Abstract
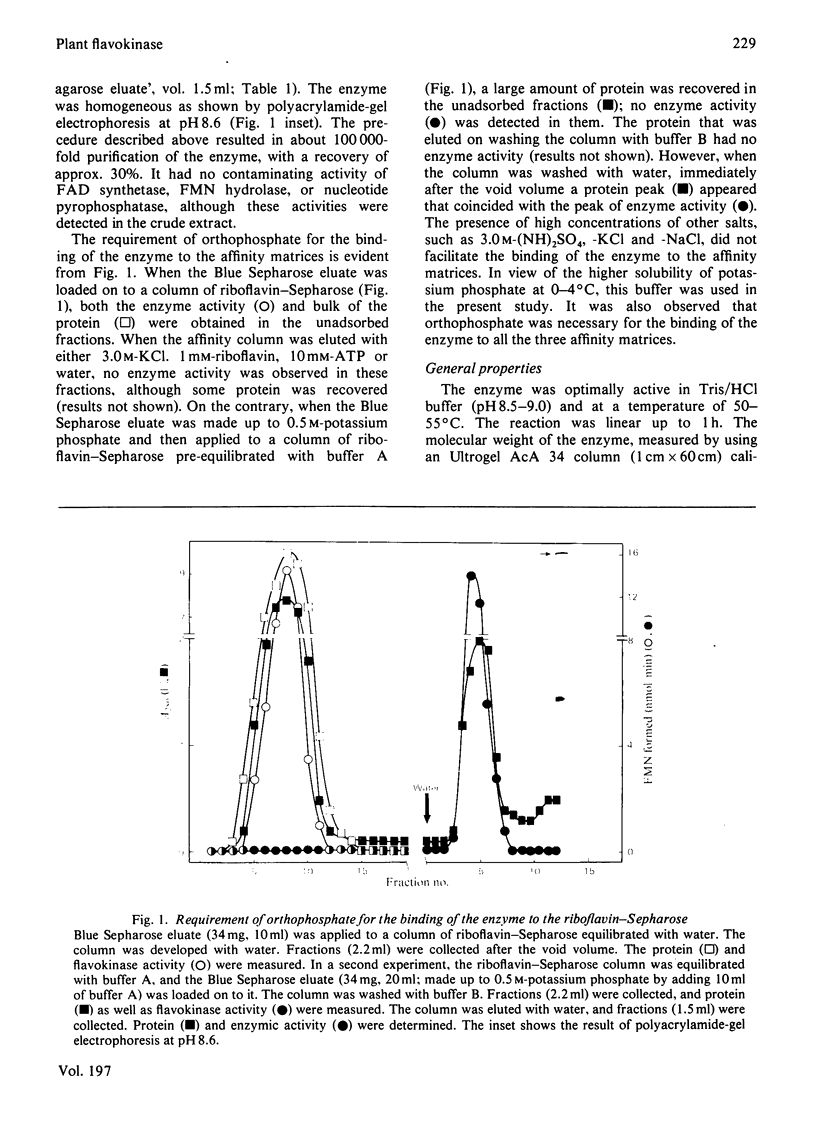
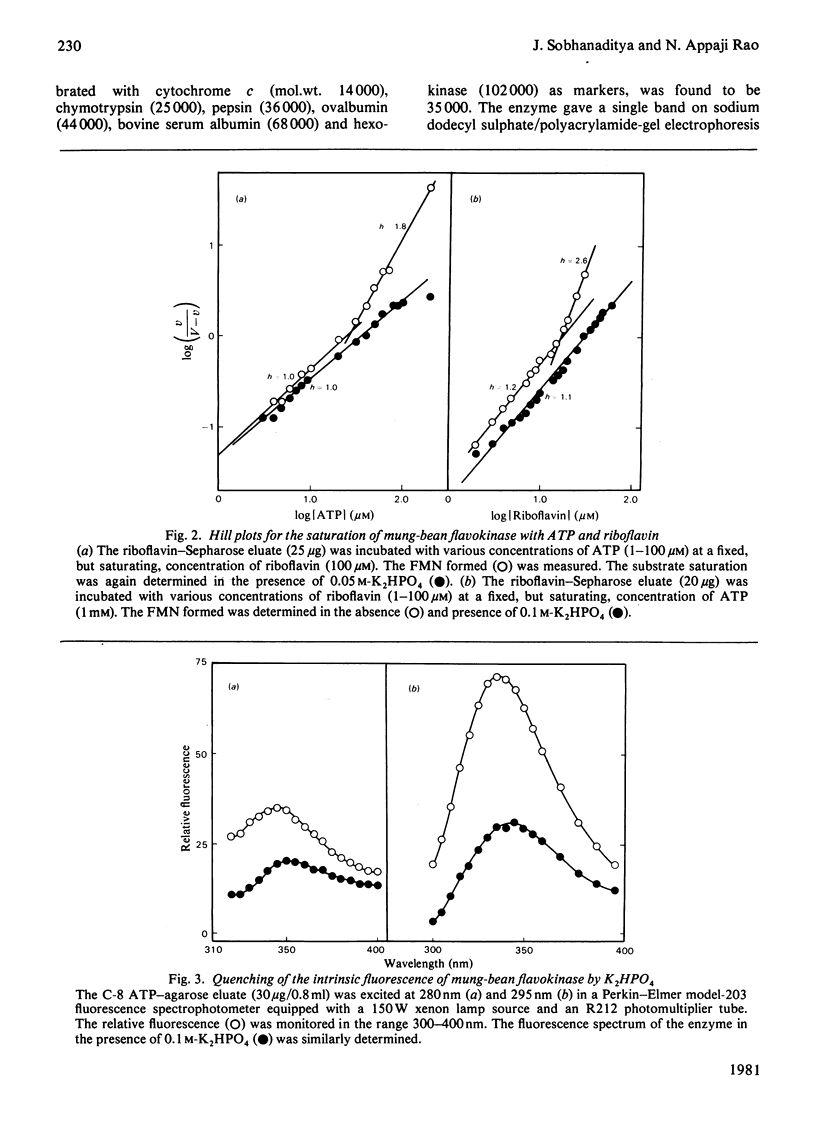
Flavokinase was purified, for the first time from a plant source [mung bean (Phaseolus aureus)] by affinity chromatography in the presence of orthophosphate and by using C-8 ATP-agarose (ATP linked through the C-8 position to beaded agarose), Cibacron Blue and riboflavin--Sepharoses. An altered substrates-saturation pattern was observed in the presence of K2HPO4. The conformational changes of the enzyme in the presence of K2HPO4 were monitored by fluorescence spectroscopy. These results highlight the regulatory nature of this enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRACHAN L., COLOWICK S. P., KAPLAN N. O. The reactivity of the bound DPN of muscle triose phosphate dehydrogenase. Biochim Biophys Acta. 1957 Apr;24(1):141–154. doi: 10.1016/0006-3002(57)90157-9. [DOI] [PubMed] [Google Scholar]
- Balakrishnan C. V., Vaidyanathan C. S., Rao N. A. Studies on nucleotidases in plants. Isolation and properties of the monomeric form of the crystalline and homogeneous mung bean nucleotide pyrophosphatase. Eur J Biochem. 1977 Aug 15;78(1):95–102. doi: 10.1111/j.1432-1033.1977.tb11717.x. [DOI] [PubMed] [Google Scholar]
- GIRI K. V., KRISHNASWAMY P. R., RAO N. A. Occurrence of flavokinase activity in plants. Nature. 1957 Jun 1;179(4570):1134–1135. doi: 10.1038/1791134b0. [DOI] [PubMed] [Google Scholar]
- GIRI K. V., KRISHNASWAMY P. R., RAO N. A. Studies on plant flavokinase. Biochem J. 1958 Sep;70(1):66–71. doi: 10.1042/bj0700066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- KEARNEY E. B., ENGLARD S. The enzymatic phosphorylation of riboflavin. J Biol Chem. 1951 Dec;193(2):821–834. [PubMed] [Google Scholar]
- Krishnan N., Rao N. A. Studies on nucleotide pyrophosphatase. I. Partial purification and properties of a sheep liver enzyme that catalyzes the hydrolysis of dinucleotides. Arch Biochem Biophys. 1972 Apr;149(2):336–348. doi: 10.1016/0003-9861(72)90332-3. [DOI] [PubMed] [Google Scholar]
- Kristiansen T., Sundberg L., Porath J. Studies on blood group substances. II. Coupling of blood group substane A to hydroxyl-containing matrices, including aminoethyl cellulose and agarose. Biochim Biophys Acta. 1969 Jun 17;184(1):93–98. [PubMed] [Google Scholar]
- MITSUDA H., TOMOZAWA Y., KAWAI F. STUDIES ON PLANT FLAVOKINASE. II. THE PURIFICATION AND SOME PROPERTIES OF BEAN FLAVOKINASE. J Vitaminol (Kyoto) 1963 Jun 10;9:142–148. [PubMed] [Google Scholar]
- Mayhew S. G., Wassink J. H. A continuous fluorometric assay for flavokinase. Properties of flavokinase from Peptostreptococcus elsdenii. Biochim Biophys Acta. 1977 Jun 10;482(2):341–347. doi: 10.1016/0005-2744(77)90247-9. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, McCormick D. B. Affinity chromatographic purification and properties of flavokinase (ATP:riboflavin 5'-phosphotransferase) from rat liver. J Biol Chem. 1980 Feb 25;255(4):1335–1338. [PubMed] [Google Scholar]
- Reddy A. R., Ananthanarayanan V. S., Rao N. A. Studies on nucleotidases in plants: fluorescence and kinetic properties of nucleotide pyrophosphatase from mung bean (Phaseolus aureus) seedlings. Arch Biochem Biophys. 1979 Nov;198(1):89–96. doi: 10.1016/0003-9861(79)90398-9. [DOI] [PubMed] [Google Scholar]
- Sadasivam S., Shanmugasundaram E. R. Studies on the flavokinase of Solanum nigrum L. Enzymologia. 1966 Oct 31;31(4):203–208. [PubMed] [Google Scholar]
- Schramm V. L., Fullin F. A. Kinetics of adenosine monophosphate nucleosidase inactivation by phosphate and protection by substrate and allosteric activator. J Biol Chem. 1978 Apr 10;253(7):2161–2167. [PubMed] [Google Scholar]
- Spencer R., Fisher J., Walsh C. Preparation, characterization, and chemical properties of the flavin coenzyme analogues 5-deazariboflavin, 5-deazariboflavin 5'-phosphate, and 5-deazariboflavin 5'-diphosphate, 5'leads to5'-adenosine ester. Biochemistry. 1976 Mar 9;15(5):1043–1053. doi: 10.1021/bi00650a015. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Lowenstein J. M. Adenylate deaminase from rat muscle. Regulation by purine nucleotides and orthophosphate in the presence of 150 mM KCl. J Biol Chem. 1979 Sep 25;254(18):8994–8999. [PubMed] [Google Scholar]



