution of categorical variables; Mann-Whitney nonparametric test was used to compare the distribution of continuous variables.
Compared to patients with ER-neg BC, those with ER-low tumors more commonly had lobular histology and were less likely to have HER2-0 status, possibly due to a positive association between HER2-signaling and ER-expression. No differences in key clinic-pathological features such as stage, nodal status, grade, or proliferation rate were noted. ER-low patients were less frequently treated with chemotherapy, including NACT, but received ET more frequently.
The non-consecutively treated cohort of patients with ER-int tumors, compared with ER-neg and ER-low, showed differences in several clinic-pathological characteristics (Supplementary Table 1, available online), which may be related partly to different inherent biology of ER-int tumors and partly to the selection procedure (cohort enriched in patients with disease relapse).
Survival analyses revealed no significant differences between ER-low and ER-neg patients both in terms of RFS (5 years RFS 70.9% vs 74.9%, log-rank P = .181; HR 1.26 [95% CI = 0.90 to 1.78]) and OS (79.3% vs 82.2%, log-rank P = .223; HR 1.27 [95% CI = 0.86 to 1.87]) (Supplementary Figure 2, A and B, available online). This observation was consistent at a 60-months landmark analysis, where no difference was noted for both RFS (log-rank P = .105; HR 1.84 [95% CI = 0.87 to 3.90]) and OS (log-rank P = .202; HR 1.57 [95% CI = 0.78 to 3.15]) (Supplementary Figure 2, C and D, available online), despite numerically higher rates of late distant relapses in the ER-low subgroup (Supplementary Table 2, available online). Similar results were obtained when directly comparing the outcome of ER-low and ER-neg among the selected group of patients exposed to systemic chemotherapy (5 years RFS 72.0% vs 76.7%, log-rank P = .182; HR 1.29 [95% CI = 0.89 to 1.87]); 5 years OS 80.2% vs 83.9%, log-rank P = .308; HR 1.25 [95% CI = 0.81 to 1.92]) (Supplementary Figure 3, available online).
TILs density according to ER status
We assessed TILs in 846 samples, 647 ER-neg, 119 ER-low, and 80 ER-int (Supplementary Figure 1, available online).
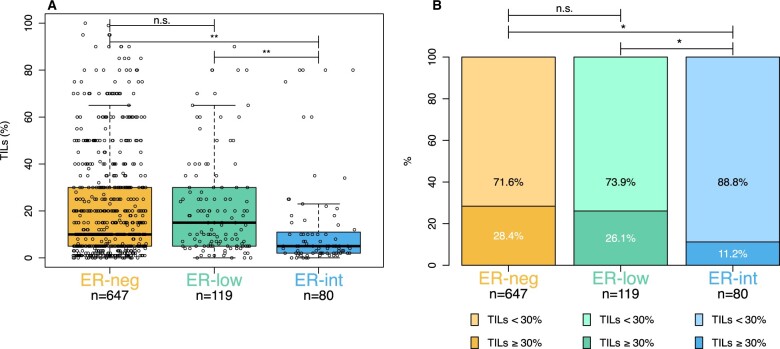
TILs were similar in ER-neg and ER-low BC (median 10%, interquartile range [IQR] [5-30] vs 15%, [5-30]; P > .999) (Figure 1, A). In contrast, TILs were statistically significantly lower in ER-int (median 5%, IQR [2-11]) compared with both ER-low (P < .001) and ER-neg (P < .001) BC specimens (Figure 1, A). To address the potential influence of tumor-intrinsic features on our analysis, we evaluated the distribution of TILs within ER status according to stage, grade, and Ki67, showing similar influence of grade and Ki67 on TIL density in both ER-neg and ER-low tumors (Supplementary Table 3, available online).
Figure 1.
Distribution of tumor-infiltrating lymphocytes (TILs) as a continuous (1% increase) (A) and categorical variable (≥30% cutoff) (B), stratified by estrogen receptor (ER) status: ER-negative (ER-neg, ER <1%), ER-low (ER 1%-9%), and ER-intermediate (ER-int, ER 10%-50%). TILs = tumor-infiltrating lymphocytes; *P < .05, **P < .001; n.s. = nonsignificant.
Similar proportions of patients with high TILs (≥30%) were observed in ER-neg and ER-low groups (28.4% vs 26.1%, P = .594). In contrast, ER-int samples showed a lower proportion of patients with high TILs (11.2%) compared with both ER-neg (P = .001) and ER-low groups (P = .011) (Figure 1, B). These findings remained consistent when we separately analyzed samples from each participating institution (Supplementary Figures 4 and 5, available online).
To further explore TILs density within ER-int tumors, we divided them into two subcategories: ER 10%-30% and ER 31%-50%. Our analysis indicated that tumors with ER 10%-30% showed no significant difference in TILs density (median 9%, IQR [3-23]), compared with ER-neg (P > .999) and ER-low tumors (P = .678). Instead, tumors with the highest spectrum of ER-expression (31%-50%) had lower TILs (median 4% [IQR 2-8]) compared with both ER-neg (P < .001) and ER-low tumors (P < .001), but not statistically different from tumors with ER 10%-30% (P = .116) (Supplementary Figure 6, available online).
Immune cell densities and PD-L1 expression
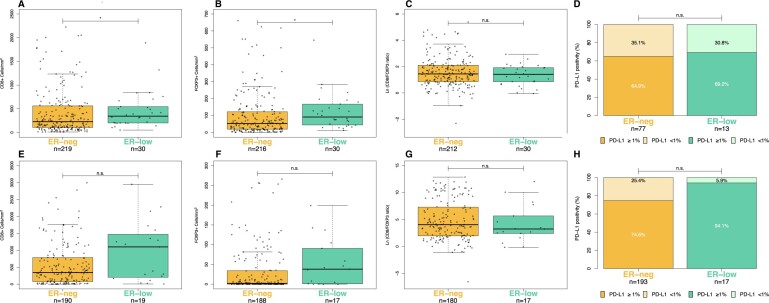
ER-low tumors showed higher densities of both CD8+ and FOXP3+ cells/mm2 compared with ER-neg BCs, and this difference reached statistical significance in the IOV cohort (P = .040 and P = .011, respectively) (Figure 2, A and B) but not in the smaller MCI cohort (P = .081 and P = .057, respectively) (Figure 2, E and F). On the other hand, the log-transformed CD8/FOXP3 ratio was similar in ER-low vs ER-neg tumors (IOV: median 1.45, IQR [0.86-2.11] vs 1.42 [0.86-1.92], P = .504; MCI: 4.04 IQR [1.97-7.30] vs 3.24 IQR [2.42-5.67] P = .400, Figure 2, C and G), and the two cohorts were also characterized by a similar rate of PD-L1 positive expression (IOV: 69.2% vs 64.9% P > .999; MCI: 94.1% vs 74.6%, P = .080, Figure 2, D and H).
Figure 2.
Distribution of CD8+ cells (cells/mm2), FOXP3+ cells (cells/mm2), the log-transformed CD8/FOXP3 ratio, and the rate of positive PD-L1 expression (≥1% cutoff) across different estrogen-receptor (ER) statuses: ER-neg (ER neg, ER <1%) and ER-low (ER 1%-9%). The top half (2A-D) presents biomarker data from the Istituto Oncologico Veneto cohort (full-face slides, stromal compartment), whereas the bottom half (2E-H) features data from the Montpellier Cancer Institute cohort (tissue micro-array, intratumoral foci). Note: To improve readability, the y-axis in Figure 2, A, B, E, and F is truncated. ER-neg = ER-negative (ER <1%), ER-low (ER 1%-9%), *P < .05; n.s. = nonsignificant.
Prognostic impact of TILs in ER-low and ER-neg BC
We examined the prognostic relevance of TILs according to ER status in 647 ER-neg and 105 ER-low cases. The median follow-up time was 8.2 years (95% CI = 7.8 to 8.7 years).
At univariate analysis, each 1% increase in TILs corresponded to a 2% reduction in the risk of RFS-event in both ER-neg (HR 0.98 [95% CI = 0.98 to 0.99], P < .001) and ER-low (HR 0.98 [95% CI = 0.96 to 1.00], P = .033) cohorts (Table 2). We also found a 2% reduction in the risk of death for each 1% TILs increase in both patient cohorts (ER-neg: HR 0.98, 95% CI [0.97 to 0.99], P < .001; ER-low: HR 0.98, 95% CI [0.96 to 1.00], P = .062).
Table 2.
Univariate and multivariate Cox analyses for relapse-free survival and overall survival in patients with estrogen receptor (ER)-negative (ER-neg, ER <1%) and ER-low (ER 1%-9%) breast cancer
| Relapse-free survival |
Overall survival |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ER-neg |
ER-low |
ER-neg |
ER-low |
||||||||||||||
| Univariate |
Multivariate |
Univariate |
Multivariate |
Univariate |
Multivariate |
Univariate |
Multivariate |
||||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Age (Cont.) | 1.02 (1.01 to 1.04) | <.001 | 1.02 (1.01 to 1.03) | .003 | 1.00 (0.98 to 1.03) | .705 | 1.01 (0.98 to 1.03) | .706 | 1.04 (1.02 to 1.05) | <.001 | 1.03 (1.01 to 1.04) | <.001 | 1.02 (0.98 to 1.05) | .087 | 1.02 (0.99 to 1.05) | .181 | |
| Grade | 1-2 | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – | ||||
| 3 | 0.78 (0.53 to 1.16) | .219 | – | – | 0.52 (0.24 to 1.11) | .089 | – | – | 0.68 (0.45 to 1.04) | .075 | – | – | 0.77 (0.29 to 2.03) | 0.594 | – | – | |
| Ki67 (Cont.) | 1.00 (0.99 to 1.00) | .198 | – | – | 0.99 (0.97 to 1.01) | .218 | – | – | 1.00 (0.99 to 1.01) | .369 | – | – | 0.99 (0.97 to 1.01) | 0.195 | – | – | |
| Stage | I | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||
| II | 2.05 (1.40 to 3.02) | <.001 | 2.45 (1.67 to 3.72) | <.001 | 1.08 (0.52 to 2.22) | .844 | 1.11 (0.51 to 2.40) | .801 | 1.87 (1.22 to 2.89) | .004 | 2.12 (1.37 to 3.29) | <.001 | 1.13 (0.51 to 2.48) | .770 | 1.43 (0.62 to 3.29) | .408 | |
| III | 4.57 (2.93 to 7.12) | <.001 | 5.21 (3.27 to 8.32) | <.001 | 1.49 (0.61 to 3.68) | .383 | 1.43 (0.52 to 3.94) | .494 | 4.32 (2.64 to 7.06) | <.001 | 4.82 (2.91 to 8.01) | <.001 | 1.06 (0.36 to 3.16) | .919 | 1.39 (0.42 to 4.60) | .594 | |
| CT exposure | No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||
| Yes | 0.39 (0.25 to 0.60) | <.001 | 0.44 (0.27 to 0.71) | <.001 | 0.56 (0.23 to 1.35) | .196 | 0.59 (0.19 to 1.86) | .369 | 0.31 (0.20 to 0.48) | <.001 | 0.41 (0.24 to 0.69) | <.001 | 0.40 (0.16 to 0.99) | .047 | 0.64 (0.20 to 2.08) | .455 | |
| TILs (1% incr.) | 0.98 (0.98 to 0.99 | <.001 | 0.99 (0.98 to 0.99) | <.001 | 0.98 (0.96 to 1.00) | .020 | 0.97 (0.96 to 1.00) | .037 | 0.98 (0.97 to 0.99) | <.001 | 0.99 (0.98 to 1.00) | .002 | 0.98 (0.96 to 1.00) | .062 | 0.98 (0.95 to 1.00) | .066 | |
All the variables that resulted significantly associated with outcome in univariate analysis at least in one group (ER-neg or ER-low) were included in multivariate analyses for both groups. CI= confidence interval; Cont. = continuous; HR = hazard ratio; Incr. = increase; CT = chemotherapy; TILs = tumor-infiltrating lymphocytes.
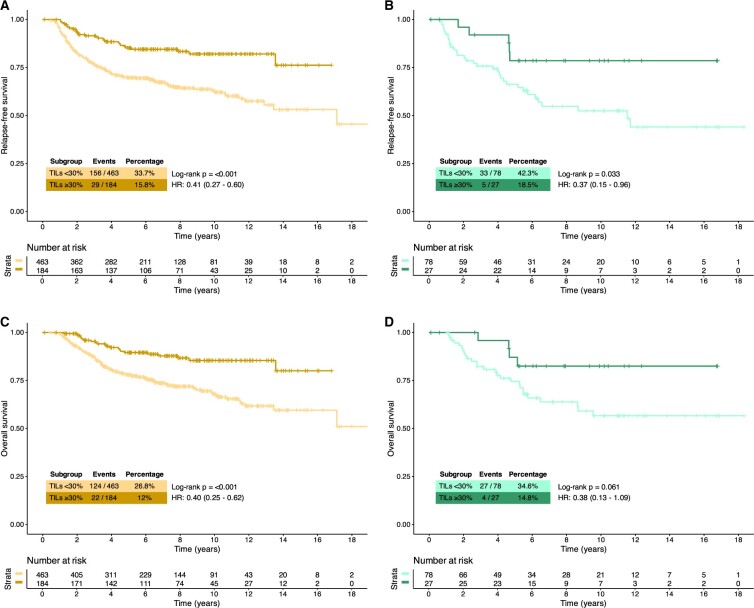
When TILs were dichotomized based on a ≥30% cutoff (Figure 3, A and B), we found that high TILs were associated with statistically significantly improved RFS in both ER-neg (5 year RFS 85.2% vs 69.8%, log-rank P < .001, HR 0.41 [95% CI = 0.27 to 0.60]) and ER-low (5-year RFS 78.6% vs 66.2%, log-rank P = .033, HR 0.37 [95% CI = 0.15 to 0.96]) cohorts. We found similar findings when OS was used as a clinical outcome, with results reaching statistical significance for ER-neg (5-year OS 89.6% vs 78.0%, log-rank P < .001; HR 0.40 [95% CI = 0.25 to 0.62]) and pointing to the same direction for ER-low (5 year 87.1% vs 74.5%, log-rank P = .061; HR 0.38 [95% CI = 0.13 to 1.09]) (Figure 3, C and D).
Figure 3.
Kaplan-Meier survival curves in patients with estrogen receptor (ER)-negative (ER-neg) and ER-low breast cancer (A, relapse-free survival in ER-neg; B, relapse-free survival in ER-low; C, overall survival in ER-neg; D, overall survival in ER-low) according to tumor-infiltrating lymphocytes (TILs) at ≥30% cutoff. HR = hazard ratio
Results of univariate analyses were confirmed by multivariate analyses adjusting for age, stage, chemotherapy exposure (Table 2), and when factoring ER expression (ER-neg vs ER-low) as a covariate (Supplementary Table 4, available online).
Gene-expression analysis
Gene-expression analyses were performed on 65 ER-low cases, matched to 39 ER-neg tumors. Twelve ER-int samples served as unmatched controls.
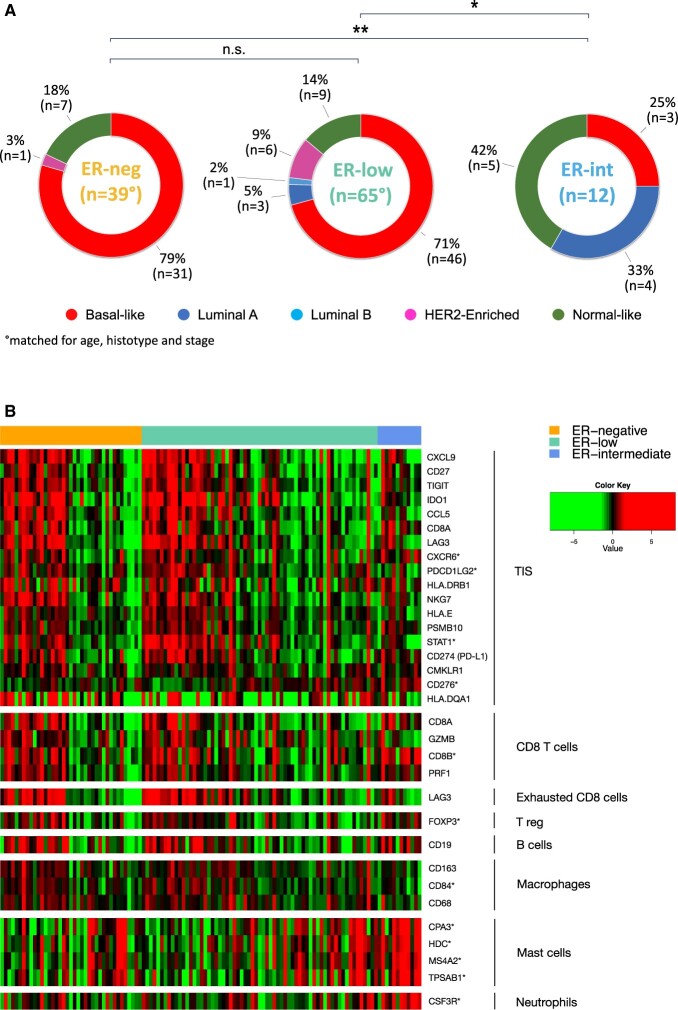
Both ER-neg and ER-low tumors exhibited a similar distribution in PAM50-intrinsic subtypes (P = .396), primarily featuring basal-like tumors (79%, n = 31, and 71%, n = 46, respectively) (Figure 4, A). Conversely, the ER-int group differed statistically significantly from both ER-low (P = .002) and ER-neg patients (P < .001), with basal-like tumors making up only 25% of the cases.
Figure 4.
A) Distribution of PAM50 intrinsic subtypes in patients with estrogen receptor (ER)-negative (ER-neg, ER <1%), ER-low (ER 1%-9%), and ER-intermediate (ER-int, ER 10%-50%) breast cancer; asterisks (*P < .05, **P < .001) mark a statistically significant difference in the distribution of subtypes. B) Heatmap illustrating differential expression of immune genes, clustered by ER status (ER-neg, ER-low, and ER-int) and ordered according to decreasing density of TILs: colors indicate mRNA expression levels, higher in the case of red, and lower for green. The graph is further segmented according to genes associated with tumor inflammation signature (TIS) and various immune cells, such as CD8 T cells, exhausted T cells, Tregs, B cells, macrophages, mast cells, and neutrophils. An asterisk (*) marks gene differentially expressed in ER-low vs ER-int samples as identified by SAM analysis (FDR <5%). ER = estrogen receptor; ER-neg = ER-negative (ER neg, ER <1%); ER-low (ER 1%-9%); ER-int = ER-intermediate (ER 10%-50%); FDR = false-discovery rate; n.s. = non significant; SAM = significance analysis of microarrays; TIS = tumor inflammation signature.
Basal-like subtype showed statistically significantly higher TILs compared with other subtypes in both ER-low (median 20%, range [0-80%] vs 6% [1-40%], P < .001) and ER-int samples (53% [25-80%] vs 5% [0-10%], P = .036), whereas no significant difference in TILs was observed in ER-neg tumors (P = .503).
SAM analysis of 776 genes revealed that only three were differentially expressed in ER-low compared with ER-neg tumors (GATA3, upregulated; EDN1 and PROM1, downregulated) (Supplementary Table 5, available online). When focusing on basal-like tumors (n = 77), only EDN1 and PROM1 genes remained differentially downregulated in ER-low (Supplementary Table 6, available online). In contrast, ER-low samples showed a distinct expression pattern compared with ER-int, with a statistically significantly higher expression of 53 genes and a lower expression of 398 genes (Supplementary Table 7, available online).
Comparing the expression of 164 immune-related genes in ER-low and ER-neg tumors, we found no significant differences in the expression of genes related to antigen presentation, cytokine and chemokine signaling, immune infiltration, TGF-beta signaling (Figure 4, B), or the characterization of immune cells (functionally annotated in Supplementary Table 8, available online). However, 86 genes, including 4 mast-cell-related genes, showed statistically significantly different expression levels between ER-low to ER-int tumors (Supplementary Table 9, available online).
Discussion
Our multicentric study reveals that ER-low and ER-neg BCs share similar immune and gene expression characteristics, differing significantly from ER-int tumors. We uniquely demonstrated that high TILs in ER-low BC independently indicate a positive prognosis.
Our clinical outcome analyses showed no significant differences in RFS and OS between the ER-low and ER-negative cohorts, with even a numerically higher rate of relapses in ER-low tumors. Importantly, both groups exhibited comparable pCR rates when treated with NACT, aligning with previous studies (11,13,15,43-45) and contrasting sharply with the limited response rates generally seen in hormone-receptor-positive/HER2- BC (46, 47).
Our observation that ER-low and ER-neg BCs have similar TILs density, which is instead lower in ER-int BC specimens, is remarkable. Indeed, ER-neg BC specimens typically exhibit higher levels of TILs when compared to hormone-receptor-positive/HER2-negative BCs (23,48), owing to the generally higher immunogenic background of ER-neg tumors, which contrasts the “cold” immune-suppressive TME often observed in hormone-receptor-positive/HER2-negative BC (21,49,50). Notably, in this study, we found that high levels of TILs were comparably associated with a more favorable prognosis in both ER-neg and ER-low BC patients.
Consistently, we observed a similar ratio of CD8/FOXP3 positive cells in ER-low and ER-neg tumor specimens, suggesting a similar polarization of the TME (40). Again in contrast with the acknowledged low expression of PD-L1 in hormone-receptor-positive BCs (51), we also identified a high positivity rate in ER-low tumors, akin to ER-neg. Together, these data support the existence of similar immune dynamics across ER-expression levels up to 9%.
In our gene-expression analysis, ER-low and ER-neg BC samples showed no major transcriptional differences, including an enrichment in basal-like subtypes, consistent with findings in previous studies (15-18). Notably, no immune-related gene was differentially expressed between these groups. In contrast, ER-int tumors displayed a distinct immune profile, characterized by increased expression of several mast cell-related genes. This aligns with previous findings that higher ER levels correlated with mast cell presence (16,52), a trait potentially contributing to the promotion of a luminal phenotype (53,54).
Our data provide strong evidence that ER-low and ER-neg are immunologically and biologically similar entities. Although ER IHC-staining was conceived as a predictive biomarker for ET benefit, the relationship between ER nuclear expression and specific immune-suppressive features typical of ER-positive tumors (55), which may dampen responses to ICIs (21), appears to be nonlinear. Our study shows that tumors with ER levels up to 9% exhibit similar CD8/FOXP3 ratio, PD-L1 expression, and GEP, indicating a marked immune and molecular divergence beginning at ER-int expression levels. This partially aligns with a recent report confirming similar immune features in ER-neg and ER-low BC (16). However, that study, despite reporting a higher prevalence of basal-like subtypes in ER-neg and ER-low compared with ER-int tumors, did not observe significant differences in TME across a broader range of ER expression levels (0% to 50%). This observation aligns with our exploratory observation of similar TIL density in patients with ER up to 30%, corroborating the potential of identifying a group of immune-active tumors within the broader ER-positive spectrum.
The biologic heterogeneity within ER-positive/HER2-negative BCs plays a critical role in determining the efficacy of CT, ET (56,57), and ICIs (34-36,58).
Luminal tumors are sensitive to ET (59,60), whereas basal-like tumors resist ET and cyclin-dependent kinase 4/6 inhibitors (61) but are more responsive to chemotherapy (62-65). Molecular subtyping combined with immune features may help identify ER-expressing tumors sensitive to immunotherapy across ER levels (32,66,67). For instance, in the I-SPY2 trial, among ER-positive/HER2-negative BC classified as high-risk on MammaPrint, a basal-like intrinsic subtype was associated with a 67% pCR with pembrolizumab added to NACT (66). Furthermore, the GIADA trial (32) reported that the co-occurrence of a basal-like intrinsic subtype and high TILs in premenopausal patients with ER ≥10%/HER2-negative BC and a luminal B-like IHC profile could accurately predict pCR after ICI-based neoadjuvant treatment and ET. Exploring the presence of this immune-responsive basal-like/high-TILs phenotype in our cohort, we observed higher TILs in ER-low and ER-int BC with basal-like tumors compared with non-basal-like tumors.
Recent trials have underscored a distinct activity of ICIs in the ER-low subgroups (34-36), mirroring those of ER-neg patients (36,68) and supported by the similar immune dynamics seen in our study. The NeoPACT phase II trial demonstrated comparable pCR rates in ER-low (56%) and ER-neg patients (58%) with pembrolizumab-NACT (36). In the Keynote-756 trial, ER-low patients experienced a 25.6% increase in pCR rates from the addition of pembrolizumab to NACT, much higher than the mere 8% seen in patients with ER 10%-100% (34). Strikingly, this delta is even larger than the 13.6% increase shown in the Keynote-522 trial, which led to pembrolizumab’s approval for ER-neg breast cancer (26). Similarly, the addition of nivolumab to NACT in the Checkmate 7FL trial resulted in a 27.0% increase in pCR rate in ER-low patients and 29.3% in those with ER ≤50%, compared to just 7.4% increase in patients with ER >50% (35). A correlation between pCR rates and the expression of PD-L1 (34, 35) and TILs (35) was seen in those trials across the spectrum of ER-positive tumors, which suggests the potential of a biologically informed, response-oriented subtyping of BC (67).
Our study has several strengths. It represents the largest study to provide immune-transcriptomic profiling of patients with ER-low BC, offering significant insights into this understudied population. The multicenter design of our study and the available long-term follow-up data enhance the generalizability and robustness of our findings. Conscious of unique approaches to tissue-handling protocols in place at the two institutions involved in our digital-pathology workflow, results regarding those analyses have been presented separately, a distinction that provides a robust and nuanced overview of immunological features.
This study also has some limitations, including its retrospective nature and the relatively small sample size of ER-low tumors. Treatment imbalances between the ER-low and ER-neg cohorts might have influenced our clinical outcome analyses and should be considered when interpreting our findings. First, patients with ER-low BC tumors were less frequently exposed to chemotherapy and more frequently managed with surgery upfront compared with ER-neg patients, although post-neoadjuvant tailoring of adjuvant treatment based on the response rate to NACT was not broadly employed in our cohort. Moreover, ET was not frequently administered, reflecting current clinical practice, as oncologists are generally less prone to prescribe ET in ER-low tumors (12,69,70) due to the limited survival benefit reported in earlier studies (2-6) and the notable side effects associated with ET (71). Our study’s limited sample size precludes a definitive evaluation of the impact of these therapeutic decisions on the prognosis of patients with ER-low tumors. In this regard, the numerically worse prognosis we observed in ER-low compared with ER-neg tumors, with an even higher incidence of distant relapses, may support further discussion on the role of ET for selected patients with ER-low tumors (72). Nonetheless, the comparable survival between ER-low and ER-neg tumors seen in our study, consistent with larger cohorts (11,73), underscores the urgent need to generate robust evidence to guide the clinical trajectory of patients with ER-low tumors.
The comparison of TILs in the non-consecutively treated ER-int cohorts warrants caution, due to limited sample size and the potential selection bias.
Potential analytical challenges stemming from the absence of a centralized review of both ER status (74,75) and TIL density cannot be excluded; however, we believe that these issues were mitigated. Tumor samples were evaluated by experienced and dedicated BC pathologists at single pathology units within high-volume comprehensive cancer centers. ER status was locally reviewed, and TILs were quantified on whole-slides following standardized recommended guidelines (37) and using reference images (76). The consistency in TILs distribution of ER-low and ER-neg tumors across our participating institutions further supports our findings and TILs’ established reproducibility (76,77).
The use of SP142 antibody to define PD-L1 positivity in our cohort warrants caution, because this assay has only partial overlap with PD-L1 expression levels defined using 22C3 antibody (78), the antibody used to define pembrolizumab eligibility in the metastatic setting. Still, a cutoff of ≥1% using SP142 has been shown to be predictive of nivolumab benefit in ER-positive patients treated in the Checkmate 7FL trial (35), reinforcing the biological role of evaluating PD-L1 status using SP142 in our cohort.
Moving forward, efforts to personalize cancer treatment in ER-low tumors should focus on examining TME’s functional status and spatial distribution. The use of IHC staining for CD8, FOXP3, and PD-L1 in our cohort allowed us to evaluate key components of the immune compartment using established IHC markers. However, this TME profiling is only partial and may overlook varying immune-states (21), which could affect the efficacy of distinct immunomodulatory combinations across ER statuses. Techniques such as multiplexed single-cell spatially resolved tissue analyses could be instrumental (79) in exploring subtle variations in the immune contexture (80) related to various ER levels, potentially overlooked in our quantitative analysis. Such an approach could pave the way for truly tailored immunotherapy strategies beyond traditional IHC-based classifications, across varying ER levels (32,33).
In conclusion, our results demonstrate that ER-low and ER-neg BC are immunologically and molecularly akin, clarifying their similar clinical outcomes and responses to therapeutics, particularly to ICIs. In this regard, we believe our data contribute notably to the growing body of clinical and translational evidence calling for a reevaluation of ER-based BC classification and management. As such, we advocate for a treatment approach that aligns ER-low tumors with ER-neg, as few guidelines are starting to acknowledge (81), to avoid perpetuating the current disparities in regulatory access to effective treatments for this subgroup of patients. Crucially, this endeavor should encompass at least the inclusion of patients with ER-low and triple-negative tumors in the same clinical trials, a practice already adopted in academic trials (82,83), ensuring that the high-risk ER-low patient population is not deprived from accessing potentially transformative therapies, such as immunotherapy. The evidence in terms of benefit from ICIs, which is stemming from the small subgroups of ER-low patients enrolled in trials dedicated to ER-positive BC, could at the best result in remarkable delay in the access to this treatment option, should long-term survival endpoints support the approval of ICIs in this population.
Supplementary Material
Acknowledgments
The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Previous presentations: Mini Oral Presentation—ESMO Breast 2023—1MO Tumor immune microenvironment in ER-negative vs ER-low, HER2-neg breast cancer, https://doi.org/10.1016/j.esmoop.2023.101225.
Ethics approval and consent to participate: The study was approved by the ethics committee of the participating centers, and all relevant ethical regulations were complied with. Tumor samples were collected after approval from the respective Institutional Review Board and per the Declaration of Helsinki. Informed written consent was obtained from each patient who was alive at the time of study entry.
Contributor Information
Davide Massa, Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology (DiSCOG), University of Padova, Padova, Italy.
Claudio Vernieri, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; IFOM ETS, The AIRC Institute of Molecular Oncology.
Lorenzo Nicolè, Department of Pathology, Angelo Hospital, Mestre, Italy.
Carmen Criscitiello, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Division of Early Drug Development for Innovative Therapy, European Institute of Oncology IRCCS, Milan, Italy.
Florence Boissière-Michot, Translational Research Unit, Institut du Cancer de Montpellier, Montpellier, France.
Séverine Guiu, Department of Medical Oncology, Institut Régional Du Cancer de Montpellier (ICM), Montpellier, France; Institut de Recherche en Cancérologie de Montpellier, INSERM U1194, Montpellier University, Montpellier, France.
Angélique Bobrie, Department of Medical Oncology, Institut Régional Du Cancer de Montpellier (ICM), Montpellier, France; Institut de Recherche en Cancérologie de Montpellier, INSERM U1194, Montpellier University, Montpellier, France.
Gaia Griguolo, Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology (DiSCOG), University of Padova, Padova, Italy.
Federica Miglietta, Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology (DiSCOG), University of Padova, Padova, Italy.
Andrea Vingiani, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Department of Advanced Diagnostics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Riccardo Lobefaro, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Beatrice Taurelli Salimbeni, Division of Early Drug Development for Innovative Therapy, European Institute of Oncology IRCCS, Milan, Italy.
Claudia Pinato, UOSD Hereditary Tumors, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy.
Francesca Schiavi, UOSD Hereditary Tumors, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy.
Silvia Brich, Department of Advanced Diagnostics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Carlo Pescia, Division of Pathology, European Institute of Oncology IRCCS, Milan, Italy.
Nicola Fusco, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Division of Pathology, European Institute of Oncology IRCCS, Milan, Italy.
Giancarlo Pruneri, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Department of Advanced Diagnostics, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Matteo Fassan, Department of Medicine (DIMED), University of Padua, Padova, Italy; Veneto Institute of Oncology IOV—IRCCS, Padova, Italy.
Giuseppe Curigliano, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Division of Early Drug Development for Innovative Therapy, European Institute of Oncology IRCCS, Milan, Italy.
Valentina Guarneri, Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology (DiSCOG), University of Padova, Padova, Italy.
William Jacot, Translational Research Unit, Institut du Cancer de Montpellier, Montpellier, France; Department of Medical Oncology, Institut Régional Du Cancer de Montpellier (ICM), Montpellier, France; Institut de Recherche en Cancérologie de Montpellier, INSERM U1194, Montpellier University, Montpellier, France.
Maria Vittoria Dieci, Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology (DiSCOG), University of Padova, Padova, Italy.
Data availability
Due to the nature of this research, participants of this study did not give consent for their data to be shared publicly and for secondary use of data derived from the study without Ethics Committee re-evaluation. However, data can be made available upon request through a Data Transfer Agreement and after Ethics Committee approval. We encourage investigators interested in data access to request them by contacting the Department of Surgery, Oncology and Gastroenterology of the University of Padua (ricerca.discog@unipd.it).
Author contributions
Davide Massa, MD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft; Writing—review & editing), Valentina Guarneri, PhD (Conceptualization; Funding acquisition; Project administration; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing), Giuseppe Curigliano, PhD (Investigation; Project administration; Supervision; Writing—review & editing), Matteo Fassan, PhD (Investigation; Supervision; Writing—review & editing), Giancarlo Pruneri, PhD (Data curation; Investigation; Supervision; Writing—review & editing), Nicola Fusco, MD (Data curation; Investigation; Writing—review & editing), Carlo Pescia, MD (Data curation; Investigation; Project administration; Supervision; Writing—review & editing), Silvia Brich, PhD (Conceptualization; Data curation; Investigation; Methodology; Writing—review & editing), Francesca Schiavi, PhD (Data curation; Formal analysis; Methodology; Resources; Supervision; Writing—review & editing), Claudia Pinato, PhD (Data curation; Formal analysis; Investigation; Methodology; Resources; Writing—original draft; Writing—review & editing), William Jacot, PhD (Data curation; Investigation; Resources; Supervision; Writing—review & editing), Beatrice Taurelli Salimbeni, MD (Data curation; Investigation; Writing—review & editing), Andrea Vingiani, MD (Data curation; Formal analysis; Investigation; Project administration; Resources; Writing—review & editing), Federica Miglietta, PhD (Data curation; Investigation; Writing—review & editing), Gaia Griguolo, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing—original draft; Writing—review & editing), Angélique Bobrie, PhD (Data curation; Investigation; Resources; Writing—review & editing), Séverine Guiu, PhD (Data curation; Investigation; Resources; Writing—review & editing), Florence Boissière-Michot, PhD (Data curation; Investigation; Resources; Writing—review & editing), Carmen Criscitiello, PhD (Data curation; Investigation; Project administration; Supervision; Writing—review & editing), Lorenzo Nicolè, PhD (Data curation; Investigation; Methodology; Writing—review & editing), Claudio Vernieri, PhD (Data curation; Investigation; Project administration; Resources; Supervision; Writing—review & editing), Riccardo Lobefaro, MD (Data curation; Investigation; Writing—review & editing), Maria Vittoria Dieci, MD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing).
Funding
This work was supported by Associazione Italiana per la Ricerca sul Cancro ID22759 to Prof. V. Guarneri; IG 27152 to Prof. M.V. Dieci, University of Padova, Department of Surgery, Oncology and Gastroenterology DOR 2021-2023 (to VG, MVD, GG, FM; grant number not applicable), Ricerca Corrente funding from the Italian Ministry of Health (grant number not applicable). Fondazione AIRC under 5 per mille 2019 (ID. 22759 program—group leader VG). Open access funding provided by BIBLIOSAN.
Conflicts of interest
D. Massa reports, outside the submitted work, the following: travel grants: Eli Lilly.
C. Vernieri reports, outside the submitted work, the following: consultancy/advisory board for Novartis, Pfizer, Eli Lilly, Menarini, and Daiichi Sankyo; honoraria as a speaker from Novartis, Istituto Gentili, Accademia di Medicina, Eli Lilly; research grants from Roche (to the institution).
G. Griguolo reports, outside the submitted work, the following: received personal fees for consultancy/advisory role from Gilead, Seagen, Menarini; honoraria as a speaker from Eli Lilly, Novartis, MSD; travel support from Gilead.
Carmen Criscitiello received outside the submitted work, the following: personal fees for consultancy/advisory role from: Eli Lilly, Pfizer, Novartis, Seagen, Gilead, MSD, AstraZeneca, Roche, Daiichi Sankyo.
F. Miglietta reports, outside the submitted work, the following: personal fees from Roche, Novartis, and Gilead.
A. Vingiani reports, outside the submitted work, the following: speaker honoraria from Roche and Lilly.
R. Lobefaro reports, outside the submitted work, the following: Financial Interests: Daiichi Sankyo, Advisory Board; Eli Lilly, Personal; Novartis, Invited Speaker, Personal; Pfizer, Invited Speaker; Accord, Invited Speaker; Roche, Personal.
S. Guiu: reports, outside the submitted work, the following: participated in advisory board for Daiichy Sankyo, Pfizer, Menarini; SG received honoraria as a speaker from Lilly.
N. Fusco reports, outside the submitted work, the following: consulting/advisory role: MSD, Novartis, AstraZeneca, Diaceutics, Adicet Bio, Sermonix, Roche, Menarini, Gilead, Veracyte Inc. Speaker bureau: MSD, Novartis, AstraZeneca, Daiichi Sankyo, GSK, Gilead, Roche, Leica Biosystems, Lilly. Research grants: Novartis, Reply, Gilead, AstraZeneca, GSK. Travel grants: Roche.
M. Fassan reports, outside the submitted work, the following: has been involved in consulting/advisory roles in Astellas Pharma, Pierre Fabre, MSD, Astra Zeneca, Janssen, GlaxoSmithKline, Amgen, Novartis, and Roche, and received research funding from Astellas Pharma, QED Therapeutics, Diaceutics, and Macrophage Pharma.
W. Jacot reports, outside the submitted work, the following grants, personal fees, and nonfinancial support from Astra Zeneca, personal fees and nonfinancial support from Eisai, personal fees and nonfinancial support from Novartis, personal fees and nonfinancial support from Roche, personal fees and nonfinancial support from Pfizer, personal fees and nonfinancial support from Eli Lilly, personal fees from MSD, personal fees from BMS, personal fees and nonfinancial support from Chugai, personal fees from Seagen, personal fees from Gilead, grants and personal fees from Daiichi Sankyo, outside the submitted work.
G. Curigliano reports, outside the submitted work, the following grants or contracts from any entity: Merck; consulting fees: BMS, Roche, Pfizer, Novartis, Lilly, Astra Zeneca, Daichii Sankyo, Merck, Seagen, Ellipsis, Gilead, Menarini; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events: Lilly, Pfizer, Relay, Gilead, Novartis; support for attending meetings and/or travel: Daichii Sankyo.
V. Guarneri reports, outside the submitted work, the following: personal fees for advisory board membership for AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Exact Sciences, Gilead, Merck Serono, MSD, Novartis, Pfizer, Olema Oncology, Pierre Fabre; personal fees as an invited speaker for AstraZeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead, GSK, Novartis, Roche, and Zentiva; personal fees for expert testimony for Eli Lilly.
M.V. Dieci reports, outside the submitted work, the following: received personal fees for consultancy/advisory role from: Eli Lilly, Pfizer, Novartis, Seagen, Gilead, MSD, Exact Sciences, AstraZeneca, Roche, Daiichi Sankyo, Roche.
The other authors declare no conflicts of interest.
References
- 1. Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346-1366. doi: 10.1200/JClinOncol.19.02309 [DOI] [PubMed] [Google Scholar]
- 2. Chen T, Zhang N, Moran MS, Su P, Haffty BG, Yang Q.. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18(1):1-8. doi: 10.1016/j.clbc.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 3. Luo C, Zhong X, Fan Y, Wu Y, Zheng H, Luo T.. Clinical characteristics and survival outcome of patients with estrogen receptor low positive breast cancer. Breast. 2022;63:24-28. doi: 10.1016/j.breast.2022.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poon IK, Tsang JY, Li J, Chan S-K, Shea K-H, Tse GM.. The significance of highlighting the oestrogen receptor low category in breast cancer. Br J Cancer. 2020;123(8):1223-1227. doi: 10.1038/s41416-020-1009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raghav KPS, Hernandez-Aya LF, Lei X, et al. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer. 2012;118(6):1498-1506. doi: 10.1002/cncr.26431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon KH, Park Y, Kang E, et al. Effect of estrogen receptor expression level and hormonal therapy on prognosis of early breast cancer. Cancer Res Treat. 2022;54(4):1081-1090., doi: 10.4143/CRT.2021.890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai Y-W, Shao Z-M, Yu K-D.. De-escalation of five-year adjuvant endocrine therapy in patients with estrogen receptor-low positive (immunohistochemistry staining 1%-10%) breast cancer: propensity-matched analysis from a prospectively maintained cohort. Cancer. 2022;128(9):1748-1756. doi: 10.1002/cncr.34155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies C, Godwin J, Gray R, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet Lond Engl. 2011;378(9793):771-784. doi: 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harvey JM, Clark GM, Osborne CK, Allred DC.. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474-1481. doi: 10.1200/JClinOncol.1999.17.5.1474 [DOI] [PubMed] [Google Scholar]
- 10. Yi M, Huo L, Koenig KB, et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol Off J Eur Soc Med Oncol. 2014;25(5):1004-1011. doi: 10.1093/annonc/mdu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paakkola N-M, Karakatsanis A, Mauri D, Foukakis T, Valachis A.. The prognostic and predictive impact of low estrogen receptor expression in early breast cancer: a systematic review and meta-analysis. ESMO Open. 2021;6(6):100289. doi: 10.1016/j.esmoop.2021.100289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoder R, Kimler BF, Staley JM, et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer. 2022;8(1):80. doi: 10.1038/s41523-022-00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landmann A, Farrugia DJ, Zhu L, et al. Low estrogen receptor (ER)-positive breast cancer and neoadjuvant systemic chemotherapy: is response similar to typical ER-positive or ER-negative disease? Am J Clin Pathol. 2018;150(1):34-42. doi: 10.1093/ajcp/aqy028 [DOI] [PubMed] [Google Scholar]
- 14. Dieci MV, Griguolo G, Bottosso M, et al. Impact of estrogen receptor levels on outcome in non-metastatic triple negative breast cancer patients treated with neoadjuvant/adjuvant chemotherapy. NPJ Breast Cancer. 2021;7(1):101. doi: 10.1038/S41523-021-00308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villegas SL, Nekljudova V, Pfarr N, et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors—an analysis of 2765 patients from neoadjuvant clinical trials. Eur J Cancer Oxf Engl 1990. 2021;148:159-170. doi: 10.1016/j.ejca.2021.02.020 [DOI] [PubMed] [Google Scholar]
- 16. Voorwerk L, Sanders J, Keusters MS, et al. Immune landscape of breast tumors with low and intermediate estrogen receptor expression. NPJ Breast Cancer. 2023;9(1):39. doi: 10.1038/s41523-023-00543-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benefield HC, Allott EH, Reeder-Hayes KE, et al. Borderline estrogen receptor-positive breast cancers in Black and White women. J Natl Cancer Inst. 2020;112(7):728-736. doi: 10.1093/jnci/djz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins T, Kantor O, Harrison B, et al. Defining the biology of estrogen receptor-low-positive breast cancer. Ann Surg Oncol. 2023;31(4):2244-2252. doi: 10.1245/s10434-023-14835-z [DOI] [PubMed] [Google Scholar]
- 19. Lovejoy LA, Turner CE, Wells JM, Shriver CD, Ellsworth RE.. Heritability of low ER staining/HER2-breast tumors: are we missing an opportunity for germline testing? Genes (Basel). 2020;11(12):1469. doi: 10.3390/genes11121469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanford RA, Song J, Gutierrez-Barrera AM, et al. High incidence of germline BRCA mutation in patients with ER low-positive/PR low-positive/HER-2 neu negative tumors. Cancer. 2015;121(19):3422-3427. doi: 10.1002/cncr.29572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onkar SS, Carleton NM, Lucas PC, et al. The great immune escape: understanding the divergent immune response in breast cancer subtypes. Cancer Discov. 2023;13(1):23-40. doi: 10.1158/2159-8290.CD-22-0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559-569. doi: 10.1200/JClinOncol.18.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19(1):40-50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 24. El Bairi K, Haynes HR, Blackley E, et al. International Immuno-Oncology Biomarker Working Group. The tale of TILs in breast cancer: a report from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer. 2021;7(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860-867. doi: 10.1200/JClinOncol.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 26. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. https://www.nejm.org/doi/full/10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 27. Cortes J, Rugo HS, Cescon DW, et al. ; KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217-226. doi: 10.1056/NEJMOA2202809 [DOI] [PubMed] [Google Scholar]
- 28. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676-684. doi: 10.1001/jamaoncol.2019.6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804-2811. doi: 10.1158/1078-0432.CCR-17-3452 [DOI] [PubMed] [Google Scholar]
- 30. Terranova-Barberio M, Pawlowska N, Dhawan M, et al. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat Commun. 2020;11(1):3584. doi: 10.1038/s41467-020-17414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tolaney SM, Barroso-Sousa R, Keenan T, et al. Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2020;6(10):1598-1605. doi: 10.1001/jamaoncol.2020.3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dieci MV, Guarneri V, Tosi A, et al. Neoadjuvant chemotherapy and immunotherapy in luminal b-like breast cancer: results of the phase II GIADA trial. Clin Cancer Res. 2022;28(2):308-317. doi: 10.1158/1078-0432.CCR-21-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pusztai L, Yau C, Wolf DM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39(7):989-998.e5. doi: 10.1016/J.CCELL.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardoso F, McArthur HL, Schmid P, et al. LBA21 KEYNOTE-756: Phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2– breast cancer. Ann Oncol. 2023;34:S1260-S1261. doi: 10.1016/j.annonc.2023.10.011 [DOI] [Google Scholar]
- 35. Loi S, Curigliano G, Salgado RF, et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) ± NIVO in patients (pts) with high-risk, ER+ HER2− primary breast cancer (BC). Ann Oncol. 2023;34:S1259-S1260. doi: 10.1016/j.annonc.2023.10.010 [DOI] [Google Scholar]
- 36. Sharma P, Stecklein SR, Yoder R, et al. Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer: NeoPACT phase 2 clinical trial. JAMA Oncol. 2023;10(2):227-235. doi: 10.1001/jamaoncol.2023.5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salgado R, Denkert C, Demaria S, et al. International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: recommendations by an International TILS Working Group 2014. Ann Oncol. 2015;26(2):259-271. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30(12):1941-1949. doi: 10.1093/annonc/mdz395 [DOI] [PubMed] [Google Scholar]
- 39. Gruosso T, Gigoux M, Manem VSK, et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest. 2019;129(4):1785-1800. doi: 10.1172/JCI96313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asano Y, Kashiwagi S, Goto W, et al. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 2016;103(7):845-854. doi: 10.1002/bjs.10127 [DOI] [PubMed] [Google Scholar]
- 41. Dieci MV, Tsvetkova V, Griguolo G, et al. Integration of tumour infiltrating lymphocytes, programmed cell-death ligand-1, CD8 and FOXP3 in prognostic models for triple-negative breast cancer: analysis of 244 stage I–III patients treated with standard therapy. Eur J Cancer. 2020;136(2):7-15. doi: 10.1016/j.ejca.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 42. Parker JS, Mullins M, Cheang MCU, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160-1167. doi: 10.1200/JClinOncol.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dieci MV, Carbognin L, Miglietta F, et al. Incorporating weekly carboplatin in anthracycline and paclitaxel-containing neoadjuvant chemotherapy for triple-negative breast cancer: propensity-score matching analysis and TIL evaluation. Br J Cancer. 2023;128(2):266-274. doi: 10.1038/s41416-022-02050-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fujii T, Kogawa T, Dong W, et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol. 2017;28(10):2420-2428. doi: 10.1093/annonc/mdx397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohara AM, Naoi Y, Shimazu K, et al. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res Treat. 2019;173(3):533-543. doi: 10.1007/s10549-018-5020-7 [DOI] [PubMed] [Google Scholar]
- 46. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 47. Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796-1804. doi: 10.1200/JClinOncol.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 48. Stanton SE, Adams S, Disis ML.. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354-1360. doi: 10.1001/jamaoncol.2016.1061 [DOI] [PubMed] [Google Scholar]
- 49. Dieci MV, Miglietta F, Guarneri V.. Immune infiltrates in breast cancer: recent updates and clinical implications. Cells. 2021;10(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yau C, Osdoit M, van der Noordaa M, et al. ; I-SPY 2 Trial Consortium. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149-160. doi: 10.1016/S1470-2045(21)00589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miglietta F, Griguolo G, Guarneri V, Dieci MV.. Programmed cell death ligand 1 in breast cancer: technical aspects, prognostic implications, and predictive value. Oncologist. 2019;24(11):e1055-e1069. doi: 10.1634/THEONCOLOGIST.2019-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. della Rovere F, Granata A, Familiari D, D’Arrigo G, Mondello B, Basile G.. Mast cells in invasive ductal breast cancer: different behavior in high and minimum hormone-receptive cancers. Anticancer Res. 2007;27(4B):2465-2471. [PubMed] [Google Scholar]
- 53. Majorini MT, Colombo MP, Lecis D.. Few, but efficient: the role of mast cells in breast cancer and other solid tumors. Cancer Res. 2022;82(8):1439-1447. doi: 10.1158/0008-5472.CAN-21-3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Majorini MT, Cancila V, Rigoni A, et al. Infiltrating mast cell-mediated stimulation of estrogen receptor activity in breast cancer cells promotes the luminal phenotype. Cancer Res. 2020;80(11):2311-2324. doi: 10.1158/0008-5472.CAN-19-3596 [DOI] [PubMed] [Google Scholar]
- 55. Segovia-Mendoza M, Morales-Montor J.. Immune tumor microenvironment in breast cancer and the participation of estrogen and its receptors in cancer physiopathology. Front Immunol. 2019;10:348. doi: 10.3389/fimmu.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Griguolo G, Bottosso M, Vernaci G, Miglietta F, Dieci MV, Guarneri V.. Gene-expression signatures to inform neoadjuvant treatment decision in HR+/HER2− breast cancer: available evidence and clinical implications. Cancer Treat Rev. 2022;102:102323. doi: 10.1016/J.CTRV.2021.102323 [DOI] [PubMed] [Google Scholar]
- 57. Falato C, Schettini F, Pascual T, Brasó-Maristany F, Prat A.. Clinical implications of the intrinsic molecular subtypes in hormone receptor-positive and HER2-negative metastatic breast cancer. Cancer Treat Rev. 2023;112:102496., doi: 10.1016/j.ctrv.2022.102496 [DOI] [PubMed] [Google Scholar]
- 58. Licata L, Barreca M, Galbardi B, et al. Breast cancers with high proliferation and low ER-related signalling have poor prognosis and unique molecular features with implications for therapy. Br J Cancer. 2023;129(12):2025-2033. doi: 10.1038/s41416-023-02477-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor–rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342-2349. doi: 10.1200/JClinOncol.2010.31.6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Griguolo G, Dieci MV, Generali DG, et al. Abstract PS5-14: Gene-expression profiling and response to neoadjuvant endocrine treatment in the phase II LETLOB trial. Cancer Res. 2021;81(suppl 4):PS5-14. doi: 10.1158/1538-7445.SABCS20-PS5-14 [DOI] [Google Scholar]
- 61. Prat A, Chaudhury A, Solovieff N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39(13):1458-1467. doi: 10.1200/JClinOncol.20.02977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cejalvo JM, Pascual T, Fernández-Martínez A, et al. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat Rev. 2018;67:63-70. doi: 10.1016/j.ctrv.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 63. Groenendijk FH, Treece T, Yoder E, et al. Estrogen receptor variants in ER-positive basal-type breast cancers responding to therapy like ER-negative breast cancers. NPJ Breast Cancer. 2019;5(1):15. doi: 10.1038/s41523-019-0109-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Prat A, Fan C, Fernández A, et al. Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med. 2015;13:303. doi: 10.1186/s12916-015-0540-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitworth PW, Beitsch PD, Pellicane JV, et al. ; NBRST Investigators Group. Distinct neoadjuvant chemotherapy response and 5-year outcome in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast tumors that reclassify as basal-type by the 80-gene signature. J Clin Oncol Precis Oncol. 2022;6(1):e2100463. doi: 10.1200/PO.21.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huppert LA, Rugo HS, Pusztai L, et al. ; I-SPY2 Consortium. Pathologic complete response (pCR) rates for HR+/HER2- breast cancer by molecular subtype in the I-SPY2 trial. J Clin Oncol. 2022;40(suppl 16):504-504. doi: 10.1200/JClinOncol.2022.40.16_suppl.504 [DOI] [Google Scholar]
- 67. Wolf DM, Yau C, Wulfkuhle J, et al. ; I-SPY2 Investigators. Redefining breast cancer subtypes to guide treatment prioritization and maximize response: predictive biomarkers across 10 cancer therapies. Cancer Cell. 2022;40(6):609-623.e6. doi: 10.1016/J.CCELL.2022.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmid P, Cortes J, Dent R, et al. ; KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556-567. doi: 10.1056/NEJMOA2112651/SUPPL_FILE/NEJMOA2112651_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 69. Lin CM, Jaswal J, Vandenberg T, Tuck A, Brackstone M.. Weakly hormone receptor–positive breast cancer and use of adjuvant hormonal therapy. Curr Oncol. 2013;20(6):e612-e613. doi: 10.3747/co.20.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Purrington KS, Gorski D, Simon MS, et al. Racial differences in estrogen receptor staining levels and implications for treatment and survival among estrogen receptor positive, HER2-negative invasive breast cancers. Breast Cancer Res Treat. 2020;181(1):145-154. doi: 10.1007/s10549-020-05607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burstein HJ. systemic therapy for estrogen receptor–positive, HER2-negative breast cancer. N Engl J Med. 2020;383(26):2557-2570. doi: 10.1056/NEJMra1307118 [DOI] [PubMed] [Google Scholar]
- 72. Choong GMY, Hoskin TL, Boughey JC, Ingle JN, Goetz MP.. The impact of adjuvant endocrine therapy (AET) omission in ER-low (1-10%) early-stage breast cancer. J Clin Oncol. 2024;42(16_suppl):513-513. doi: 10.1200/JClinOncol.2024.42.16_suppl.513 [DOI] [Google Scholar]
- 73. Acs B, Hartman J, Sönmez D, Lindman H, Johansson ALV, Fredriksson I.. Real-world overall survival and characteristics of patients with ER-zero and ER-low HER2-negative breast cancer treated as triple-negative breast cancer: a Swedish population-based cohort study. Lancet Reg Health Eur 2024;40:100886. doi: 10.1016/j.lanepe.2024.100886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25(25):3846-3852. doi: 10.1200/JClinOncol.2007.11.9453 [DOI] [PubMed] [Google Scholar]
- 75. Makhlouf S, Althobiti M, Toss M, et al. The clinical and biological significance of estrogen receptor-low positive breast cancer. Mod Pathol. 2023;36(10):100284. doi: 10.1016/j.modpat.2023.100284 [DOI] [PubMed] [Google Scholar]
- 76. Kos Z, Roblin E, Kim RS, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27(2):249-256. doi: 10.1093/annonc/mdv571 [DOI] [PubMed] [Google Scholar]
- 78. Rugo HS, Loi S, Adams S, et al. PD-L1 immunohistochemistry assay comparison in atezolizumab plus nab-paclitaxel-treated advanced triple-negative breast cancer. J Natl Cancer Inst. 2021;113(12):1733-1743. doi: 10.1093/JNCI/DJAB108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Massa D, Tosi A, Rosato A, Guarneri V, Dieci MV.. Multiplexed in situ spatial protein profiling in the pursuit of precision immuno-oncology for patients with breast cancer. Cancers. 2022;14(19):4885-4885. doi: 10.3390/cancers14194885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nederlof I, Hajizadeh S, Sobhani F, et al. Spatial interplay of lymphocytes and fibroblasts in estrogen receptor-positive HER2-negative breast cancer. NPJ Breast Cancer. 2022;8(1):56., doi: 10.1038/s41523-022-00416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loibl S, André F, Bachelot T, et al. ; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;35(2):159-182. doi: 10.1016/j.annonc.2023.11.016 [DOI] [PubMed] [Google Scholar]
- 82. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920-928. doi: 10.1038/s41591-019-0432-4 [DOI] [PubMed] [Google Scholar]
- 83. Conte PF, Dieci MV, Bisagni G, et al. A-BRAVE trial: a phase III randomized trial with avelumab in early triple-negative breast cancer with residual disease after neoadjuvant chemotherapy or at high risk after primary surgery and adjuvant chemotherapy. J Clin Oncol. 2024;42(suppl 17):LBA500-LBA500. doi: 10.1200/JClinOncol.2024.42.17_suppl.LBA500 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the nature of this research, participants of this study did not give consent for their data to be shared publicly and for secondary use of data derived from the study without Ethics Committee re-evaluation. However, data can be made available upon request through a Data Transfer Agreement and after Ethics Committee approval. We encourage investigators interested in data access to request them by contacting the Department of Surgery, Oncology and Gastroenterology of the University of Padua (ricerca.discog@unipd.it).