Abstract
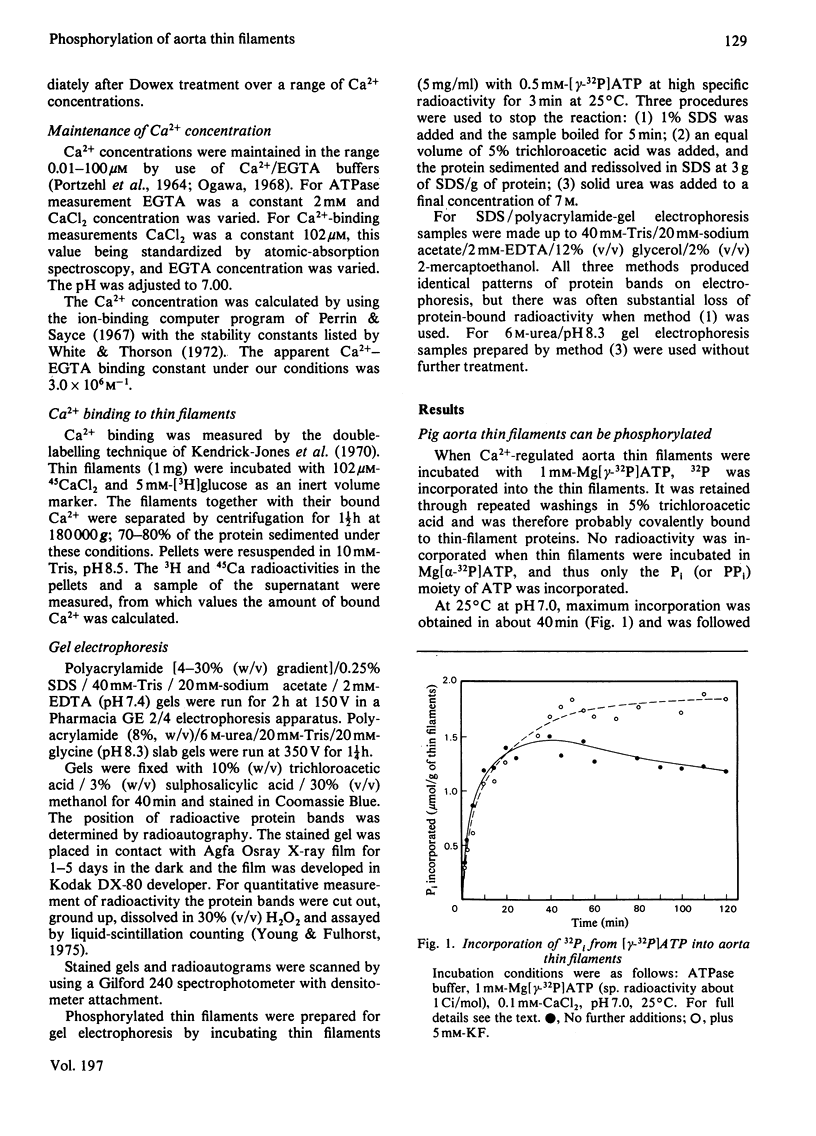
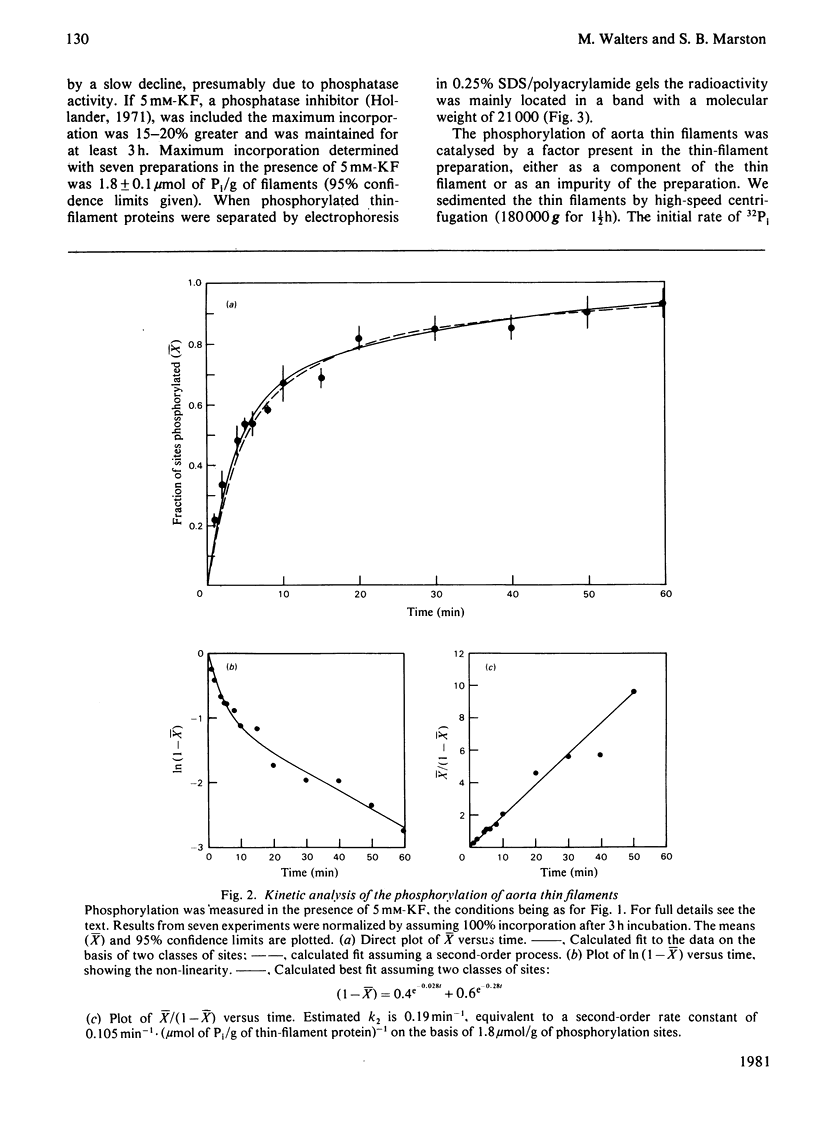
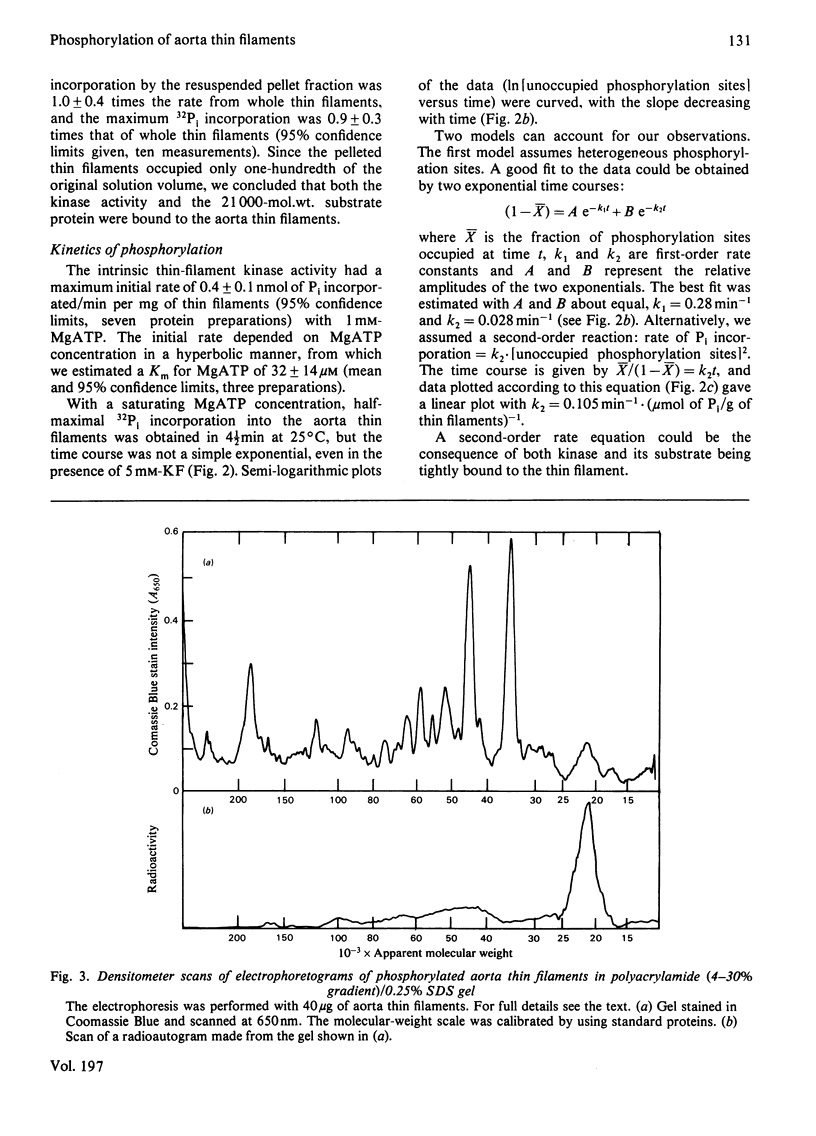
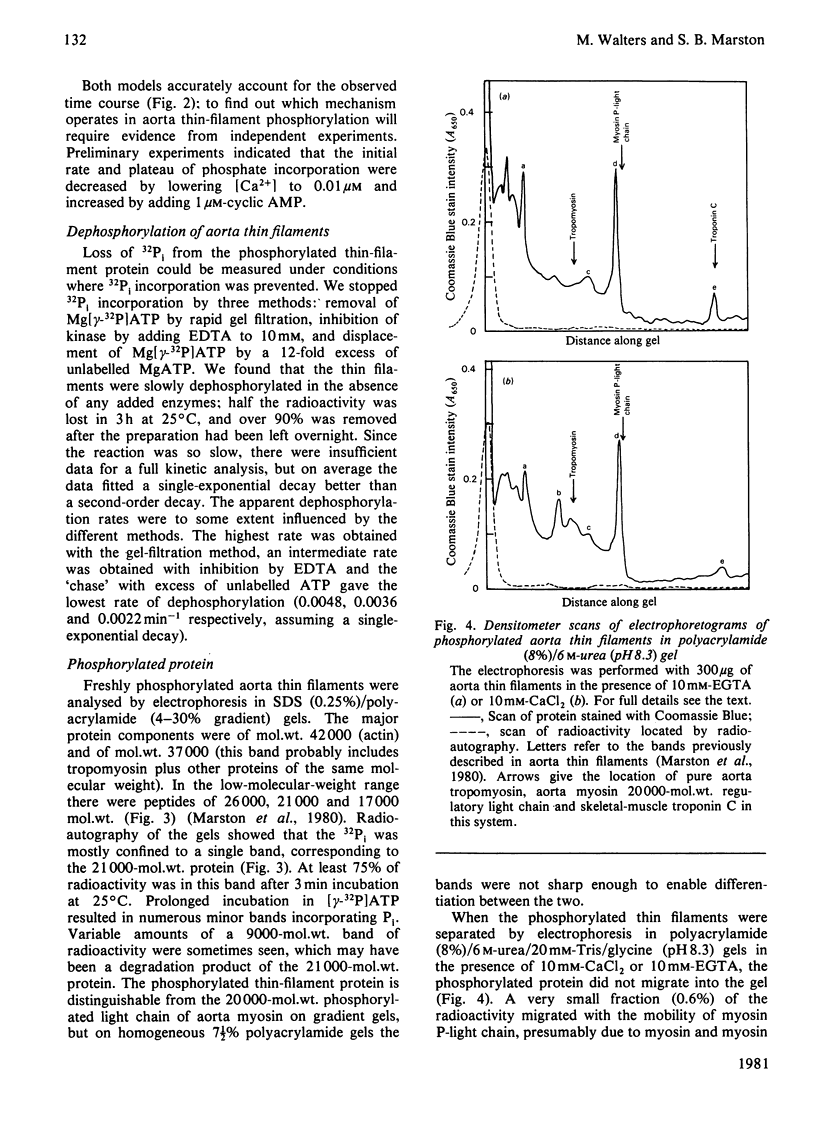
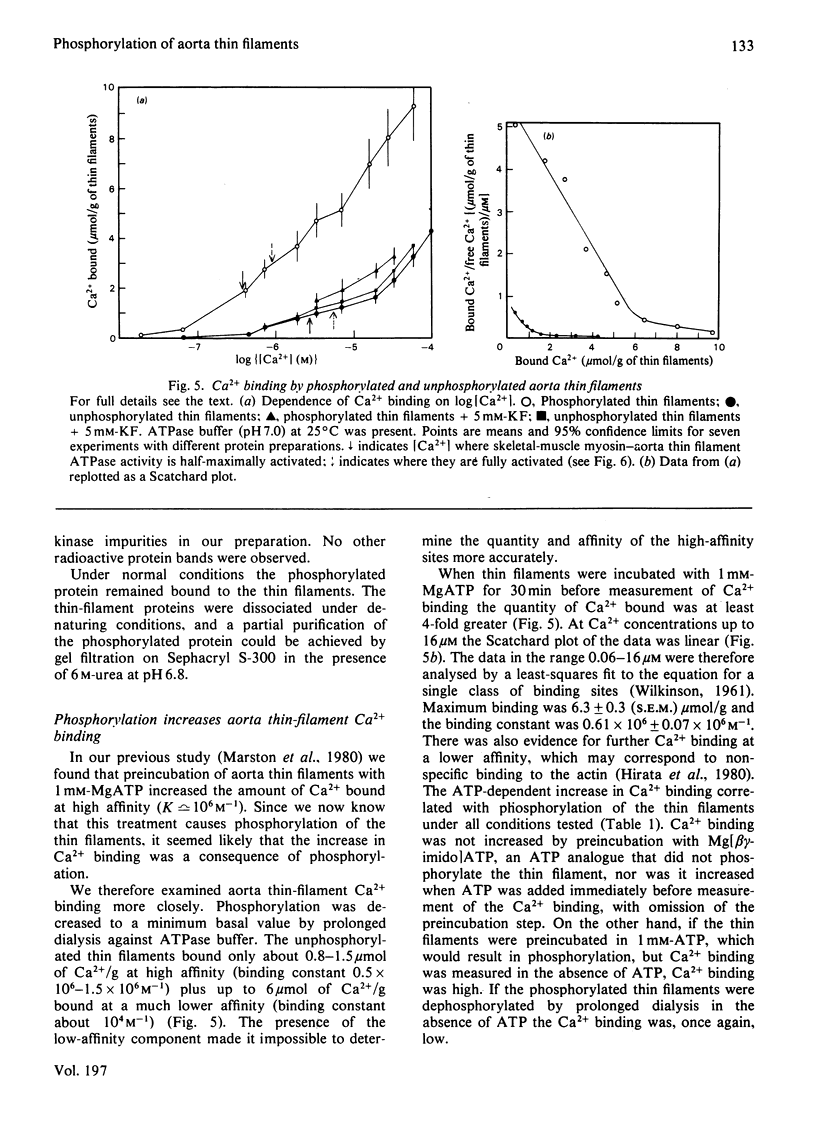
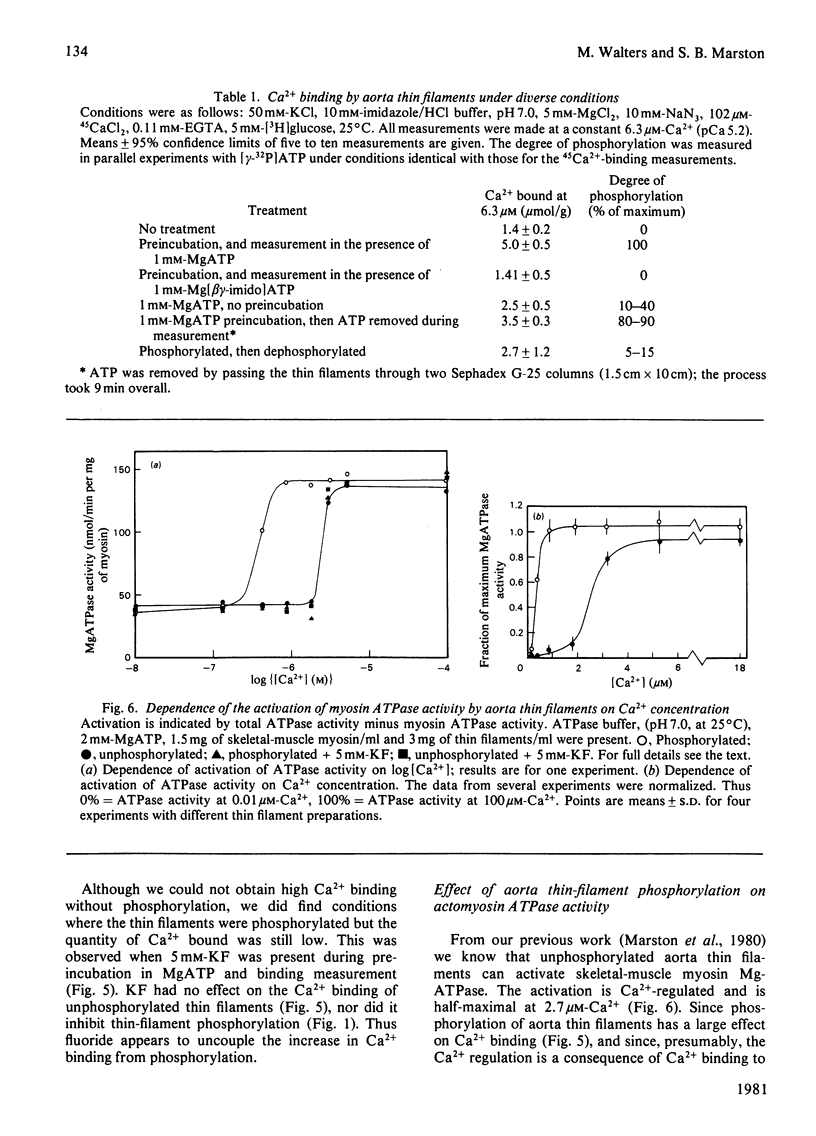
The thin filaments of vascular smooth muscle (pig aorta) contain a Ca2+-sensitive regulatory system that resembles troponin-tropomyosin [Marston, Trevett & Walters (1980) Biochem. J. 185, 355-365]. Our thin-filament preparations also contain enzymes that phosphorylate and dephosphorylate a specific protein. Initial rate of phosphorylation was 0.42 +/- 0.10 (95% confidence limits) mumol of Pi/min per g of thin filaments; half-maximal incorporation was obtained in 4 1/2 min, and a maximum of 1.8 +/- 0.1 mumol of Pi/g of thin filaments was incorporated after 40 min (conditions: 1 mM-MgATP, 60 mM-MgATP, 60 mM-KCl, 10 mM-imidazole, pH 7.0, 5 mM-MgCl2, 10 mM-NaN3, 0.5 mM-dithiothreitol, 0.1 mM-CaCl2, 25 degrees C). On gel electrophoresis in polyacrylamide (4-30% gradient)/0.25% sodium dodecyl sulphate gel over 75% of protein-bound phosphate was in a single protein of mol.wt. 21000. On electrophoresis in polyacrylamide (8%)/6 M-urea (pH 8.6) gel the phosphoprotein remained at the origin. Phosphorylation was associated with an increase in the concentration of high-affinity (K congruent to 10(6) M-1) Ca2+-binding sites from 0.8-1.5 to 6.3 mumol of Ca2+/g of thin filaments. Phosphorylation also changed the regulatory properties of the skeletal-muscle myosin-aorta thin-filament MgATPase; maximum activity was unaltered, but the phosphorylated thin filaments required only 0.36 microM-Ca2+ for half-activation compared with 2.7 microM-Ca2+ for unphosphorylated thin filaments. The possible regulatory role of thin-filament phosphorylation is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy M. O., Williams D., Sharkey E. M., Hartshorne D. J. A relationship between Ca2+ sensitivity and phosphorylation of gizzard actomyosin. Biochem Biophys Res Commun. 1976 Mar 8;69(1):35–41. doi: 10.1016/s0006-291x(76)80268-9. [DOI] [PubMed] [Google Scholar]
- Barron J. T., Bárány M., Bárány K. Phosphorylation of the 20,000-dalton light chain of myosin of intact arterial smooth muscle in rest and in contraction. J Biol Chem. 1979 Jun 25;254(12):4954–4956. [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Stull J. T. Calcium binding to cardiac troponin and the effect of cyclic AMP-dependent protein kinase. FEBS Lett. 1977 Jan 15;73(1):101–104. [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Daniel J. L., Adelstein R. S. Isolation and properties of platelet myosin light chain kinase. Biochemistry. 1976 Jun 1;15(11):2370–2377. doi: 10.1021/bi00656a019. [DOI] [PubMed] [Google Scholar]
- DiSalvo J., Gruenstein E., Schmidt C. Relationships between Ca2+, myosin light chains, and ATPase in bovine aortic actomyosin: presence of Ca2+-requiring inactivation factor. Proc Soc Exp Biol Med. 1979 Nov;162(2):337–341. doi: 10.3181/00379727-162-40677. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Toyo-Oka T., Nonmura Y. Gizzard Troponin. J Biochem. 1975 Oct;78(4):859–861. doi: 10.1093/oxfordjournals.jbchem.a130976. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Mikawa T., Nonomura Y., Ebashi S. Ca2+ regulation in vascular smooth muscle. II. Ca2+ binding of aorta leiotonin. J Biochem. 1980 Feb;87(2):369–378. doi: 10.1093/oxfordjournals.jbchem.a132757. [DOI] [PubMed] [Google Scholar]
- Hirata M., Mikawa T., Nonomura Y., Ebashi S. Ca2+ regulation in vascular smooth muscle. J Biochem. 1977 Dec;82(6):1793–1796. doi: 10.1093/oxfordjournals.jbchem.a131879. [DOI] [PubMed] [Google Scholar]
- Hsiao K. J., Sandberg A. R., Li H. C. The role of ATP and divalent cations in the regulation of a cardiac phosphorylase phosphatase (phosphoprotein phosphatase) of Mr = 35,000. J Biol Chem. 1978 Oct 10;253(19):6901–6907. [PubMed] [Google Scholar]
- Jahnke U., Heilmeyer L. M., Jr Comparison of the Mg2+ and Ca2+ binding properties of troponin complexes P1-TI2C and TI2C. Eur J Biochem. 1980 Oct;111(2):325–332. doi: 10.1111/j.1432-1033.1980.tb04945.x. [DOI] [PubMed] [Google Scholar]
- Katzinski L., Mrwa U. A Ca2+-sensitive myosin light chain kinase, regulating pig carotid smooth muscle actomyosin ATPase. Experientia. 1980 Mar 15;36(3):282–283. doi: 10.1007/BF01952276. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. The informational role of calcium in the cytosol. Adv Cyclic Nucleotide Res. 1979;11:1–26. [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Solaro R. J., Ford G. D. Nature of the calcium regulatory system of bovine arterial actomyosin. Blood Vessels. 1979;16(1):26–34. doi: 10.1159/000158187. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol. 1980 Jun 5;139(4):573–600. doi: 10.1016/0022-2836(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Trevett R. M., Walters M. Calcium ion-regulated thin filaments from vascular smooth muscle. Biochem J. 1980 Feb 1;185(2):355–365. doi: 10.1042/bj1850355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Ebashi S. Does phosphorylation of myosin light chain have direct relation to regulation in smooth muscle? J Biochem. 1977 Dec;82(6):1789–1791. doi: 10.1093/oxfordjournals.jbchem.a131878. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Hirata M., Ebashi S., Kakiuchi S. Involvement of an acidic protein in regulation of smooth muscle contraction by the tropomyosin-leiotonin system. J Biochem. 1978 Dec;84(6):1633–1636. doi: 10.1093/oxfordjournals.jbchem.a132290. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Toyo-oka T., Nonomura Y., Ebashi S. Essential factor of gizzard "troponin" fraction. A new type of regulatory protein. J Biochem. 1977 Jan;81(1):273–275. doi: 10.1093/oxfordjournals.jbchem.a131447. [DOI] [PubMed] [Google Scholar]
- Murphy R. A. Filament organization and contractile function in vertebrate smooth muscle. Annu Rev Physiol. 1979;41:737–748. doi: 10.1146/annurev.ph.41.030179.003513. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Paul R. J., Glück E., Rüegg J. C. Cross bridge ATP utilization in arterial smooth muscle. Pflugers Arch. 1976 Feb 24;361(3):297–299. doi: 10.1007/BF00587295. [DOI] [PubMed] [Google Scholar]
- Perrin D. D., Sayce I. G. Computer calculation of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta. 1967 Jul;14(7):833–842. doi: 10.1016/0039-9140(67)80105-x. [DOI] [PubMed] [Google Scholar]
- Perry S. V. The regulation of contractile activity in muscle. Biochem Soc Trans. 1979 Aug;7(4):593–617. doi: 10.1042/bst0070593. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ regulation of muscle contraction. Biochemistry. 1974 Jun 18;13(13):2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Reddy Y. S. Phosphorylation of cardiac regulatory proteins by cyclic AMP-dependent protein kinase. Am J Physiol. 1976 Nov;231(5 Pt 1):1330–1336. doi: 10.1152/ajplegacy.1976.231.5.1330. [DOI] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Bremel R. D. Preparation and properties of vertebrate smooth-muscle myofibrils and actomyosin. Eur J Biochem. 1975 Jun 16;55(1):49–60. doi: 10.1111/j.1432-1033.1975.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol. 1977 Jun 5;112(4):559–576. doi: 10.1016/s0022-2836(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Maxwell L. C., Ruegg J. C., Bohr D. F. Preparation and properties of a calcium ion-sensitive actomyosin from arteries. Am J Physiol. 1970 Nov;219(5):1366–1372. doi: 10.1152/ajplegacy.1970.219.5.1366. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Manning D. R., High C. W., Blumenthal D. K. Phosphorylation of contractile proteins in heart and skeletal muscle. Fed Proc. 1980 Apr;39(5):1552–1557. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W., Fulhorst H. W. Recovery of S35 radioactivity from protein-bearing polyacrylamide gel. Anal Biochem. 1965 May;11(2):389–391. doi: 10.1016/0003-2697(65)90030-8. [DOI] [PubMed] [Google Scholar]


