Abstract
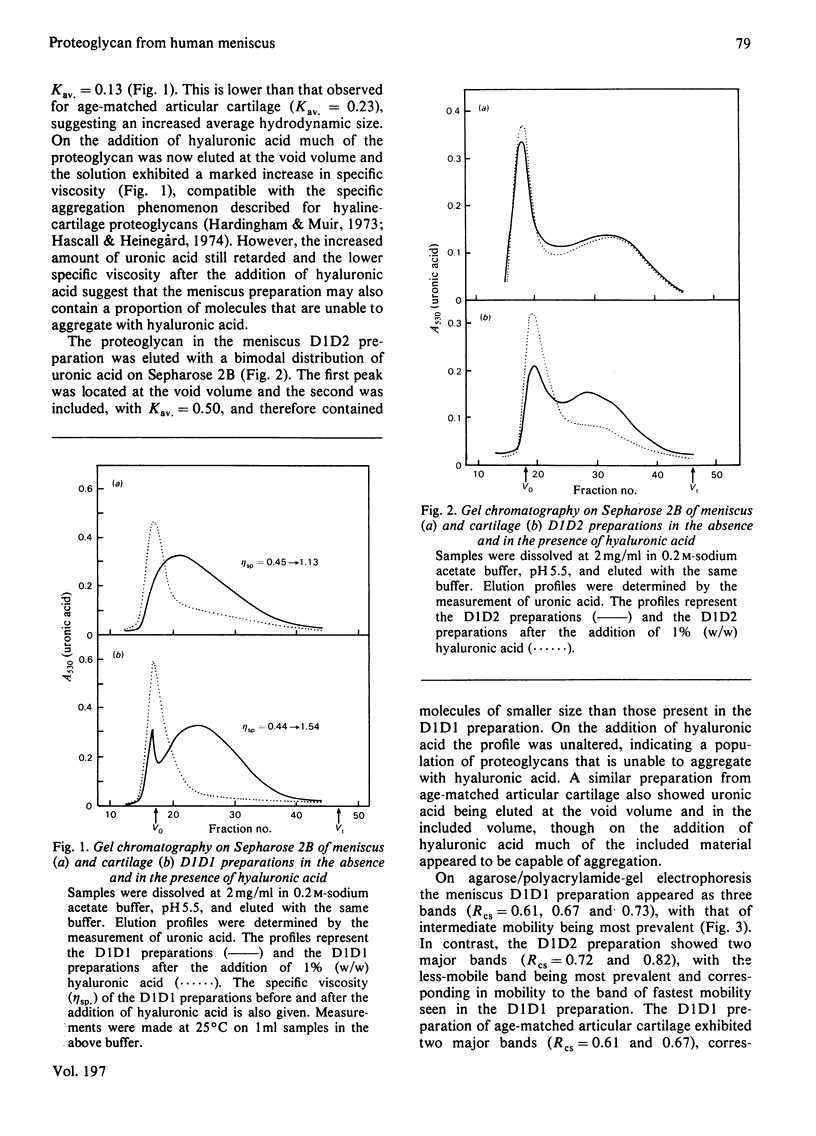
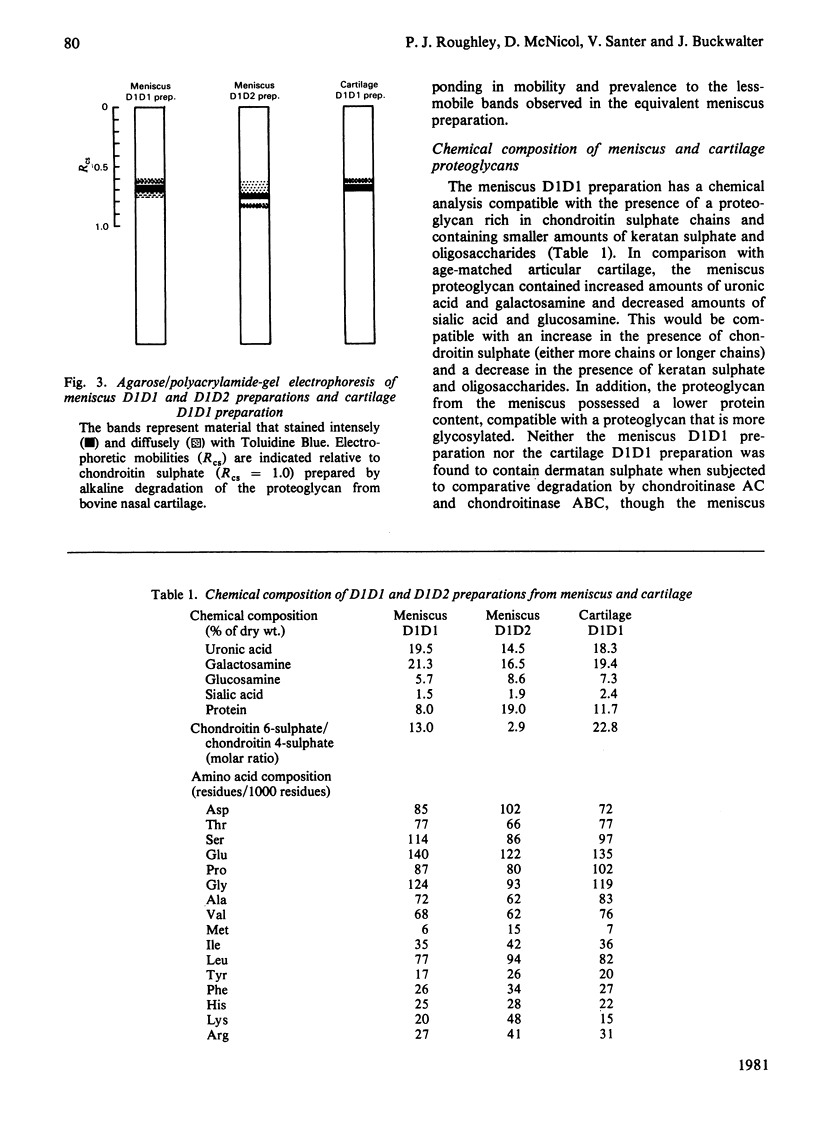
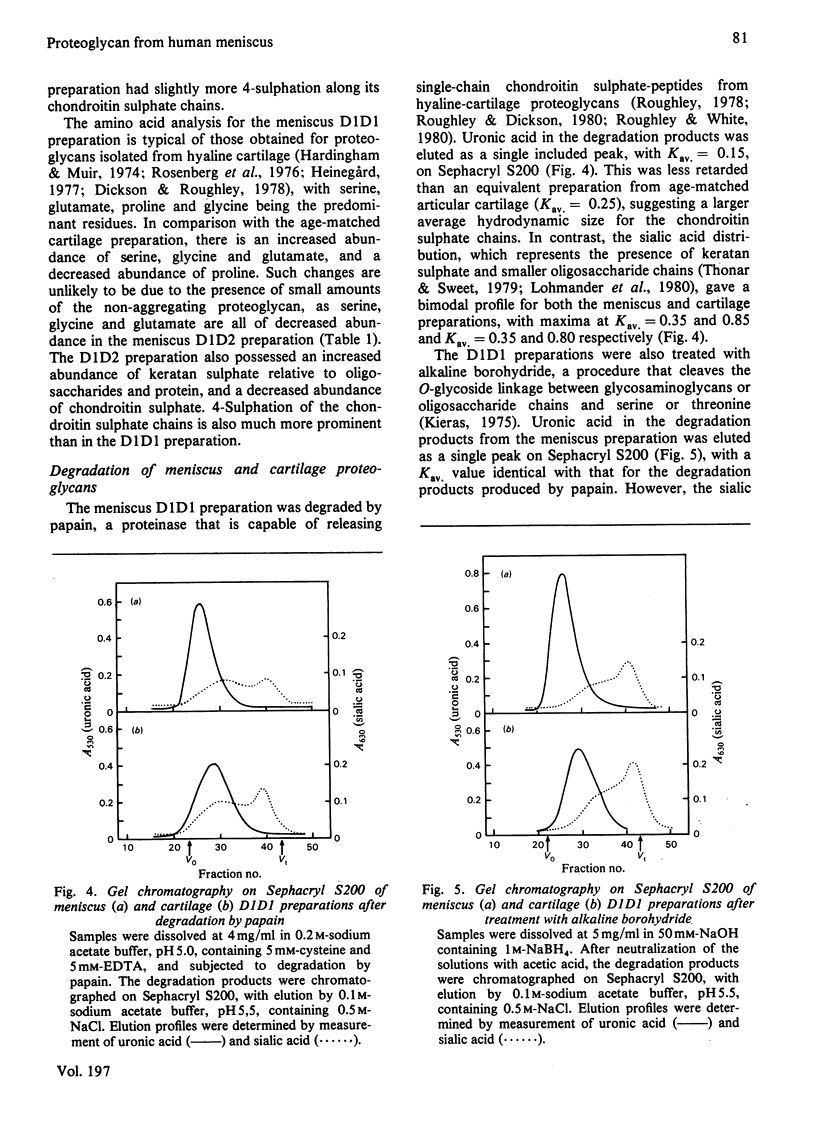
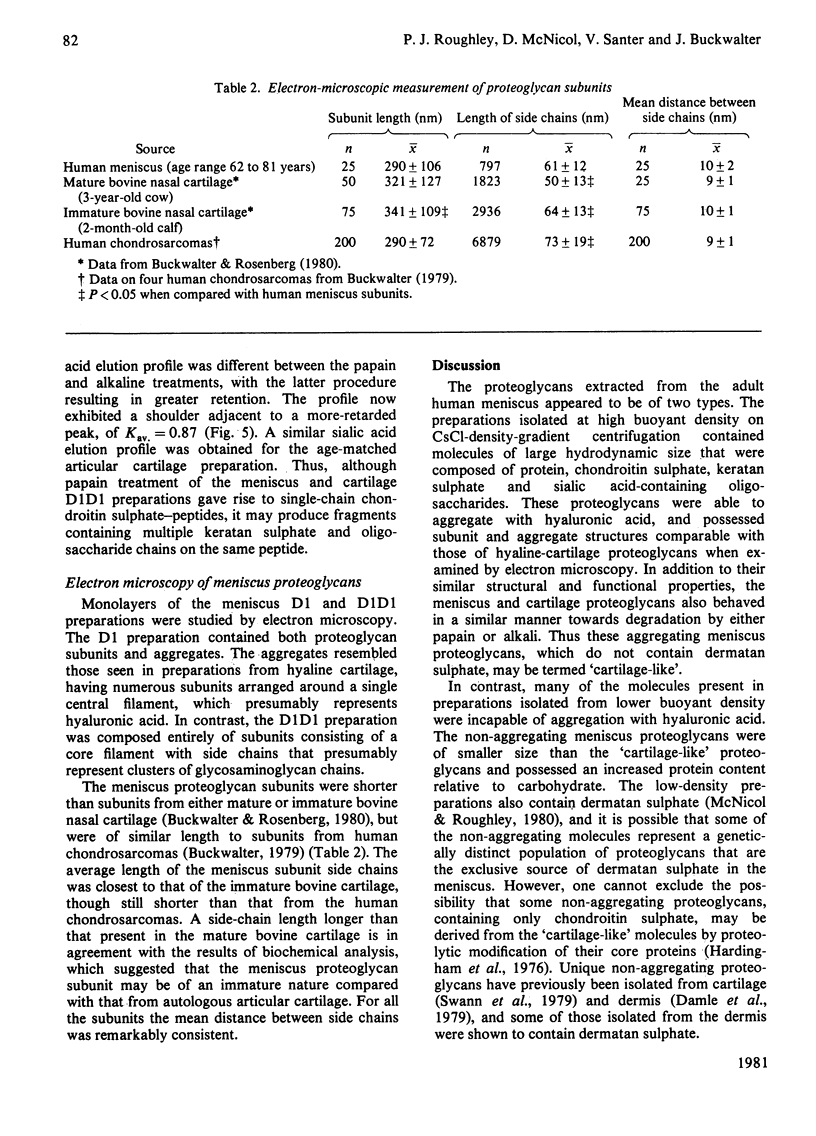
Proteoglycans were extracted from the adult human meniscus under dissociative conditions and purified by CsCl-density-gradient centrifugation. The preparations of highest density contained proteoglycan that possessed the ability to interact with hyaluronic acid, was of large subunit size and was composed of chondroitin sulphate, keratan sulphate and sialic acid-containing oligosaccharides. This 'cartilage-like' proteoglycan also exhibited subunit and aggregate structures analogous to those of hyaline-cartilage proteoglycans when examined by electron microscopy. However, the composition of this proteoglycan was more comparable with proteoglycans from immature cartilage than from age-matched cartilage. The preparations from lower density, which were enriched in dermatan sulphate, contained smaller proteoglycan that was not able to interact with hyaluronic acid. This non-aggregating proteoglycan may be structurally distinct from the 'cartilage-like' proteoglycan, which does not contain dermatan sulphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Damle S. P., Kieras F. J., Tzeng W. K., Gregory J. D. Isolation and characterization of proteochondroitin sulfate from pig skin. J Biol Chem. 1979 Mar 10;254(5):1614–1620. [PubMed] [Google Scholar]
- De Luca S., Lohmander L. S., Nilsson B., Hascall V. C., Caplan A. I. Proteoglycans from chick limb bud chondrocyte cultures. Keratan sulfate and oligosaccharides which contain mannose and sialic acid. J Biol Chem. 1980 Jul 10;255(13):6077–6083. [PubMed] [Google Scholar]
- Dickson I. R., Roughley P. J. A comparative study of the proteoglycan of growth cartilage of normal and rachitic chicks. Biochem J. 1978 Jun 1;171(3):675–682. doi: 10.1042/bj1710675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Ingman A. M., Taylor T. K. Variations in collagen, non-collagenous proteins, and hexosamine in menisci derived from osteoarthritic and rheumatoid arthritic knee joints. J Rheumatol. 1975 Mar;2(1):100–107. [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Kieras F. J. Alkaline borohydride treatment of cartilage keratan sulfate: a comparison of two reaction conditions. Carbohydr Res. 1975 May;41:339–343. doi: 10.1016/s0008-6215(00)87037-8. [DOI] [PubMed] [Google Scholar]
- Koseki M., Kimura A., Tsurumi K. Micro determination of unsaturated disaccharide formed by the action of acidic glycosaminoglycan-endoeliminases. An application of the thiobarbituric acid method to the assay of D-gluco-4-enepyranosyluronic acid-containing disaccharides. J Biochem. 1978 Feb;83(2):553–558. doi: 10.1093/oxfordjournals.jbchem.a131943. [DOI] [PubMed] [Google Scholar]
- Krause W. R., Pope M. H., Johnson R. J., Wilder D. G. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976 Jul;58(5):599–604. [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- McNicol D., Roughley P. J. Extraction and characterization of proteoglycan from human meniscus. Biochem J. 1980 Mar 1;185(3):705–713. doi: 10.1042/bj1850705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Strider W., Margolis R., Gallo G., Lee-Huang S. Isolation and characterization of proteoglycans from human chondrosarcomas. J Biol Chem. 1978 Feb 25;253(4):1279–1289. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Rosenberg L., Wolfenstein-Todel C., Margolis R., Pal S., Strider W. Proteoglycans from bovine proximal humeral articular cartilage. Structural basis for the polydispersity of proteoglycan subunit. J Biol Chem. 1976 Oct 25;251(20):6439–6444. [PubMed] [Google Scholar]
- Roughley P. J. A comparative study of the glycosaminoglycan-peptides obtained after degradation of cartilage proteoglycan by different proteinases, and their use in the characterization of different proteoglycans. Connect Tissue Res. 1978;6(3):145–153. doi: 10.3109/03008207809152624. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P., Dickson I. Factors influencing proteoglycan size in rachitic-chick growth cartilage. Biochem J. 1980 Jan 1;185(1):33–39. doi: 10.1042/bj1850033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann D. A., Powell S., Sotman S. The heterogeneity of cartilage proteoglycans. Isolation of different types of proteoglycans from bovine articular cartilage. J Biol Chem. 1979 Feb 10;254(3):945–954. [PubMed] [Google Scholar]
- Thonar E. J., Sweet M. B. An oligosaccharide component in proteoglycans of articular cartilage. Biochim Biophys Acta. 1979 May 1;584(2):353–357. doi: 10.1016/0304-4165(79)90281-2. [DOI] [PubMed] [Google Scholar]
- Wasserman L., Ber A., Allalouf D. Use of thin-layer chromatography in the separation of disaccharides resulting from digestion of chondroitin sulphates with chondroitinases. J Chromatogr. 1977 Jun 11;136(2):342–347. doi: 10.1016/s0021-9673(00)86291-3. [DOI] [PubMed] [Google Scholar]


