Abstract
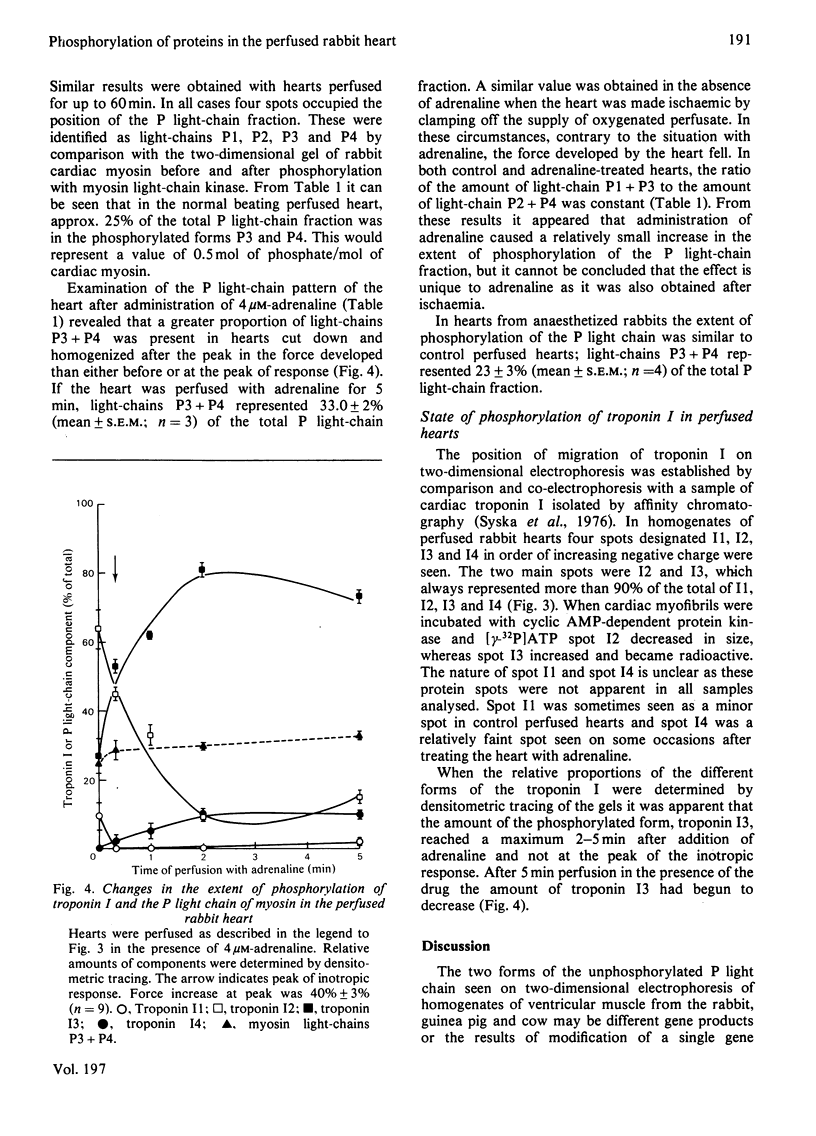
1. Two-dimensional electrophoresis has been used to study the extent of phosphorylation of the P light chain of myosin and troponin I in the rabbit beating heart. 2. A procedure has been developed that eliminates endogenous protein phosphatase activity during homogenization and sample preparation for electrophoresis. 3. Evidence has been obtained for two unphosphorylated forms of the P light chain in myosin from the ventricle of the rabbit, guinea pig and cow. 4. In vivo and in the rabbit perfused beating heart about 25% of the P light-chain fraction is in the phosphorylated form. 5. Intervention with adrenaline produced a slight increase in the extent of phosphorylation that reached a maximum after the peak in inotropic response. A similar increase was obtained with ischaemia in the absence of adrenaline. 6. The changes in phosphorylation of the major forms of troponin I identified by electrophoresis occurred after the peak of response to adrenaline and were compatible with previous results.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Arce C. A., Barra H. S., Rodriguez J. A., Caputto R. Tentative identification of the amino acid that binds tyrosine as a single unit into a soluble brain protein. FEBS Lett. 1975 Jan 15;50(1):5–7. doi: 10.1016/0014-5793(75)81027-1. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson N., Solaro R. J., Perry S. V. Changes in phosphorylation of P light chain of myosin in perfused rabbit heart. Nature. 1976 Dec 23;264(5588):801–802. doi: 10.1038/264801a0. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., Yeoh G. P., Thomas M. A., Higginbottom L. Structural differences in the heavy chains of rat ventricular myosin isoenzymes. FEBS Lett. 1979 Jan 15;97(2):330–334. doi: 10.1016/0014-5793(79)80115-5. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Small D. A., Howe E., Solaro R. J. Isolation of cardiac myofibrils and myosin light chains with in vivo levels of light chain phosphorylation. Biochim Biophys Acta. 1979 Nov 1;587(4):628–637. doi: 10.1016/0304-4165(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Jeacocke S. A., England P. J. Phosphorylation of myosin light chains in perfused rat heart. Effect of adrenaline and increased cytoplasmic calcium ions. Biochem J. 1980 Jun 15;188(3):763–768. doi: 10.1042/bj1880763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. J., Perry S. V. Phosphorylation of rabbit cardiac-muscle troponin I by phosphorylase kinase. The effect of adrenaline. Biochem J. 1980 Nov 1;191(2):547–554. doi: 10.1042/bj1910547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. J., Solaro R. J., Perry S. V. The site of phosphorylation of troponin I in the perfused rabbit heart. The effect of adrenaline. Biochem J. 1980 Feb 1;185(2):505–513. doi: 10.1042/bj1850505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M., Perry S. V., Ottaway J. Myosin light-chain phosphatase. Biochem J. 1976 Sep 1;157(3):687–697. doi: 10.1042/bj1570687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemrick S. M. The phosphorylated L2 light chain of skeletal myosin is a modifier of the actomyosin ATPase. J Biol Chem. 1980 Sep 25;255(18):8836–8841. [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E. M., Perry S. V. Purification and properties of myosin light-chain kinase from fast skeletal muscle. Biochem J. 1977 Oct 1;167(1):137–146. doi: 10.1042/bj1670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E., Perry S. V., Thomas M. A. Myosin light-chain kinase, a new enzyme from striated muscle. FEBS Lett. 1974 May 1;41(2):292–296. doi: 10.1016/0014-5793(74)81232-9. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Solaro R. J., Pang D. C., Briggs F. N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971 Aug 6;245(1):259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Manning D. R., High C. W., Blumenthal D. K. Phosphorylation of contractile proteins in heart and skeletal muscle. Fed Proc. 1980 Apr;39(5):1552–1557. [PubMed] [Google Scholar]
- Syska H., Wilkinson J. M., Grand R. J., Perry S. V. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem J. 1976 Feb 1;153(2):375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]