Abstract
Background
The MEK inhibitor, selumetinib, reduces plexiform neurofibroma (PN) in pediatric patients with neurofibromatosis type 1 (NF1). Its safety and efficacy in adults with PN and effectiveness in other NF1 manifestations (eg, neurocognitive function, growth reduction, and café-au-lait spots) are unknown.
Methods
This open-label, phase II trial enrolled 90 pediatric or adult NF1 patients with inoperable, symptomatic, or potentially morbid, measurable PN (≥3 cm). Selumetinib was administered at doses of 20 or 25 mg/m2 or 50 mg q 12 hours for 2 years. Pharmacokinetics, PN volume, growth parameters, neurocognitive function, café-au-lait spots, and quality of life (QoL) were evaluated.
Results
Fifty-nine children and 30 adults (median age, 16 years; range, 3–47) received an average of 22 ± 5 (4–26) cycles of selumetinib. Eighty-eight (98.9%) out of 89 per-protocol patients showed volume reduction in the target PN (median, 40.8%; 4.2%–92.2%), and 81 (91%) patients showed partial response (≥20% volume reduction). The response lasted until cycle 26. Scores of neurocognitive functions (verbal comprehension, perceptual reasoning, processing speed, and full-scale IQ) significantly improved in both pediatric and adult patients (P < .05). Prepubertal patients showed increases in height score and growth velocity (P < .05). Café-au-lait spot intensity decreased significantly (P < .05). Improvements in QoL and pain scores were observed in both children and adults. All adverse events were CTCAE grade 1 or 2 and were successfully managed without drug discontinuation.
Conclusions
Selumetinib decreases PN volume in the majority of pediatric and adult NF1 patients while also showing efficacy in nonmalignant diverse NF1 manifestations.
Trial Registration
Cris.nih.go.kr Identifier (KCT0003700).
Keywords: Café-au-Lait spots, mitogen-activated protein kinase kinases, neurofibromatosis 1, neurofibroma, plexiform
Key Points.
This study is the first to demonstrate the efficacy of selumetinib in both children and adults with NF1 and inoperable plexiform neurofibromas and in other manifestations such as neurocognitive function, growth reduction, and café-au-lait spots.
Selumetinib exhibited diverse therapeutic effects without serious adverse events.
Neurofibromatosis type 1 (NF1; OMIM#16220) is a rare genetic disorder affecting 1 out of 3,000 people.1 NF1 is caused by a heterozygous loss-of-function mutation in the NF1 gene2 that regulates the RAS-MAPK pathway, which is essential for cellular growth, proliferation, and differentiation.3 Its somatic loss of heterozygosity leads to representative NF1 manifestations such as café-au-lait spots, bone dysplasia, and tumor development.4 Up to 50% of patients with NF1 develop plexiform neurofibroma (PN), which has the potential for malignant transformation5 and causes significant morbidity by compressing important body structures. In the past, the standard treatment for PN was surgical removal; however, most PNs could not be completely resected due to their position and risk of complications such as nerve and vascular injury, and 40%–50% of PNs progress after surgery.6
Recently, the oral selective MEK inhibitor selumetinib has been reported to decrease the size of PN in pediatric NF1.7,8 In a phase II trial, selumetinib (25 mg/m2 q 12 hours, 28 days/cycle) resulted in at least 20% reduction in PN volume after a median of 8 cycles in 74% of pediatric NF1 patients without subsequent disease progression in most patients. Selumetinib also showed potential clinical benefits in tumor-related disfigurements or pain with improvement in quality of life (QoL).8
Although PNs have received a significant amount of attention in relation to the use of selumetinib in pediatric NF1 patients, their safety and efficacy in adult patients have remained unknown. Moreover, NF1 entails many other comorbidities, including learning disabilities, reduced growth, and café-au-lait spots.9 However, the effect of selumetinib on these comorbidities is largely unknown. In addition, there are conflicting data regarding whether Asian patients with NF1 have different pharmacokinetics of selumetinib compared to those of Caucasian patients.10–12 In the study by Dymond et al., higher drug exposure (30%) was suggested in Asians10; in contrast, other studies11,12 reported that body surface area rather than ethnicity was associated with drug exposure.
In these regards, we analyzed the feasibility, efficacy, and safety of selumetinib on the reduction of PN in Korean pediatric and adult NF1 patients with pharmacokinetic analysis. We also evaluated, for the first time, the potential therapeutic effects of selumetinib on neurocognitive functions, growth parameters, and cutaneous manifestations in patients with NF1.
Methods
Trial Outlines and Participants
This is a single-arm phase II trial developed and conducted by the Medical Genetics Center at Asan Medical Center in Seoul, Korea (KCT0003700, https://cris.nih.go.kr/cris/search/detailSearch.do/19080). The study was approved by the Institutional Review Board of Asan Medical Center (approval no. 2019-0199) and the Ministry of Food and Drug Safety. Informed consent was obtained from each patient or his/her guardian prior to enrollment.
Patients with either NF1 or mosaic NF1 according to the revised diagnostic criteria13 and at least one measurable inoperable PN (≥3 cm) and significant symptoms/comorbidities such as sensory or motor deficit, respiratory difficulty, or disfigurement or the potential to develop symptoms/comorbidities due to the inoperable measurable PN were included (Supplementary Table 1).
Trial Design
The primary outcomes were the safety and pharmacokinetic properties of selumetinib in Korean patients with NF1 and PN, and secondary outcomes were the efficacies of selumetinib in PN reduction, operability, pain, QoL, neurocognitive functions, and gene expression profiles (Supplementary Table 2). This manuscript included the analytic findings for the primary outcome of the trial.
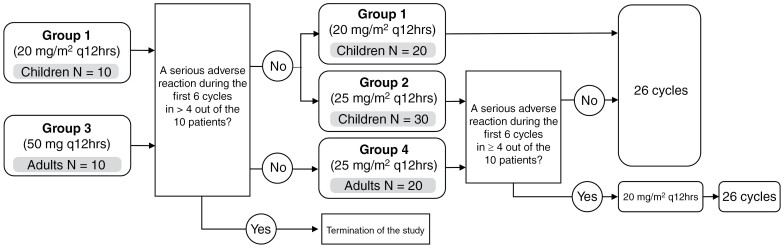
Selumetinib was administered orally 20 or 25 mg/m2 or 50 mg q 12 hours on a continuous dosing schedule (28-day cycle). The dose of selumetinib was calculated based on body surface area. Patients were enrolled in 2 stages, with separation between pediatric and adult groups based on age (Figure 1 and Supplementary Method 1).
Figure 1.
Study flow diagram.
Safety Assessment
Clinical examinations, laboratory evaluations, ophthalmologic examinations, echocardiography, and electrocardiography were performed at baseline and regularly throughout the trial (Supplementary Table 3). AE was graded based on the CTCAE version 4.0.14
Pharmacokinetic Assessments
Blood samples were taken on the first day before the first dosage and 0.5, 1, 2, 3, 5, 8, between 10 and 12, 24, and between 30 and 36 hours after the first dose; in addition, a blood sample was taken on day 27 before the first dose for cycle 2 was given. Concentrations of selumetinib and its active metabolite, n-desmethyl selumetinib, were analyzed by Labcorp Bioanalytical Services LLC (Indianapolis, IN, USA; Supplementary Method 2). Noncompartmental approaches were used in the pharmacokinetic variables.
Tumor Response Evaluation
At enrollment, the treating physician identified the most clinically relevant inoperable quantifiable PN as the target PN (Supplementary Figure 1 and Appendix B of the original protocol). To measure the volume of the target lesion, image analysts conducted whole segmentation by carefully drawing the region of interest along the margin of the target lesion to cover the entire volume of the tumor. AsanJ, an in-house software developed from ImageJ (National Institute of Health, USA, https://imagej.net), was utilized for semiautomatic volumetric analysis.15 Volumetric examination of the MRI of target PNs assessed the tumor response according to the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) International Collaboration.6 Tumor response assessment was conducted in accordance with the prescribed protocol schedule (Baseline, post cycles 6, 12, 18, and 26).
Response Criteria
Partial response (PR) was defined as a decrease in tumor volume of 20% or more from baseline for at least 4 weeks. An increase in the tumor volume of more than 20% from baseline was considered to signify disease progression, and tumor volume changes of less than 20% from baseline were considered a stable disease.
Neurocognitive Examination
For the neurocognitive examination, we used the Korean versions of the Wechsler Preschool and Primary Scale of Intelligence (K-WPPSI),16 the Wechsler Intelligence Scale for Children, fourth edition (K-WISC-IV),17 and the Wechsler Adult Intelligence Scale fourth edition (K-WAIS-IV)18 to evaluate patients under 6 years old, between 6 and 15 years old, and 16 years and older, respectively. Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), working memory index, Processing Speed Index (PSI), and Full-Scale Intelligence Quotient (FSIQ) were also measured. The severity of intellectual disability was determined using IQ scores (borderline: 70–79; mild: 50–69; moderate: 35–49; severe: < 35).16–18 The Advanced Test of Attention19,20 and the Seoul Computerized Neurocognitive Function Test21 were used to assess the attention level of patients aged 5–18 years and those aged 19 years and above, respectively. A Z score greater than 1.5 in patients aged 5–18 years and a T score lower than 40 in patients 19 years and older were used to determine attention deficit.18,19 Neurocognitive examinations were conducted in accordance with the prescribed protocol schedule (baseline, post cycles 12, and 26). The changes in scores for each item were evaluated from baseline across the study period using a 2-tailed Wilcoxon signed-rank test against baseline (*P value < .05).
Growth Parameters
Height, growth velocity, Tanner stage, bone age (left wrist), and blood levels of insulin-like growth factor I (IGF1) and insulin-like growth factor-binding protein 3 (IGF-BP3) were measured.
Café-au-Lait Spots
Representative café-au-lait spots were photographed using a white paper background and a scale as part of the routine clinical practice throughout the study (not included in the protocol). For image analysis, ImageJ was downloaded from the NIH website.22 The digital image is converted into Log R image and the brightness is increased to a practical level (process > math > multiply, then input the appropriate number, usually 2–5). The area of interest is determined as the same in each image of a single patient (see right) and the intensity score is measured.
Assessment of Quality of Life and Pain
QoL and pain were assessed with PRO measures. The PedsQL 4.0 Generic Core Scales and a 10-point scale Numeric Pain Rating Scale (NPS)/Wong-Baker Faces Pain Rating Scale (WBS) were used in patients aged 3–18 years.23,24 There are different forms for parents according to the age of the patients (toddler: 2–4; young child: 5–7; child: 8–12; adolescent: 13–18). For this study, children aged 8 to 18 completed self-report measures of the PedsQL, while parents or legal guardians of children aged 2 to 18 completed the parent proxy measures of the PedsQL. The Impact of NF1 on QoL (INF1-QOL) questionnaire and the Korean Neuropathic Pain Questionnaire were used in patients aged >18 years.25,26
Statistical Analyses
The estimated sample size was 90 (cf. page 75 in the original protocol). IBM SPSS Statistics for Windows, version 21 (IBM Corp.) was used (2-tailed Wilcoxon signed-rank test).
Results
Patient Characteristics
From May 2019 to December 2021, the goal enrollment of 90 participants was accomplished. As of January 2023, one child discontinued the study due to the development of a malignant peripheral nerve sheath tumor during the fifth cycle; as a result, 90 patients (children, n = 60; adults, n = 30) took selumetinib for 22 ± 5 (range, 4–26) cycles (Table 1, Supplementary Table 4). Of the 60 pediatric patients, 30 were in group 1 (20 mg/m2/dose every 12 hours) and the other 30 were in group 2 (25 mg/m2/dose every 12 hours); of the 30 adult patients, 10 were in group 3 (50 mg/dose every 12 hours) and 20 were in group 4 (25 mg/m2/dose every 12 hours; Figure 1). According to the revised diagnostic criteria of NF1,13 7 (7.8%) were identified as having mosaic NF1. The most common sites of target PNs were the neck area in children (N = 19, 31.7%) and the pelvic area in adults (N = 11, 36.7%). The most common symptoms were disfigurement in children and pain in adults. At baseline, the median target PN volume was 60.7 mL (range, 2.5–1137.5) in children and 84.5 mL (range, 5.7–546.8) in adults. At the time of analysis, the median number of total treatment cycles was 21 cycles (range, 4–26) in children and 26 cycles (range, 16–26) in adults.
Table 1.
Baseline Characteristics of the Study Patients
| Children (3 ≥ Age < 19 years) | Adults (≥19 years) | |||||
|---|---|---|---|---|---|---|
| Group 1 (20 mg/m2 q 12 h) |
Group 2 (25 mg/m2 q 12 h) |
Subtotal | Group 3 (50 mg q 12 h) |
Group 4 (25 mg/m2 q 12 h) |
Subtotal | |
| Patients enrolled—no. | 30 | 30 | 60 | 10 | 20 | 30 |
| NF1 | 29 | 29 | 58 | 10 | 18 | 28 |
| Mosaic NF1 | 1 | 3 | 4 | 1 | 2 | 3 |
| Sex—no. | ||||||
| Male | 15 | 20 | 35 | 6 | 14 | 20 |
| Female | 15 | 10 | 25 | 4 | 6 | 10 |
| Median age at enrollment (range)—year | 9.0 (4.0–18.0) | 8.5 (3.0–18.0) | 8.0 (3.0–18.0) | 34.0 (19.0–42.0) | 24.0 (19.0–47.0) | 26.5 (19.0–47.0) |
| Median performance status score (range) | 100 (70–100) | 100 (70–100) | 100 (70–100) | 90 (70–100) | 95 (70–100) | 90 (70–100) |
| Target location of plexiform neurofibroma—no. | ||||||
| abdomen | 2 | 1 | 3 | 2 | 2 | |
| ankle | 1 | 1 | ||||
| arm | 2 | 3 | 5 | |||
| arm, pelvis | 1 | 1 | ||||
| arm, pelvis, thigh | 1 | 1 | ||||
| arm, shoulder, back, pelvis | 1 | 1 | ||||
| buttock | 1 | 1 | ||||
| chest | 1 | 1 | 1 | 1 | ||
| chest wall | 1 | 1 | ||||
| chest, leg | 1 | 1 | ||||
| eye | 4 | 4 | ||||
| face | 1 | 1 | ||||
| face, chest | 1 | 1 | ||||
| face, paraspinal | 1 | 1 | ||||
| face, pelvis | 1 | 1 | ||||
| foot | 1 | 1 | ||||
| leg | 1 | 3 | 4 | 1 | 1 | 2 |
| liver | 1 | 1 | ||||
| neck | 6 | 9 | 15 | 4 | 1 | 5 |
| neck and pelvis | 1 | 1 | ||||
| neck, abdomen | 1 | 1 | ||||
| neck, back | 1 | 1 | ||||
| neck, chest | 1 | 1 | ||||
| neck, face | 1 | 1 | ||||
| neck, pelvis | 1 | 1 | 1 | 2 | 2 | |
| orbit | 1 | 1 | ||||
| paraspinal | 2 | 2 | 4 | 1 | 1 | |
| paraspinal, chest | 1 | 1 | ||||
| pelvis | 5 | 3 | 8 | 2 | 5 | 7 |
| pelvis, chest | 1 | 1 | ||||
| thoracic paraspinal | 1 | 1 | 1 | 1 | ||
| thoracic paraspinal, leg | 1 | 1 | ||||
| Median target plexiform neurofibroma volume at enrollment (range)—mL | 50.2 (3.4–922.6) | 70.6 (2.5–1137.5) | 60.7 (2.5–1137.5) | 71.2 (15.3–278.6) | 92.2 (5.7–546.8) | 84.5 (5.7–546.8) |
| Documented neurofibroma–related complication at baseline—no. (%) | ||||||
| Café-au-lait spot | 29 (96.7) | 30 (100) | 59 (98.3) | 10 (100.0) | 19 (95.0) | 29 (96.7) |
| Diffuse cutaneous neurofibroma | 14 (46.7) | 13 (43.3) | 27 (45.0) | 9 (90.0) | 14 (70.0) | 23 (76.7) |
| Mental retardation | 7 (23.3) | 7 (23.3) | 14 (23.3) | 3 (30.0) | 3 (15.0) | 6 (20.0) |
| Attention deficient hyperactivity disorder | 15 (50.0) | 12 (40.0) | 27 (45.0) | 2 (20.0) | 5 (25.0) | 7 (23.3) |
| Autism | 0 | 0 | 0 | 0 | 1 (5.0) | 1 (3.3) |
| Seizure | 1 (3.3) | 0 | 1 (1.7) | 0 | 0 | 0 |
| Hypertension | 0 | 2 (6.7) | 2 (3.3) | 2 (20.0) | 2 (10.0) | 4 (13.3) |
| Cardiac abnormality | 1 (3.3) | 0 | 1 (1.7) | 0 | 2 (10.0) | 2 (6.7) |
| Hearing abnormality | 3 (10.0) | 1 (3.3) | 4 (6.7) | 1 (10.0) | 3 (15.0) | 4 (13.3) |
| Optic pathway abnormality | 3 (10.0) | 3 (10.0) | 6 (10.0) | 0 | 0 | 0 |
| Dysplasia of bone | 12 (40.0) | 10 (33.3) | 22 (36.7) | 1 (10.0) | 10 (50.0) | 11 (36.7) |
| Median cycle at analysis (range) | 21 (4–26) | 22 (16–26) | 21 (4–26) | 26 | 26 (16–26) | 26 (16–26) |
NF1, neurofibromatosis type 1; no., number.
Adverse Events
A total of 422 AEs occurred in 90 patients (median per patient, 5; range, 0–15; Supplementary Table 5). The most common AE was paronychia (n = 62, 14.7%), followed by acneiform rash (n = 61, 14.5%) and skin infection (n = 59, 14.0%). Except for 20 (4.7%) cases of CTCAE grade 2 AEs, all other AEs were grade 1. The frequency of AE was the highest in cycle 1 (Supplementary Figure 2) and decreased as the cycles progressed. Supportive therapy eased all AEs regardless of severity and drug discontinuation was not required.
Tumor Volume Reduction
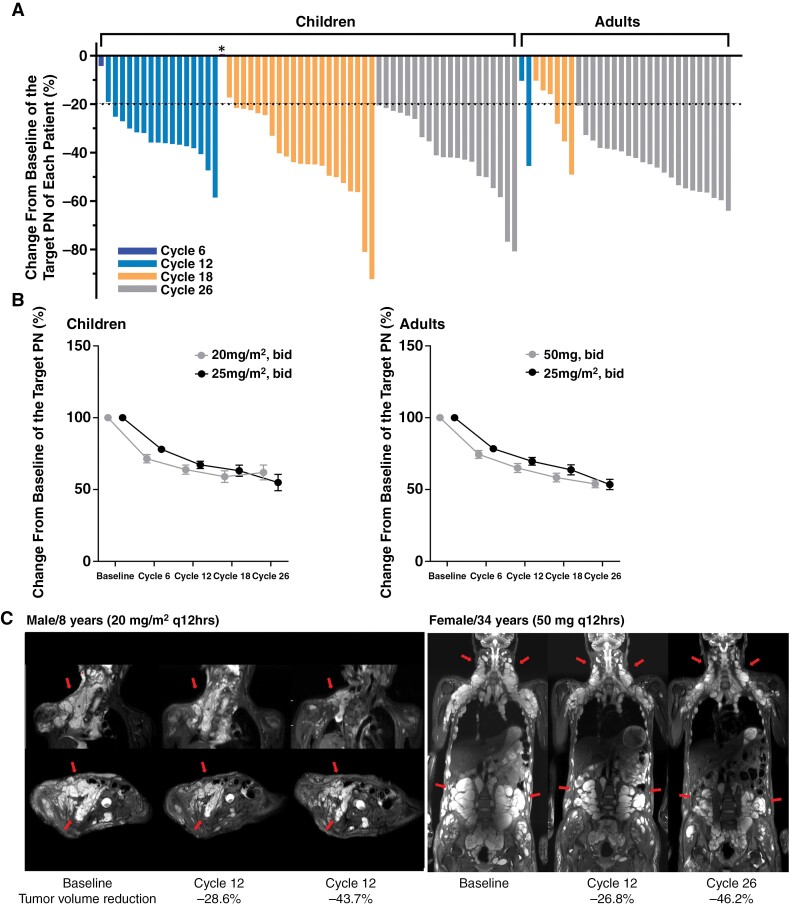
At the last follow-up, 88 patients (97.8%) out of 90 patients in the intention-to-treat group showed a median reduction of 40.8% (range, 4.2%–92.2%) in the PN volume (Supplementary Table 6), except for 1 child (S51) who did not experience any change in volume. For per-protocol analysis with 89 patients evaluated, the response rate was 98.9% (88 out of 89 patients). In the per-protocol group, 81 (91%) patients showed PR (Figure 2A). Specifically, PR was achieved by 60 (67.4%) patients by cycle 6, then an additional 16 (18.0%) patients by cycle 12, and an additional 5 (5.6%) patients by cycle 18. Notably, PR was achieved in all 42 patients who received 26 cycles of selumetinib. Two patients (S27, S28 in Supplement Table 6) experienced a confirmed PR after 6 cycles of treatment. Subsequently, in cycles 12 and 18, there was a volume increase of 5.3% and 3.4%, respectively. However, the volume decreased again to PR grades compared to the baseline in cycle 26. While the tumor-reducing response lasted until cycle 26, the most pronounced reduction was observed between baseline and cycle 6 (Figure 2B). There was no significant difference in the degree of volume reduction between the dose groups (Supplementary Figures 3 and 4); however, a higher proportion of patients achieved PR in the higher dosage group: 89.7% (n = 29, 20 mg/m2 q 12 hours) versus 96.7% (n = 30, 25 mg/m2 q 12 hours) in children and 80% (n = 20, 25 mg/m2 q 12 hours) versus 100% (n = 10, 50 mg q 12 hours) in adults (Supplementary Figure 5).
Figure 2.
Changes in tumor volume. (A) Percentage changes in the targeted tumor volume in each patient divided according to the number of cycles received. The dotted line denotes the threshold for partial response, which was defined as ≥20% reduction in tumor volume. *One child showed no response with an increased volume change of 0.7%. (B) Percentage changes in the targeted tumor volume in pediatric and adult patients according to the drug dosage. Each graph represents the mean and standard error of the means of each group. (C) Regular MRI scans of representative patients at baseline, cycle 12, and cycle 26. Left panel: an 8-year-old child in group 1. Right panel: a 34-year-old female 6 in group 3 (arrows, target lesion).
At the last evaluation, no patients showed disease progression while taking selumetinib, and there was no need for dose reduction or discontinuation due to toxic effects or poor compliance. After the selumetinib treatment, total surgical resection of the target PN was not possible in the subjects due to its location. However, partial resection was considered in 12 (13.5%) patients, such as for eyelid reconstruction or the removal of redundant skin. Anecdotal data from 2 cases revealed that extensive PNs that were close to compressing the spinal cord had decreased to the point that surgery was no longer necessary, along with a significant reduction in discomfort (Figure 2C).
Neurocognitive Assessment
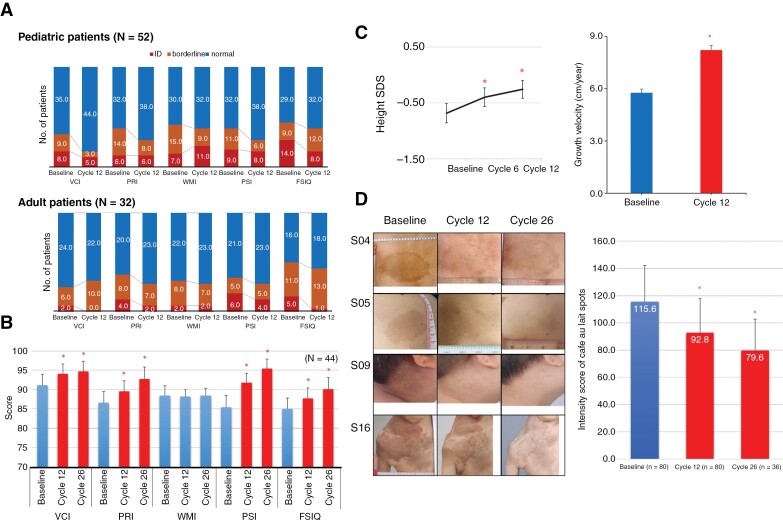
After cycle 12, patients generally showed improvements in VCI (children), PSI (children and adults), and FSIQ (children and adults) scores (K-WISC-IV, K-WPPSI, and K-WAIS-IV tests; Supplementary Tables 7,8). Specifically, the improvements were statistically significant for VCI and FSIQ (group 1), and PSI (groups 1 and 3). The proportion of patients with borderline (FSIQ; 70~79) or overt intellectual disability (FSIQ < 70) in terms of VCI (children), PRI (children and adults), PSI (children and adults), and FSIQ (children and adults) decreased because of this improvement (Figure 3A). Statistical improvements were observed in the VCI, PRI, PSI, and FSIQ scores of 19 pediatric and 25 adult patients who underwent 26 cycles of selumetinib compared to their baseline scores.
Figure 3.
Effects of selumetinib on Neurocognitive function, growth parameter, and café-au-lait spots. (A) Proportion of children (n = 54) and adult (n = 30) patients with normal (blue), borderline (orange), or intellectual disability (ID; red) at baseline and cycle 12 according to VCI, PRI, WMI, PSI, and FSIQ scores. (B) Cognitive functioning ratings of 44 patients at baseline, cycle 12, and cycle 26. Mean ± S.E. *P < .05 versus baseline. *P < .05 versus baseline. (C) Standard deviation (SD) scores of heights and growth velocity of 37 prepubertal patients at baseline, cycle 12, and cycle 26. Mean ± S.D. *P < .05 versus baseline. (D) Representative images of patients who showed improvements in café-au-lait spots. Intensity score of Café au lait spots Mean ± S.D. *P < .05 versus baseline. VCI, verbal comprehension index; PRI, perceptual reasoning index; WMI, working memory index; PSI, processing speed index; FSIQ, Full-Scale IQ.
(Figure 3B). The attention scores showed a wide individual variability (Supplementary Tables 7 and 8).
Growth Assessment
Thirty-seven patients (16 males and 21 females) were in the prepubertal stage at the time of enrollment, and their growth parameters during selumetinib treatment were assessed. The Standard deviation (SD) score of height and growth velocity was −0.7 ± 1.1 (range, −3.5–−0.9), and the height SD score increased gradually throughout the treatment period (Figure 3C, left). The growth velocity significantly increased from 5.8 ± 1.3 cm/year to 8.2 ± 1.5 cm/year (Figure 3C, right). The SD scores of IGF-1 and IGF-BP and the difference between bone age and chronological age did not significantly change during the treatment (Supplementary Tables 9 and 10; Figure 6).
Café-au-Lait Spots
Café-au-Lait Spots intensity score, as measured by the Image J program, had decreased from 115.6 ± 26.6 (Baseline, n = 79) to 92.8 ± 25.1 (Cycle 12, n = 79; P < .001) and 79.6 ± 23.2 (Cycle 26, n = 36; (P < .001; Figure 3D, Supplementary Figure 7).
Quality of Life and Pain
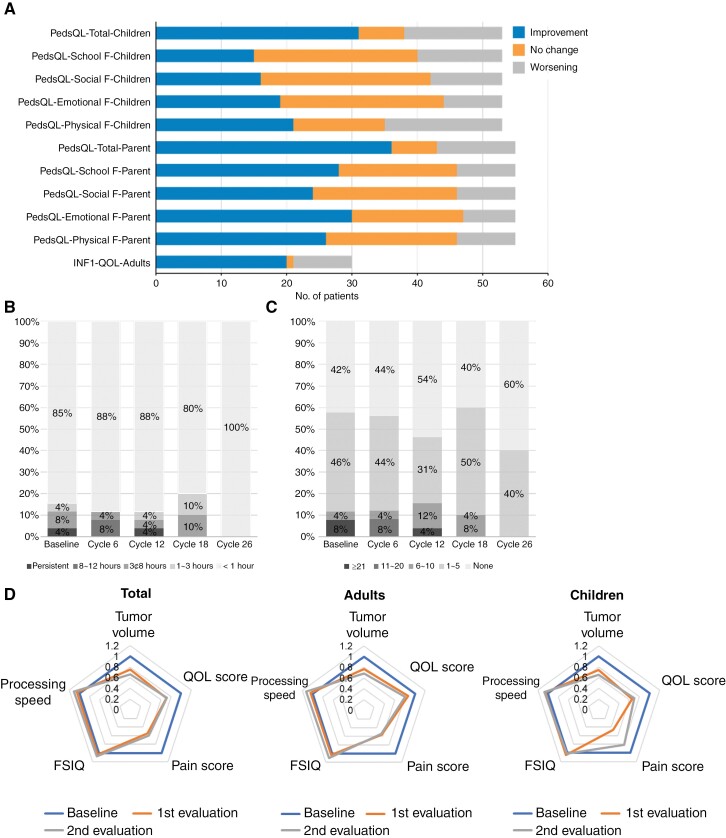
After receiving selumetinib, 63.6% of children and 78.6% of their parents reported an improvement in their QoL (Figure 4A). Specifically, the QoL of children significantly improved after cycles 6 (P = .02) and 26 (P = .004) compared to the baseline (Supplementary Figure 8A). While all domains of QoL scores showed improvement, physical functioning (47.3%) had the highest proportion of children who reported improvements, and emotional functioning (66.1%) had the highest proportions of parents who reported improvement (Figure 4A, Supplementary Figure 9). In adult patients, 61.5% reported an improvement in their QoL after selumetinib treatment (Figure 4A), and the temporal trajectory of QoL scores over time differed from that in children, with cycle 18 showing the highest QoL (Supplementary Figure 8B). When the depression and anxiety scores were compared separately, 26.7% of the patients in the survey showed improvement, and 50% of the 16 patients with a score of 1 or higher prior to taking the drug also showed improvement. (Supplementary Table 11).
Figure 4.
Effects of selumetinib on quality of life and pain. (A) Number of pediatric patients, their parents, and adult patients who reported improvements (blue), no change (orange), or worsening (gray) in their quality of life between baseline and cycle 26. Total scores and subscores (ie, school, social, emotional, and physical functioning) of quality of life are shown. (B) Duration of pain during the last 24 hours in adult patients. (C) Frequency of pain during the last 24 hours in adult patients. (D) Radial charts showing the scores of 5 fields (tumor volume, QOL score, pain score, FSIQ, processing speed) that were assessed at baseline, first evaluation, and second evaluation.
In terms of pain, both the duration and frequency of pain decreased in adult patients (Figure 4, B and C). At the latest evaluation, the duration of recent pain was less than 1 hour in all patients, and 60% reported that the pain had completely disappeared. In pediatric patients, the pain score showed a decreasing trend over time (Supplementary Figure 10A). Adult patients reported significant reductions in pain at cycle 6 compared with baseline (Supplementary Figure 10B).
Pharmacokinetics
A total of 82 patients were included in the pharmacokinetic analysis (Supplementary Figure 11; Table 12). In children, the Cmax and AUC0-12hr increased with higher doses. In adults, group 3 showed a higher degree of drug exposure compared with group 4. Group 3 (adults) had the highest AUC0-12hr, while group 2 (children) had the highest Cmax.
We compared our results on Korean children with those of the SPRINT study, which did not include Asian patients. The Cmax in Korean children was 30% to 65% higher than that in the SPRINT study, and the AUC0–12h after 1 dose of selumetinib was 7% to 17% higher in Korean children as well (Supplementary Table 13).
Discussion
Our single-arm phase II trial on selumetinib highlights multiple previously unrecognized beneficial therapeutic effects of selumetinib in children and adults with NF1 and PN. The PR rate of our subjects was 91% (81/89), which was comparable or slightly higher than those reported in the phase I (71%)7 and phase II (74%)8 studies of the SPRINT trial. Importantly, a continued response was observed during the study period in all patients with PR, and the measures of pain and QoL also improved, with the most significant changes in pain occurring during the first 18 cycles. The changes in QoL in adults were modest compared to those in children.
None of our patients experienced AEs that were grade 3 or higher. Most of the AEs occurred in cycle 1 and decreased significantly afterward (Supplementary Figure 2), indicating that the risk of AEs may not increase with longer treatment durations. An adverse event evaluation was conducted using the same methods as the SPRINT study. The subjects received regular instruction on education and preventive measures for skin and oral care as specified in the study protocol. In addition, avoiding strenuous exercise was recommended to prevent myositis (personal observation). There were no worsening of cutaneous or musculoskeletal adverse effects in patients who adhered to the therapeutic guidelines. The most common AEs in a phase I trial of Caucasian patients were acneiform rash, gastrointestinal symptoms, and asymptomatic increases in creatine kinase (CK).7 In our patients, the occurrence rates of gastrointestinal symptoms and asymptomatic CK elevation were low.
We found that children and adult patients with NF1 showed improvements in neurocognitive functions after receiving selumetinib treatment. Especially, the proportion of patients with overt or borderline intellectual disability decreased after undergoing more treatment cycles. The improvement may be attributed to the restoration of RAS-MAPK activity by selumetinib.27 It is possible that improvements in pain and motor dysfunction may have affected the neurocognitive function and attention; moreover, better scores in neurocognitive assessments may simply reflect increased proficiency due to repeated examinations. However, it is important to consider potential bias from the examiners and find ways to minimize these issues, such as using computerized tests or alternative measures with different forms. However, considering that previous studies28,29 reported similar beneficial results of different MEK inhibitors on the cognitive functions of NF1 patients, it may be worthwhile to investigate these promising effects further through randomized controlled, long-term trials.
Another possible therapeutic effect of selumetinib shown in our study is the improvement of growth profiles in prepubertal NF1 children, which might also be associated with the restoration of RAS-MAPK activity.30 Short stature is one of the characteristic manifestations of genetic disorders (RASopathy) caused by germline gain-of-function mutations in the RAS-MAPK pathway, such as Noonan syndrome, and growth hormone therapy is recommended for children with short stature (< −2 SD score). However, growth hormone treatment can be controversial for NF1 patients because of the risks of tumor growth. Therefore, our results suggest a potential indication for selumetinib treatment in children with NF1 who have short stature. Our observation needs to be validated through age- and sex-matched, randomized controlled trials with detailed endocrine and auxology studies. Moreover, we found that café-au-lait spots became fainter with selumetinib treatment, and this effect increased with a longer duration of treatment. This is an encouraging result considering that café-au-lait spots are one of the psychological and social burdens causing mental conflict on NF1 patients.31 Also, the only currently available treatment using laser has shown limited efficacy.32 However, as avoidance of sun exposure or the natural tendency for café-au-lait spots to fade with increasing age in post-pubertal patients should be considered as other affecting factors, these preliminary results should be validated through age-adjusted, randomized controlled trials.
Finally, our study is the first official report to demonstrate the therapeutic efficacy of selumetinib in NF1 adults with PN.33 Most adult patients showed PR as well as multifaceted therapeutic benefits, including improvements in neurocognitive functions, as observed in pediatric patients. The diverse clinical benefits of selumetinib are depicted as radar charts in Figure 4D. It is noteworthy that the degree of improvement in PN volume reduction was more remarkable in children than in adults, which suggests the importance of early treatment to enhance the therapeutic efficacy of selumetinib.
Regarding the pharmacokinetic properties of selumetinib (Supplementary Table 13), Cmax was 30%–65% higher in our patients than in Caucasian patients, while AUC0-12hr was comparable or slightly higher than in Caucasians by 7%–17%. In contrast to previous studies,10–12 we cannot conclude that higher drug exposure is expected in Asian populations. However, the increase in Cmax and AUC0-12hr suggests higher drug exposure in Korean or Asian patients compared to Caucasian patients, which partially explains the high rate of PR achievement of PN in Korean patients. It is important to note that despite the higher Cmax, no serious adverse events were observed in our patients, and a higher number of patients who received a higher dosage of selumetinib (25 mg/m2 q 12 hours in children and 50 mg q 12 hours in adults) achieved PR. Therefore, it seems feasible to adopt the same dosage of 25 mg/m2/dose q 12 hours in Asian patients as well.
The limitations of our study should be addressed. As our study has a single institutional design, the generalizability of its conclusion may be limited. Furthermore, as a single-arm analysis, there was no control group available for comparison. The QoL scores might have been influenced by the location and growth rate of PN, as well as the degree of PN-related pain and the extent of PN reduction. Furthermore, the complete establishment of the causal effect of selumetinib remains elusive due to the lack of comprehensive endocrine and auxology studies to ascertain the growth effect. Additionally, the reported alleviation of café-au-lait spots with MEK inhibitors may have been influenced by factors such as sun avoidance or the inherent propensity for café-au-lait spots to fade with age in post-pubertal patients. Finally, an optimal dosage of selumetinib cannot be recommended because no significant differences were observed among the different dosage groups in terms of safety and efficacy. However, it is worth noting that a larger number of patients achieved PR with higher dosages. More studies are required to investigate the pharmacokinetic properties among various ethnic groups. Additionally, longer-term evaluations are needed to compare the safety and efficacy among different dosage groups, especially considering the current indication and activity of selumetinib in NF1-related PN, rather than as a generic NF1 drug addressing multiple manifestations of the disease. As part of an ongoing study, the gene and protein expression profiles are being investigated to comprehend the molecular network that underlies the therapeutic effects of selumetinib in NF1.
In conclusion, our phase II trial of selumetinib on Korean pediatric and adult patients with NF1 showed encouraging results in reducing PN regardless of age. Importantly, selumetinib demonstrated a wide range of beneficial effects on the systemic manifestations of NF1. Considering its significant effectiveness in both children and adults with NF1, continued and sustained treatment with selumetinib should be considered along with safety monitoring.
Supplementary material
Supplementary material is available online at Neuro-Oncology (https://academic.oup.com/neuro-oncology).
Acknowledgments
We deeply appreciate the patients and their families for participating in this study. We also thank Dr. D Wade Clapp (Indiana University School of Medicine), Alexion, AstraZeneca Rare Disease and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (Rahway, NJ, USA) for providing a courtesy review of the publication.
Contributor Information
Hyery Kim, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Hee Mang Yoon, Department of Radiology and Research Institute of Radiology, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Eun Key Kim, Department of Plastic Surgery, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Young Shin Ra, Department of Neurosurgery, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Hyo-Won Kim, Department of Psychiatry, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Mi-Sun Yum, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Min-Jee Kim, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Jae Suk Baek, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Yu Sub Sung, Department of Convergence Medicine, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Sang Min Lee, Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Hyeong-Seok Lim, Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Byung Joo Lee, Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Hyun Taek Lim, Orthopia Eye Clinic, Seoul, Republic of Korea; Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Dohyung Kim, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Jihee Yoon, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Hyunwoo Bae, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Soojin Hwang, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Yun-Ha Choi, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Kyung Ah Kim, Medical Genetics Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
In Hee Choi, Department of Genetic Counseling, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Seung Won Lee, Radiation Oncology Laboratory, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Su-Jung Park, School of Korean Medicine, Pusan National University, Pusan, Korea.
Beom Hee Lee, Orthopia Eye Clinic, Seoul, Republic of Korea; Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Funding
This research was supported in part by an externally sponsored research program (ESR-17-12847) by AstraZeneca (provision of selumetinib and funding for study), in collaboration with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (Rahway, NJ, USA), a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HR21C0198), and the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (grant number: NRF-2022R1A2C2091689).
Conflict of interests
HK, and BHL have participated on an Advisory Board on a Round Table Discussion of Selumetinib for Astrazeneca.
Authorship statement
H.K. and B.H.L. designed the study. H.K., H.M.Y., E.K.K., Y.S.R., H.W.K., M.S.Y., M.J.K., J.S.B., B.J.L., H.T.L., D.K., J.Y., H.B., S.H., Y.A.C., K.A.K., and B.H.L. performed clinical and radiological examinations. H.K., H.M.Y., H.W.K., J.S.B., Y.S.S., S.M.L., H.S.L., K.K.K., I.H.C., S.W.L., S.J.P., D.W.C., and B.H.L. analyzed the data. H.K., H.M.Y., S.H., and B.H.L. drafted the manuscript, and H.K., H.M.Y., S.J.P., and B.H.L. revised the manuscript. All authors read and approved the final manuscript.
Data availability
Anonymized participant data will be made available when the trials are complete, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of proposal, data can be shared through a secure online platform after signing a data access agreement.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Asan Medical Center (Seoul, Korea) and the Ministry of Food and Drug Safety, Korea.
Consent for publication
No personally identifiable data are contained in this manuscript.
References
- 1. Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. [DOI] [PubMed] [Google Scholar]
- 2. Xu GF, O’Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62(3):599–608. [DOI] [PubMed] [Google Scholar]
- 3. Bergoug M, Doudeau M, Godin F, et al. Neurofibromin structure, functions and regulation. Cells. 2020;9(11):2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paria N, Cho TJ, Choi IH, et al. Neurofibromin deficiency-associated transcriptional dysregulation suggests a novel therapy for tibial pseudoarthrosis in NF1. J Bone Miner Res. 2014;29(12):2636–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang E, Yoon HM, Lee BH.. Neurofibromatosis type I: Points to be considered by general pediatricians. Clin Exp Pediatr. 2021;64(4):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: Relationship to age and body weight. Neurology. 2007;68(9):643–647. [DOI] [PubMed] [Google Scholar]
- 7. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang E, Kim YM, Seo GH, et al. Phenotype categorization of neurofibromatosis type I and correlation to NF1 mutation types. J Hum Genet. 2020;65(2):79–89. [DOI] [PubMed] [Google Scholar]
- 10. Dymond AW, Elks C, Martin P, et al. Pharmacokinetics and pharmacogenetics of the MEK1/2 inhibitor, selumetinib, in Asian and Western healthy subjects: A pooled analysis. Eur J Clin Pharmacol. 2017;73(6):717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schalkwijk S, Zhou L, Cohen-Rabbie S, et al. Population pharmacokinetics and exposure-response of selumetinib and its N-desmethyl metabolite in pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas. Cancer Chemother Pharmacol. 2021;88(2):189–202. [DOI] [PubMed] [Google Scholar]
- 12. Suenobu S, Terashima K, Akiyama M, et al. Selumetinib in Japanese pediatric patients with neurofibromatosis type 1 and symptomatic, inoperable plexiform neurofibromas: An open-label, phase I study. Neurooncol. Adv.. 2023;5(1):vdad054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Legius E, Messiaen L, Wolkenstein P, et al. ; International Consensus Group on Neurofibromatosis Diagnostic Criteria (I-NF-DC). Revised diagnostic criteria for neurofibromatosis type 1 and legius syndrome: An international consensus recommendation. Genet Med. 2021;23(8):1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed November 27, 2023. [Google Scholar]
- 15. Suh CH, Lee JH, Chung MS, et al. MRI predictors of malignant transformation in patients with inverted papilloma: A decision tree analysis using conventional imaging features and histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2021;22(5):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park HW, Kwak KJ, Park KB.. Korean-Wechsler preschool and primary scale of intelligencee (K-WPPSI). Seoul: Seoul Community Rehabilitation Center; 1996. [Google Scholar]
- 17. Hwang K, Oh SW.. Validity of the K-WISC-IV short forms. Korean J Clin Psychol. 2017;36(3):381–390. [Google Scholar]
- 18. Choe A-Y, Hwang S-T, Kim J-H, et al. Validity of the K-WAIS-IV short forms. Korean J Clin Psychol. 2014;33(2):413–428. [Google Scholar]
- 19. Shin MS, Choi H, Kim H, et al. A study of neuropsychological deficit in children with obsessive-compulsive disorder. Eur Psychiatry. 2008;23(7):512–520. [DOI] [PubMed] [Google Scholar]
- 20. Cho S-Z, Chun S-Y, Hong K-E, Shin M-S.. A study of the development and standardization of ADHD diagnostic system. J Korean Acad Child Adolesc Psychiatry. 2000;11(1):91–99. [Google Scholar]
- 21. Bae DS, Lee JB, Ban YG.. Computerized neurocognitive function test. Seoul: Hana Medical Publishing; 2005. [Google Scholar]
- 22. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varni JW, Seid M, Kurtin PS.. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 24. Hockenberry MJ, Rodgers CC, Wilson D.. Wong’s essentials of pediatric nursing. 11st ed. St. Louis, Missouri: Elsevier; 2022. [Google Scholar]
- 25. Ferner RE, Thomas M, Mercer G, et al. Evaluation of quality of life in adults with neurofibromatosis 1 (NF1) using the Impact of NF1 on Quality Of Life (INF1-QOL) questionnaire. Health Qual Life Outcomes. 2017;15(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yun D-J, Oh J, Kim B-J, et al. Development of Korean neuropathic pain questionnaire for neuropathic pain screening and grading: A pilot study. J Korean Neurol Assoc. 2012;30(1):15–25. [Google Scholar]
- 27. Li W, Cui Y, Kushner SA, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15(21):1961–1967. [DOI] [PubMed] [Google Scholar]
- 28. Walsh KS, Wolters PL, Widemann BC, et al. Impact of MEK inhibitor therapy on neurocognitive functioning in NF1. Neurol Genet. 2021;7(5):e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lalancette E, Cantin E, Routhier ME, et al. Impact of trametinib on the neuropsychological profile of NF1 patients. J Neurooncol. 2024;167(3):447–454. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez F, Gaete X, Cassorla F.. Etiology and treatment of growth delay in noonan syndrome. Front Endocrinol (Lausanne). 2021;12:691240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foji S, Mohammadi E, Sanagoo A, Jouybari L.. The patients’ experiences of burden of neurofibromatosis: A qualitative study. Iran J Nurs Midwifery Res. 2021;26(4):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belkin DA, Neckman JP, Jeon H, Friedman P, Geronemus RG.. Response to laser treatment of cafe au lait macules based on morphologic features. JAMA Dermatol. 2017;153(11):1158–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helen G, Coyne OS, Gross AM, et al. Phase II trial of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886 Hydrogen Sulfate) in adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN) [abstract]. J Clin Oncol. 2020;38(15_suppl):3612. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized participant data will be made available when the trials are complete, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of proposal, data can be shared through a secure online platform after signing a data access agreement.