ABSTRACT
Background
Patients with autosomal dominant polycystic kidney disease (ADPKD) represent >10% of patients awaiting kidney transplantation. These patients are prone to potentially severe urinary tract (UTI) and liver cyst infections after transplantation. Whether such infections compromise outcome is unclear.
Methods
Between 2000 and 2017 we performed 193 kidney transplantations in patients with ADPKD. In 189 patients, we assessed the occurrence, frequency, and severity of infection episodes requiring inpatient treatment and their impact on graft and patient outcomes compared with 189 matched controls. Risk factors were analyzed by uni- and multivariable analyses.
Results
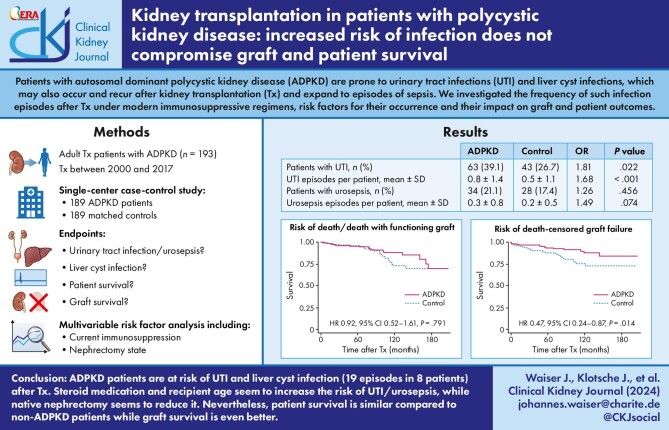
During a mean observation period of 77 months UTIs occurred more frequently in ADPKD patients (39.1% vs. 26.7%, P = .022; 0.8 ± 1.4 vs. 0.5 ± 1.1 episodes, P < .001). Eight ADPKD patients suffered from 19 episodes of liver cyst infection. Steroid medication (RR 3.04; P < .001) and recipient age (RR 1.05; P = .003) increased the risk for UTI/urosepsis, while nephrectomy reduced it (unilateral, RR 0.60; P = .088; bilateral, RR 0.45; P = .020). Patient survival was similar in both groups. The risk of graft failure was lower in ADPKD patients [hazard ratio (HR) 0.67; P = .047] due to a lower risk of death-censored graft loss (HR 0.47; P = .014). Donor age (HR 1.34; P = .002) and rejection (HR 8.47; P < .001) were risk factors for death-censored graft loss.
Conclusions
ADPKD patients are at increased risk of UTI and liver cyst infection after transplantation. Steroid medication and recipient age seem to increase the risk of UTI/urosepsis, while nephrectomy seems to reduce it. Nevertheless, patient survival was similar compared to non-ADPKD patients and death-censored graft survival even better.
Keywords: infection, kidney transplantation, polycystic kidney disease, risk factors, survival
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
-
•
Patients with autosomal dominant polycystic kidney disease (ADPKD) are prone to urinary tract infections and liver cyst infections, which may also occur and recur after kidney transplantation. However, the frequency of such infection episodes after transplantation under modern immunosuppressive regimens, risk factors for their occurrence and their impact on graft and patient outcomes are unclear.
This study adds:
-
•
In this single-center study comparing 189 ADPKD patients with an equal number of matched controls, we found that significantly more patients (OR 1.81) experienced significantly more episodes (OR 1.68) of urinary tract infection requiring inpatient treatment and that liver cyst infections occurred in 4% of patients. Nevertheless, patient survival was similar and graft survival, especially death-censored graft survival even better. Recipient age and steroid treatment seem to increase the risk of infection while nephrectomy of the native kidneys seems to reduced it.
Potential impact:
-
•
The observation of similar survival rates and lower rates of graft loss despite an increased risk of infection is reassuring for ADPKD patients considering kidney transplantation. Awareness of the increased infection risk may facilitate early diagnosis and treatment. Our findings suggest that a reduction in infection risk should be weight into risk benefit assessments for nephrectomy and that steroid sparing immunosuppressive regimes may mitigate infection risk.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease and the fourth leading cause of kidney failure accounting for ∼10% of patients requiring kidney replacement therapy [1–4]. For patients with ADPKD and kidney failure kidney transplantation (Tx) is the preferred treatment option [5]. In 2021, >16% of all patients, who were added to the Eurotransplant waiting list in Germany suffered from ADPKD [6]. After Tx, these patients may experience episodes of urinary tract infection (UTI) and cyst infection of their native kidneys and liver. These episodes may recur and expand to episodes of sepsis. In this case-control study, we compared the occurrence, frequency, and severity of UTIs and liver cyst infections in a cohort of hospitalized ADPKD patients, who had received a kidney transplant at our center, with a cohort of matched control kidney transplant patients, who suffered from other causes of kidney failure. In addition, we analyzed patient survival, graft survival and graft function. Risk factors for the respective endpoints were analyzed by uni- and multivariable analysis.
MATERIALS AND METHODS
In 2017, we searched our electronic database system ‘Tbase’ [7] for patients who had received a kidney allograft between 1 January 2000 and 31 October 2017. We found 1478 Tx events during this period. In 272 cases the individual chart contained the term *cyst*, *zyst*, *ADPKD*, or the ICD-10 code *Q61*. We excluded 37 cases, in which Tx was performed under the age of 18 years, and 42 cases in which the diagnosis could not be confirmed by either family history, pathology or imaging [8]. This resulted in a group of 193 Tx events. Patients with any other cause of kidney failure were matched with the ADPKD cohort using the following criteria: donor type (living or deceased donor), recipient gender, recipient age (±5 years), and date of Tx (±1 year). Hereby, we were able to identify 189 matched pairs.
Notably, only patients without current and historical, preformed donor-specific HLA antibodies (2000–2008: ELISA, Lambda Antigen Tray LAT, One Lambda; since 2008: Luminex Single Antigen Bead Assay, One Lambda) and negative preoperative complement-dependent cytotoxicity crossmatch using isolated donor-derived T and B lymphocytes were accepted for transplantation. Because the immunological risk in all patients was considered low, thymoglobulin induction was not used.
Both groups were compared concerning the following endpoints: UTI, urosepsis, liver cyst infection, death, overall graft failure, death-censored graft failure, and renal function measured as serum creatinine-based estimated glomerular filtration rate (eGFRcr) calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [9]. Patients were censored at the time of graft failure. Risk factors for the previously mentioned endpoints were calculated by univariable and multivariable analyses. In the analysis of UTI and urosepsis patients were censored from the time at which both native kidneys had been removed.
A diagnosis of UTI was made in patients with typical signs and symptoms of UTI including blood tests and a positive urine culture. We did not distinguish between lower and upper UTI, because in our experience such a differentiation is generally difficult in renal allograft recipients. A diagnosis of urosepsis was based on the sepsis-2 criteria including positive urine and blood cultures [10]. Liver cyst infection was diagnosed by magnetic resonance imaging or positron-emission computed tomography [11] in combination with blood tests and blood cultures. Only infection episodes requiring hospitalization were recorded. The necessity of inpatient treatment was caused by either the severity of disease or the necessity of intravenous (IV) antibiotic treatment or both.
The Charlson Comorbidity Index (CCI) was calculated at the time of Tx. The CCI is a common, validated and widely accepted instrument used to predict 10-year mortality [12]. The CCI was further adapted in kidney failure patients [13] and kidney transplant recipients [14, 15]. Kidney failure as comorbid condition was considered present in all patients, irrespective of graft function. Serious ongoing conditions such as dementia, acquired immunodeficiency syndrome, and malignancy represent an absolute contraindication for Tx and therefore were not present.
The study was conducted in accordance with the principles of the Declaration of Helsinki, as revised in 2013 and the Declaration of Istanbul. The study was approved by the Institutional Ethics Committee of the Charité (EA1/048/14).
Statistical analyses
The risk of death, overall graft failure, and death-censored graft failure for ADPKD patients and matched controls was analyzed by Cox-proportional hazard models. Conditional logistic regression analysis was applied to compare the likelihood for UTI and urosepsis between ADPKD and matched controls. The association of defined risk factors with survival outcomes (death, death-censored graft failure, overall graft failure) was estimated by Cox-proportional hazard models. The likelihood for UTI or urosepsis during the initial Tx hospitalization was analyzed by a generalized linear model with robust error variances, the assumption of a Poisson distribution for onset of infections and the log link function including the defined risk factors and initial therapy after Tx. The entire observation period was divided into single treatment episodes based on immunosuppressive therapy administered during follow-up. Episodes of UTI or urosepsis were assigned to the treatment episode during which they occurred. The resulting patient-treatment episode data were analyzed by a multilevel mixed-effects Poisson regression model including the time under treatment as exposure to assess the association between UTI or urosepsis with time-depended therapy and defined baseline risk factors. The course of eGFR during follow-up was analyzed by a multilevel mixed-effect linear regression model. A worst-case imputation for missing eGFR values was performed in case of death-censored graft failure by 5 ml/min/1.73m². All defined risk factors were tested in univariable models for each outcome. LASSO (least absolute shrinkage and selection operator) regression was performed to identify a multivariable model for each defined outcome. LASSO is suited for models with high levels of multicollinearity. Relevant variables were included in the model regardless of their significance in univariate association with the outcome by LASSO. Continuously distributed variables were included as continuous in the regression models, the unit for interpretation is given in the tables. Variables resulting in <10 cases in cells in a cross table were not included in the regression analysis to assure reliable estimates. All statistical analyses were performed with SAS v.9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Table 1 shows patient characteristics at the time of transplantation. As expected, more patients in the ADPKD group underwent nephrectomy before Tx as compared to the control group. All other patient characteristics were similar between groups.
Table 1:
Patient characteristics at transplantation.
| ADPKD complete n = 193 | ADPKD matched-pair n = 189 | Control matched-pair n = 189 | P value | |
|---|---|---|---|---|
| Recipient age (years), mean ± SD | 55.1 ± 10.2 | 55.1 ± 10.1 | 55.2 ± 10.2 | .924 |
| Waiting time (months), mean ± SD | 47.9 ± 42.1 | 48.2 ± 42.4 | 50.1 ± 37.0 | .643 |
| Female recipient, n (%) | 81 (42.0) | 80 (42.3) | 80 (42.3) | |
| Recipient HbA1c, mean ± SD | 5.4 ± 0.7 | 5.6 ± 0.8 | 5.7 ± 0.9 | .254 |
| Recipient BMI (kg/m2), mean ± SD | 26.0 ± 3.9 | 26.0 ± 3.9 | 26.2 ± 4.9 | .661 |
| Recipient CCI score, mean ± SD | 3.6 ± 1.3 | 3.6 ± 1.3 | 4.0 ± 1.8 | .069 |
| Unilateral nephrectomy before Tx, n (%) | 61 (31.6) | 61 (32.3) | 12 (6.3) | <.001 |
| Bilateral nephrectomy before Tx, n (%) | 22 (11.4) | 22 (11.6) | 9 (4.8) | .015 |
| Underlying cause of chronic kidney disease | ||||
| Diabetic nephropathy, n (%) | 18 (9.5) | |||
| Vascular nephropathy, n (%) | 19 (10.1) | |||
| Primary glomerulopathy, n (%) | 70 (37.0) | |||
| Systemic disease, n (%) | 8 (4.2) | |||
| Hereditary kidney disease, n (%) | 10 (5.3) | |||
| Obstructive nephropathy/chronic pyelonephritis, n (%) | 26 (13.8) | |||
| Miscellaneous, n (%) | 13 (6.9) | |||
| Undetermined, n (%) | 25 (13.2) | |||
| vPRA %, mean ± SD | 6.5 ± 21.5 | 6.6 ± 21.7 | 11.3 ± 26.5 | .061 |
| Donor age (years), mean ± SD | 54.1 ± 13.9 | 54.2 ± 14.0 | 53.8 ± 14.8 | .787 |
| Donor eGFRcr (ml/min/1.73 m2), mean ± SD | 83.4 ± 24.6 | 83.6 ± 24.7 | 84.5 ± 24.7 | .723 |
| Living donor, n (%) | 68 (35.2) | 65 (34.4) | 65 (34.4) | |
| Preemptive Tx, n (%) | 23 (11.9) | 22 (11.6) | 12 (6.4) | .072 |
| AB0 compatible Tx, n (%) | 177 (91.7) | 174 (92.1) | 183 (96.8) | .073 |
| Steroids, n (%) | 193 (100.0) | 189 (100.0) | 189 (100.0) | |
| Basiliximab, n (%) | 183 (94.8) | 179 (94.7) | 177 (93.7) | .660 |
| Cyclosporine A, n (%) | 85 (44.0) | 83 (43.9) | 83 (43.9) | |
| Tacrolimus, n (%) | 104 (53.9) | 102 (54.0) | 102 (54.0) | |
| Everolimus, n (%) | 3 (1.6) | 3 (1.6) | 2 (1.1) | .653 |
| Belatacept, n (%) | 1 (0.5) | 1 (0.5) | 3 (1.6) | .315 |
| Mycophenolate, n (%) | 179 (92.7) | 175 (92.6) | 182 (96.3) | .116 |
| Fingolimod, n (%) | 2 (1.0) | 2 (1.1) | 1 (0.5) | .562 |
| Sotrastaurin, n (%) | 12 (6.2) | 12 (6.3) | 5 (2.7) | .082 |
Immunosuppression refers to the initial treatment after Tx.
Note: in the control group one patient concomitantly received cyclosporine A and everolimus.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; eGFRcr, creatinine-based estimated glomerular filtration rate; SD, standard deviation; Tx, transplantation; vPRA, virtual panel reactive antibodies (Eurotransplant Reference Laboratory, HLA database version 4).
Chi-square test for categorical variables, Wilcoxon signed-rank-test for continuously distributed variables.
The mean observation time of the complete ADPKD cohort (n = 193) was 78.9 ± 51.9 months. The mean observation time of the matched-pair cohorts (n = 189) was 79.4 ± 52.0 (ADPKD) and 74.4 ± 48.6 months (control), respectively.
After Tx, altogether 29 endogenous kidneys were removed in the ADPKD group; both kidneys in five patients, the first kidney in nine patients and the second kidney in 10 patients. In the control cohort, 16 endogenous kidneys were removed after Tx, both kidneys in three patients and the first kidney in 10 patients (P = .161).
Posttransplant immunological data including calcineurin-inhibitor trough levels, frequencies and types of rejection, drugs used for the treatment of rejection, and white blood cell (WBC) counts according to groups are shown in Table 2. There were no differences between both groups except for the number of pre- and posttransplant WBC counts, which were significantly lower in ADPKD patients as compared to the control group.
Table 2:
Immunological parameters after transplantation.
| ADPKD complete n = 193 | ADPKD matched-pair n = 189 | Control matched-pair n = 189 | P value | |
|---|---|---|---|---|
| Cyclosporin A trough level | ||||
| Month 1–3, median (IQR) | 177 (162–195) | 177 (162–195) | 176 (155–196) | .562 |
| Month 4–12, median (IQR) | 110 (96–123) | 110 (95–122) | 112 (102–124) | .224 |
| Month >12, median (IQR) | 92 (84–102) | 91 (84–103) | 93 (84–103) | .733 |
| Tacrolimus trough level | ||||
| Month 1–3, median (IQR) | 9.95 (9.10–11.00) | 10.00 (9.10–11.00) | 9.83 (8.55–11.10) | .277 |
| Month 4–12, median (IQR) | 7.35 (6.50–8.25) | 7.32 (6.50–8.26) | 7.15 (6.35–8.35) | .719 |
| Month >12, median (IQR) | 6.00 (5.30–6.81) | 6.00 (5.30–6.82) | 6.00 (5.49–6.70) | .832 |
| Number of patients with rejection, n (%) | 43 (22.3) | 41 (21.7) | 41 (21.7) | |
| TCMR, n (%) | 38 (19.7) | 36 (19.1) | 33 (17.5) | .690 |
| ABMR, n (%) | 5 (2.6) | 5 (2.7) | 5 (2.7) | |
| Mixed TCMR/ABMR, n (%) | 1 (0.5) | 1 (0.5) | 5 (2.7) | .100 |
| Number of rejection episodes, mean ± SD | 0.25 ± 0.51 | 0.24 ± 0.51 | 0.29 ± 0.55 | .699 |
| TCMR, mean ± SD | 0.20 ± 0.40 | 0.19 ± 0.39 | 0.17 ± 0.38 | .691 |
| ABMR, mean ± SD | 0.03 ± 0.16 | 0.03 ± 0.16 | 0.03 ± 0.16 | |
| Mixed TCMR/ABMR, mean ± SD | 0.01 ± 0.07 | 0.01 ± 0.07 | 0.03 ± 0.17 | .101 |
| Rejection treatment | ||||
| Steroid pulse, n | 49 | 47 | 55 | .354 |
| Thymoglobulin, n | 2 | 2 | 4 | .410 |
| Bortezomib, n | 1 | 1 | 4 | .177 |
| Plasmapheresis/IVIG, n | 6 | 6 | 9 | .491 |
| Rituximab, n | 0 | 0 | 1 | .317 |
| WBC count (×109/l) | ||||
| Pretransplant, median (IQR) | 8.00 (6.26–9.97) | 7.97 (6.22–9.96) | 9.46 (7.73–11.77) | <.001 |
| Day 1–14, median (IQR) | 8.55 (6.82–10.25) | 8.41 (6.81–10.18) | 9.61 (7.89–11.19) | <.001 |
| Day 15–month 3, median (IQR) | 7.78 (6.43–9.48) | 7.74 (6.40–9.48) | 8.57 (6.75–10.11) | .011 |
| Month 4–12, median (IQR) | 6.23 (5.09–7.65) | 6.21 (5.09–7.56) | 7.17 (5.82–8.80) | <.001 |
Abbreviations: ABMR, antibody mediated rejection; ADPKD, polycystic kidney disease; IQR, interquartile range; IVIG, intravenous immunoglobulins; SD, standard deviation; TCMR, T cell mediated rejection.
Chi-square test for categorical variables; Mann–Whitney U-test for continuously distributed variables.
UTI and liver cyst infection episodes
Episodes of UTI/urosepsis requiring hospitalization are summarized in Table 3a. The total number of UTI episodes during the entire observation period was 126 vs. 79 (ADPKD vs. non-ADPKD), the total number of urosepsis episodes was 50 vs. 36. Altogether, 41 vs. 27 patients suffered from recurrent UTI/urosepsis episodes. During the initial hospital stay at the time of Tx, the number of patients who experienced UTI/urosepsis was not different between both cohorts. By contrast, the number of patients with UTI during the entire observation period was higher in the ADPKD cohort compared to the control cohort. Also, the number of UTI episodes per patient was higher in the ADPKD group. The number of patients with urosepsis and the number of urosepsis episodes per patient were slightly higher in the ADPKD group, but differences were not statistically significant. Table 3b shows a comparison of the risk of UTI and urosepsis between ADPKD patients and subgroups of the control group. Compared to ADPKD patients the risk of UTI and urosepsis was lower in patients with primary glomerulopathy, but higher in patients with obstructive nephropathy/chronic pyelonephritis.
Table 3a:
UTI and urosepsis: matched-pair comparison between ADPKD patients and control group.
|
ADPKD
(n = 161) |
Control
(n = 161) |
OR a | 95%CI | P value | |
|---|---|---|---|---|---|
| Patients with UTI or urosepsis during initial hospitalization, n (%) | 14 (8.8) | 12 (8.2) | 1.09 | 0.49; 2.43 | .841 |
| Entire observation period | |||||
| Patients with UTI, n (%) | 63 (39.1) | 43 (26.7) | 1.81 | 1.09; 3.02 | .022 |
| UTI episodes per patient, mean ± SD | 0.8 ± 1.4 | 0.5 ± 1.1 | 1.68 | 1.27; 2.24 | <.001 |
| Patients with urosepsis, n (%) | 34 (21.1) | 28 (17.4) | 1.26 | 0.68; 2.33 | .456 |
| Urosepsis episodes per patient, mean ± SD | 0.3 ± 0.8 | 0.2 ± 0.5 | 1.49 | 0.96; 2.29 | .074 |
| Patients with UTI or urosepsis, n (%) | 71 (44.1) | 55 (34.2) | 1.65 | 0.99; 2.74 | .055 |
| UTI and urosepsis episodes per patient, mean ± SD | 1.1 ± 1.9 | 0.7 ± 1.4 | 1.63 | 1.28; 2.01 | <.001 |
Patients were censored from the time at which both native kidneys had been removed. In 22 ADPKD patients and in 9 control patients both native kidneys had been removed before Tx resulting in 161 matched pairs (in three pairs both native kidneys had been removed before Tx in both, the ADPKD patient and the control patient).
Odds ratio for ADPKD patients versus control patients estimated by a conditional logistic regression model adjusted for unilateral nephrectomy before Tx.
Abbreviations: ADPKD, polycystic kidney disease; CI, confidence interval; OR, odds ratio; SD, standard deviation; Tx, transplantation; UTI, urinary tract infection.
Table 3b:
UTI and urosepsis during the entire observation period: comparison between ADPKD patients and control subgroups.
| ADPKD a (n = 161) | Diabetic nephropathy (n = 16) | Vascular nephropathy (n = 18) | Primary glomerulopathy (n = 59) | Systemic disease b (n = 8) | Hereditary kidney disease b (n = 7) | Obstructive nephropathy/chronic pyelonephritis (n = 20) | Miscellaneous b (n = 12) | Undetermined (n = 21) | |
|---|---|---|---|---|---|---|---|---|---|
| Patients with UTI, n (%), P | 63 (39.1) | 6 (37.5), .938 | 4 (22.2), .234 | 9 (15.3), .002 | 1 (12.5) | 1 (14.3) | 10 (50.0), 0.318 | 4 (33.3) | 6 (28.6), 0.382 |
| UTI episodes per patient, mean ± SD, P | 0.8 ± 1.4 | 0.9 ± 1.7, 0.636 | 0.5 ± 1.1, 0.253 | 0.3 ± 0.7, 0.001 | 0.1 ± 0.4 | 0.1 ± 0.4 | 1.1 ± 1.6, 0.076 | 0.3 ± 0.5 | 0.6 ± 1.1, 0.041 |
| Patients with urosepsis, n (%), P | 34 (21.1) | 4 (25.0), 0.748 | 5 (27.8), 0.358 | 7 (11.9), 0.166 | 2 (25.0) | 2 (28.6) | 4 (20.0), 0.872 | 1 (8.3) | 3 (14.3), 0.434 |
| Urosepsis episodes per patient, mean ± SD, P | 0.3 ± 0.8 | 0.5 ± 1.0, 0.366 | 0.4 ± 0.6, 0.687 | 0.1 ± 0.3, 0.028 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.7, 0.596 | 0.1 ± 0.3 | 0.2 ± 0.5, 0.097 |
| Patients with UTI or urosepsis, n (%), P | 71 (44.1) | 8 (50.0), 0.654 | 7 (38.9), 0.830 | 13 (22.0), 0.006 | 3 (37.5) | 2 (28.6) | 10 (50.0), 0.543 | 4 (33.3) | 6 (28.6), 0.178 |
| UTI and urosepsis episodes per patient, mean ± SD, P | 1.1 ± 1.9 | 1.5 ± 2.0, 0.349 | 0.8 ± 1.3, 0.548 | 0.4 ± 1.0, 0.001 | 0.5 ± 0.8 | 0.4 ± 0.8 | 1.4 ± 2.1, 0.032 | 0.4 ± 0.7 | 0.8 ± 1.3, 0.002 |
Patients were censored from the time at which both native kidneys had been removed. In 22 ADPKD patients and in 9 control patients both native kidneys had been removed before Tx resulting in 161 matched pairs (in three pairs both native kidneys had been removed before Tx in both, the ADPKD patient and the control patient).
Reference group.
Infection rates and number of infections were not compared to ADPKD patients because of limited group size.
P value for ADPKD patients versus control subgroups estimated by a conditional logistic regression model adjusted for unilateral nephrectomy before Tx.
Abbreviations: ADPKD, polycystic kidney disease; SD, standard deviation; Tx, transplantation; UTI, urinary tract infection.
In eight ADPKD patients (two females/six males) altogether 19 liver cyst infections occurred, eight of which with a septic course. One patient experienced eight episodes of liver cyst infection necessitating inpatient treatment, six of which with a septic course. The patient finally died with a functioning graft because of a septic liver cyst infection. Another patient also died because of severe liver cyst infection, but was not included in the statistics, because she died a few weeks after her kidney failed.
Patient survival, graft survival and graft function
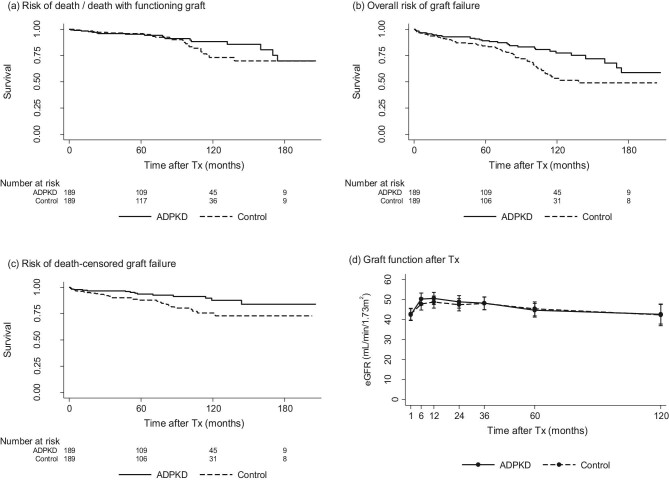
Figure 1a shows that the risk of death/death with functioning graft in ADPKD patients was comparable to matched controls. Altogether, 24/189 patients of the ADPKD cohort died, 7/24 because of infectious events, 7/24 because of malignancy, and 8/24 because of cardiovascular events. In 2/24 patients, the cause of death remained unknown. In the control cohort, 25/189 patients died, 4/25 because of infectious events, 5/25 because of malignancy, and 8/25 because of cardiovascular events. In 8/25 cases the cause of death was unknown.
Figure 1:
Matched-pair comparison between ADPKD patients and matched control group. (a) Risk of death/death with functioning graft (HR 0.92; 95%CI 0.52; 1.61; P = .791). (b) Overall risk of graft failure (HR 0.67; 95%CI 0.45; 0.99; P = .047). (c) Risk of death-censored graft failure (HR 0.47; 95%CI 0.24; 0.87; P = .014). (d) Graft function shown as eGFRcr (mean; 95%CI) (beta 0.26; 95%CI −2.82; 3.34; P = .868); number of ADPKD patients and control patients, at baseline: 189/189, 6 months: 182/184, 12 months: 174/179, 24 months: 164/163, 36 months: 147/148, 60 months: 113/109 and 120 months: 53/63. Abbreviations: ADPKD, polycystic kidney disease; CI, confidence interval; HR, hazard ratio; Tx, transplantation.
The overall risk of graft failure was lower in ADPKD patients as compared to their matched controls (Fig. 1b). This difference was mainly caused by a reduced risk of death-censored graft failure (Fig. 1c). As expected, the frequency of graft loss due to recurrence of the underlying disease was lower in the ADPKD group (0 vs. 5) and cardiorenal syndrome (1 vs. 6) was also less frequent. Graft loss due to rejection (9 vs. 12) and calcineurin-inhibitor nephrotoxicity (2 vs. 1) were comparable.
Concerning the eGFR course during follow-up estimated by eGFRcr according to the CKD-EPI formula [9] with imputation for graft loss, we observed no significant differences between both groups (Fig. 1d).
Risk factor analysis
Nearly one half of all ADPKD patients (82/193, 42.5%) experienced at least one major change of maintenance immunosuppression during the observation period, for example from a calcineurin-inhibitor containing to a calcineurin-inhibitor-free regimen. In 27/193 (14.0%) patients, maintenance immunosuppression was changed more than once. Therefore, maintenance immunosuppression was excluded from the analysis of risk factors for patient survival, graft survival, and graft function. Concerning UTIs and urosepsis, however, immunosuppression at diagnosis was included.
Risk factors for UTI and urosepsis during the initial hospitalization for Tx are shown in Table 4. Immunosuppression refers to the initial treatment after Tx. Steroids were not included because at this early stage all patients received steroids. Univariable analysis indicates that tacrolimus and rejection increased the risk of UTI/urosepsis, while cyclosporine decreased it. Multivariable analysis confirmed the effect of tacrolimus and rejection.
Table 4:
Risk factors for UTI and urosepsis during the initial Tx hospitalization in ADPKD patients.
| Univariable | Multivariable b | |||||||
|---|---|---|---|---|---|---|---|---|
| UTI or urosepsis during initial Tx hospitalization | Yes n = 14 | No n = 157 | RR a | 95%CI | P value | RR a | 95%CI | P value |
| Recipient age at Tx, mean ± SD (per 5 years) | 58.2 ± 8.8 | 54.9 ± 10.7 | 1.18 | 0.92; 1.51 | .198 | 1.10 | 0.71; 1.69 | .679 |
| Waiting time, mean ± SD (per 12 months) | 47.7 ± 37.3 | 43.4 ± 38.2 | 1.09 | 0.96; 1.23 | .180 | |||
| Female recipient, n (%) (yes vs no) | 6 (42.9) | 69 (44.0) | 0.87 | 0.32; 2.35 | .781 | |||
| Recipient diabetes mellitus, n (%) (yes vs no) | 3 (21.4) | 20 (12.9) | 1.77 | 0.54; 5.84 | .346 | |||
| Recipient BMI, mean ± SD (per 1 kg/m2) | 27.4 ± 3.3 | 25.9 ± 4.0 | 1.04 | 0.92; 1.17 | .501 | |||
| Recipient CCI score, mean ± SD (per 1 point) | 3.9 ± 1.0 | 3.5 ± 1.3 | 1.35 | 0.94; 1.93 | .104 | 1.13 | 0.59; 2.19 | .707 |
| Unilateral nephrectomy before Tx, n (%) (yes vs no) | 4 (28.6) | 57 (36.3) | 0.70 | 0.21; 2.34 | .564 | |||
| Donor age, mean ± SD (per 5 years) | 59.0 ± 11.2 | 54.8 ± 13.7 | 1.00 | 0.84; 1.20 | .966 | |||
| Donor eGFRcr, mean ± SD (per 5 ml/min/1.73 m2) | 80.0 ± 27.8 | 83.9 ± 24.0 | 1.01 | 0.92; 1.12 | .781 | |||
| Living donor, n (%) (yes vs no) | 3 (21.4) | 60 (38.2) | c | |||||
| Preemptive Tx, n (%) (yes vs no) | 2 (14.3) | 21 (13.4) | 0.92 | 0.20; 4.27 | .912 | |||
| AB0 compatible Tx, n (%) (yes vs no) | 12 (85.7) | 143 (91.1) | 0.70 | 0.14; 3.34 | .650 | |||
| Basiliximab, n (%) (yes vs no) | 13 (92.9) | 148 (94.3) | 0.92 | 0.11: 7.72 | .940 | |||
| Cyclosporine A, n (%) (yes vs no) | 3 (21.4) | 69 (44.0) | 0.23 | 0.06; 0.81 | .023 | |||
| Tacrolimus, n (%) (yes vs no) | 11 (78.6) | 84 (53.5) | 4.83 | 1.35; 17.28 | .015 | 6.44 | 1.66; 25.10 | .007 |
| Mycophenolate, n (%) (yes vs no) | 12 (85.7) | 147 (93.6) | 0.34 | 0.08; 1.34 | .121 | |||
| Rejection episode, n (%) (yes vs no) | 8 (57.1) | 32 (20.4) | 3.20 | 1.18; 8.71 | .023 | 5.24 | 1.68; 16.30 | .004 |
Immunosuppression refers to the initial treatment after transplantation. Patients were censored from the time at which both native kidneys had been removed. In 22 patients both native kidneys had been removed before transplantation. Steroids were not included because at this early stage after Tx all patients received steroids. Immunosuppression applied in <10 cases was excluded from the analysis to assure reliable risk estimates: belatacept, bortezomib, everolimus, fingolimod, plasmapheresis/intravenous immunoglobulins, rituximab, sotrastaurin, thymoglobulin.
Relative risk estimated by a generalized linear model by Poisson regression with robust error variance.
Multivariable model was identified by LASSO regression.
Parameter was not analyzed because of small population of cells in cross table.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; SD, standard deviation; Tx, transplantation.
Table 5 shows the risk factors for UTI and urosepsis during the entire observation period. Maintenance immunosuppression at the time of diagnosis was included by comparing the number of episodes of UTI/urosepsis on a specific substance with the total exposure time of all ADPKD patients on this substance. According to the univariable analysis recipient age, comorbidity, donor age, donor eGFRcr, and rejection episodes as well as the use of steroids and tacrolimus influenced the risk of UTI/urosepsis. Multivariable analysis shows that only increasing recipient age and steroid medication increased the risk.
Table 5:
Risk factors for episodes of UTI and urosepsis during the entire observation period in ADPKD patients.
| Univariable | Multivariable b | |||||
|---|---|---|---|---|---|---|
| UTI and/or urosepsis during the entire observation period | RR a | 95%CI | P value | RR a | 95%CI | P value |
| Recipient age at Tx (per 5 years) | 1.06 | 1.03; 1.10 | .001 | 1.05 | 1.02; 1.08 | .003 |
| Waiting time (per 12 months) | 1.00 | 0.99; 1.01 | .396 | |||
| Female recipient (yes vs no) | 1.42 | 0.68; 2.97 | .356 | |||
| Recipient diabetes mellitus (yes vs no) | 0.58 | 0.20; 1.70 | .322 | |||
| Recipient BMI (per 1 kg/m2) | 1.00 | 0.91; 1.10 | .923 | |||
| Recipient CCI score (per 1 point) | 1.52 | 1.15; 2.02 | .003 | |||
| Unilateral nephrectomy before Tx (yes vs no) | 0.57 | 0.26; 1.24 | .157 | 0.64 | 0.32; 1.29 | .209 |
| Donor age (per 5 years) | 1.03 | 1.01; 1.06 | .011 | |||
| Donor eGFRcr (per 5 ml/min/1.73 m2) | 0.98 | 0.97; 1.00 | .040 | |||
| Living donor (yes vs no) | 0.48 | 0.22; 1.06 | .071 | |||
| Preemptive Tx (yes vs no) | 0.80 | 0.26; 2.45 | .703 | |||
| AB0compatible Tx (yes vs no) | 1.47 | 0.34; 6.41 | .605 | |||
| Steroids (yes vs no) | 3.06 | 2.12; 4.43 | <.001 | 3.04 | 2.11; 4.38 | <.001 |
| Cyclosporine A (yes vs no) | 1.87 | 0.70; 4.94 | .221 | |||
| Tacrolimus (yes vs no) | 0.57 | 0.32; 0.98 | .039 | |||
| Everolimus (yes vs no) | 0.79 | 0.39; 1.60 | .513 | |||
| Belatacept (yes vs no) | 0.47 | 0.17; 1.27 | .135 | 0.61 | 0.24; 1.56 | .306 |
| Mycophenolate (yes vs no) | 1.67 | 0.76; 3.67 | .204 | |||
| Rejection episode (yes vs no) | 2.58 | 1.11; 5.95 | .027 | 2.01 | 0.98; 4.12 | .056 |
Patients were censored from the time at which both native kidneys had been removed. In 22 patients both native kidneys had been removed before Tx. In 15 patients the second kidney was removed after Tx. Maintenance immunosuppression changed during the observation period. Immunosuppression at diagnosis is shown. Immunosuppression with <10 years of total exposure time (entire observation period of all patients) was excluded to assure reliable estimates: basiliximab, fingolimod, sotrastaurin.
Relative risk estimated by a multilevel mixed-effects Poisson regression model.
Multivariable model was identified by LASSO regression.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; Tx, transplantation.
Additionally, we compared the risk for UTI/urosepsis between ADPKD patients after removal of the first native kidney (n = 74) and ADPKD patients in whom both native kidneys were in situ (n = 110). The incidence rate of combined UTI/urosepsis episodes (related to time under risk) was reduced after removal of the first native kidney, although not significantly (RR 0.60; 95%CI 0.33; 1.08; P = .088). In comparison, the incidence rate of combined UTI/urosepsis episodes was significantly lower after nephrectomy of the second native kidney (n = 37) compared to patients in whom at least one native kidney was in situ (n = 171) (RR 0.45; 95%CI 0.23; 0.88; P = .020).
Risk factors for death/death with functioning graft are shown in Table 6. Univariable analysis indicates that recipient age, waiting time, comorbidity, donor age, and donor eGFRcr had a significant influence on both outcomes. Multivariable analysis shows that only waiting time and comorbidity significantly influenced the risk of death/death with functioning graft.
Table 6:
Risk factors for death/death with functioning graft in ADPKD patients.
| Univariable | Multivariable b | |||||||
|---|---|---|---|---|---|---|---|---|
| Death/death with functioning graft | Yes n = 25 | No n = 168 | HR a | 95%CI | P value | HR a | 95%CI | P value |
| Recipient age at Tx, mean ± SD (per 5 years) | 60.6 ± 12.1 | 54.3 ± 9.7 | 1.50 | 1.20; 1.88 | <.001 | |||
| Waiting time, mean ± SD (per 12 months) | 69.8 ± 44.9 | 44.7 ± 40.9 | 1.14 | 1.05; 1.24 | .003 | 1.12 | 1.02; 1.23 | .017 |
| Female recipient, n (%) (yes vs no) | 11 (44.0) | 70 (41.7) | 1.05 | 0.47; 2.32 | .907 | |||
| Recipient diabetes mellitus, n (%) (yes vs no) | 6 (25.0) | 22 (13.2) | 1.40 | 0.55; 3.56 | .481 | |||
| Recipient BMI, mean ± SD (per 1 kg/m2) | 25.1 ± 3.9 | 26.1 ± 3.9 | 0.96 | 0.86; 1.08 | .504 | |||
| Recipient CCI score, mean ± SD (per 1 point) | 4.5 ± 1.5 | 3.5 ± 1.2 | 1.81 | 1.36; 2.41 | <.001 | 1.74 | 1.31; 2.31 | <.001 |
| Unilateral nephrectomy before Tx, n (%) (yes vs no) | 9 (36.0) | 52 (31.0) | 1.07 | 0.47; 2.44 | .865 | |||
| Bilateral nephrectomy before Tx, n (%) (yes vs no) | 4 (16.0) | 18 (10.7) | 1.36 | 0.47; 3.99 | .570 | |||
| Donor age, mean ± SD (per 5 years) | 58.2 ± 13.9 | 53.5 ± 13.9 | 1.16 | 1.01; 1.35 | .042 | |||
| Donor eGFRcr, mean ± SD (per 5 ml/min/1.73 m2) | 73.4 ± 29.0 | 84.9 ± 23.6 | 0.93 | 0.87; 0.99 | .040 | |||
| Living donor, n (%) (yes vs no) | 4 (16.0) | 64 (38.1) | 0.35 | 0.12; 1.02 | .054 | |||
| Preemptive Tx, n (%) (yes vs no) | 1 (4.0) | 22 (13.1) | c | |||||
| AB0 compatible Tx, n (%) (yes vs no) | 25 (100.0) | 152 (90.5) | c | |||||
| Rejection episode, n (%) (yes vs no) | 5 (20.0) | 38 (22.6) | 0.98 | 0.36; 2.64 | .970 | |||
| Patients with UTI or urosepsis, n (%) (yes vs no) | 15 (60.0) | 71 (42.3) | 2.01 | 0.90; 4.52 | .090 | |||
Hazard ratio estimated by Cox-proportional hazard model.
Multivariable model was identified by LASSO regression.
Parameter was not analyzed because of small population of cells in cross table.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; Tx, transplantation; UTI, urinary tract infection.
Table 7 shows risk factors for death-censored graft failure. Univariable analysis indicates that recipient age, comorbidity, donor age, rejection, and UTI/urosepsis had a significant influence on the risk of death-censored graft failure. Multivariable analysis shows that only donor age and rejection significantly influenced the risk. UTI/urosepsis is not included in the multivariable model, because its association with death-censored graft failure is explained by rejection as shown in the combined model of both variables [hazard ratio (HR) 9.10; P < .001 for rejection and HR 2.78; P = .185 for UTI/urosepsis].
Table 7:
Risk factors for death-censored graft failure in ADPKD patients.
| Yes | No | Univariable | Multivariable b | |||||
|---|---|---|---|---|---|---|---|---|
| Death-censored graft failure | n = 17 | n = 176 | HR a | 95%CI | P value | HR a | 95%CI | P value |
| Recipient age at Tx, mean ± SD (per 5 years) | 61.6 ± 9.5 | 54.5 ± 10.1 | 1.66 | 1.22; 2.26 | .001 | |||
| Waiting time, mean ± SD (per 12 months) | 46.5 ± 32.7 | 48.1 ± 43.0 | 1.00 | 0.86; 1.17 | .975 | |||
| Female recipient, n (%) (yes vs no) | 9 (52.9) | 72 (40.9) | 1.39 | 0.50; 3.85 | .522 | |||
| Recipient diabetes mellitus, n (%) (yes vs no) | 1 (5.9) | 27 (15.5) | c | |||||
| Recipient BMI, mean ± SD (per 1 kg/m2) | 26.5 ± 3.5 | 25.9 ± 3.9 | 1.08 | 0.95; 1.23 | .242 | |||
| CCI score, mean ± SD (per 1 point) | 4.2 ± 1.3 | 3.5 ± 1.3 | 1.67 | 1.14; 2.43 | .008 | |||
| Unilateral nephrectomy before Tx, n (%) (yes vs no) | 4 (23.5) | 57 (32.4) | c | |||||
| Bilateral nephrectomy before Tx, n (%) (yes vs no) | 1 (5.9) | 21 (11.9) | c | |||||
| Donor age, mean ± SD (per 5 years) | 65.2 ± 10.3 | 53.0 ± 13.8 | 1.44 | 1.17; 1.76 | <.001 | 1.34 | 1.12; 1.61 | .002 |
| Donor eGFRcr, mean ± SD (per 5 ml/min/1.73 m2) | 73.1 ± 23.4 | 84.4 ± 24.5 | 0.93 | 0.85; 1.01 | .073 | |||
| Living donor, n (%) (yes vs no) | 3 (17.7) | 65 (36.9) | c | |||||
| Preemptive Tx, n (%) (yes vs no) | 0 (0.0) | 23 (13.1) | c | |||||
| AB0 compatible Tx, n (%) (yes vs no) | 15 (88.2) | 162 (92.1) | 0.22 | 0.05; 1.06 | .059 | |||
| Rejection episode, n (%) (yes vs no) | 12 (70.6) | 31 (17.6) | 10.34 | 3.25; 32.90 | <.001 | 8.47 | 2.63; 27.31 | <.001 |
| Patients with UTI or urosepsis, n (%) (yes vs no) | 11 (64.7) | 75 (42.6) | 3.60 | 1.14; 11.40 | .029 | |||
Hazard ratio estimated by Cox-proportional hazard model.
Multivariable model was identified by LASSO regression.
Parameter was not analyzed because of small population of cells in cross table.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; Tx, transplantation; UTI, urinary tract infection.
In Table 8, the risk factors for overall graft failure are summarized. Univariable analysis indicates that recipient age, waiting time, comorbidity, donor age, donor eGFRcr, donor type, rejection, and UTI/urosepsis all had a significant influence on the overall risk of graft failure. Supporting the results of our previous analyses on death with functioning graft (Table 6) and death-censored graft survival (Table 7), multivariable analysis confirmed that waiting time, comorbidity, donor age, and rejection all significantly contribute to the overall risk of graft failure. The effect of UTI/urosepsis on overall graft failure is explained by rejection as the joint analysis of rejection and UTI/urosepsis shows (HR 2.27; P = .016 for rejection and HR 2.34; P = .116 for UTI/urosepsis).
Table 8:
Risk factors for overall graft failure in ADPKD patients.
| Univariable | Multivariable b | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall graft failure | Yes n = 42 | No n = 151 | HR a | 95%CI | P value | HR a | 95%CI | P value |
| Recipient age at Tx, mean ± SD (per 5 years) | 61.0 ± 11.0 | 53.5 ± 9.4 | 1.56 | 1.30; 1.87 | <.001 | |||
| Waiting time, mean ± SD (per 12 months) | 60.4 ± 41.6 | 44.5 ± 41.8 | 1.10 | 1.02; 1.18 | .017 | 1.14 | 1.02; 1.27 | .025 |
| Female recipient, n (%) (yes vs no) | 20 (47.6) | 61 (40.4) | 1.17 | 0.63; 2.18 | .625 | |||
| Recipient diabetes mellitus, n (%) (yes vs no) | 7 (17.1) | 21 (14.0) | 0.90 | 0.40; 2.07 | .812 | |||
| Recipient BMI, mean ± SD (per 1 kg/m2) | 25.6 ± 3.8 | 26.0 ± 4.0 | 1.01 | 0.93; 1.10 | .857 | |||
| Recipient CCI score, mean ± SD (per 1 point) | 4.4 ± 1.4 | 3.4 ± 1.2 | 1.76 | 1.40; 2.21 | <.001 | 1.41 | 1.08; 1.85 | .013 |
| Unilateral nephrectomy before Tx, n (%) (yes vs no) | 13 (31.0) | 48 (31.8) | 0.94 | 0.48; 1.83 | .858 | |||
| Bilateral nephrectomy before Tx, n (%) (yes vs no) | 5 (11.9) | 17 (11.3) | 1.00 | 0.39; 2.56 | .999 | |||
| Donor age, mean ± SD (per 5 years) | 61.1 ± 12.9 | 52.2 ± 13.6 | 1.26 | 1.12; 1.42 | <.001 | 1.18 | 1.03; 1.35 | .014 |
| Donor eGFRcr, mean ± SD (per 5 ml/min/1.73 m2) | 73.3 ± 26.6 | 86.3 ± 23.3 | 0.93 | 0.88; 0.98 | .006 | 0.99 | 0.92; 1.05 | .658 |
| Living donor, n (%) (yes vs no) | 7 (16.7) | 61 (40.4) | 0.34 | 0.14; 0.80 | .014 | 0.98 | 0.33; 2.94 | .976 |
| Preemptive Tx, n (%) (yes vs no) | 1 (2.4) | 22 (14.6) | c | |||||
| AB0 compatible Tx, n (%) (yes vs no) | 40 (95.2) | 137 (90.7) | 0.70 | 0.16; 2.96 | 0626 | |||
| Rejection episode, n (%) (yes vs no) | 17 (40.5) | 26 (17.2) | 2.59 | 1.36; 4.92 | .004 | 2.20 | 1.10; 4.40 | .026 |
| Patients with UTI or urosepsis, n (%) (yes vs no) | 26 (61.9) | 60 (39.7) | 2.47 | 1.28; 4.75 | .007 | |||
Hazard ratio estimated by Cox-proportional hazard model.
Multivariable model was identified by LASSO regression.
Parameter was not analyzed because of small population of cells in cross table.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; HR, hazard ratio; SD, standard deviation; Tx, transplantation; UTI, urinary tract infection.
Factors influencing the eGFRcr course over time are shown in Table 9. Univariable analysis indicates that recipient age, comorbidity, donor age, donor eGFRcr, donor type, preemptive Tx, and rejection had a significant influence on the eGFRcr course. Multivariable analysis shows that increasing donor age and rejection had a negative impact on the eGFRcr course while living donor status had a positive impact.
Table 9:
Factors influencing the eGFRcr course in ADPKD patients.
| Univariable | Multivariable b | |||||
|---|---|---|---|---|---|---|
| eGFRcr course | beta a | 95%CI | P value | beta a | 95%CI | P value |
| Recipient age at Tx (per 5 years) | −3.58 | −4.77; −2.39 | <.001 | 0.13 | −1.59; 1.85 | .879 |
| Waiting time (per 1 year) | −0.39 | −1.14; 0.37 | .313 | |||
| Female recipient (yes vs no) | −1.93 | −7.29; 3.42 | .479 | |||
| Recipient diabetes mellitus (yes vs no) | 0.80 | −6.72; 8.32 | .835 | |||
| Recipient BMI (per 1 point) | −0.26 | −0.94; 0.41 | .446 | |||
| Recipient CCI score (per 1 point) | −4.92 | −6.86; −2.98 | <.001 | −1.47 | −3.94; 1.00 | 0.245 |
| Unilateral nephrectomy before Tx (yes vs no) | 3.99 | −1.66; 9.64 | .166 | |||
| Bilateral nephrectomy before Tx (yes vs no) | 4.40 | −3.86; 12.66 | .296 | |||
| Donor age (per 5 years) | −3.36 | −4.18; −2.54 | <.001 | −2.69 | −3.54; −1.84 | <.001 |
| Donor eGFRcr (per 5 ml/min/1.73 m2) | 1.25 | 0.74; 1.76 | <.001 | 0.44 | −0.02; 0.90 | .063 |
| Living donor (yes vs no) | 11.81 | 6.52; 17.10 | <.001 | 7.38 | 1.98; 12.78 | .007 |
| Preemptive Tx (yes vs no) | 9.51 | 1.38; 17.65 | .022 | 0.35 | −6.96; 7.65 | .926 |
| AB0 compatible Tx (yes vs no) | −0.50 | −10.21; 9.20 | .919 | |||
| Rejection episode (yes vs no) | −13.42 | −19.46; −7.37 | <.001 | −13.07 | −17.98; −8.16 | <.001 |
| Patients with UTI or urosepsis (yes vs no) | −0.72 | −2.57; 1.13 | .444 | |||
eGFRcr in follow-up was analyzed by a multilevel mixed-effects linear regression model.
Multivariable model was identified by LASSO regression.
Abbreviations: ADPKD, polycystic kidney disease; BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; Tx, transplantation; UTI, urinary tract infection.
DISCUSSION
In this single-center case-control study we compared the occurrence, frequency, and severity of UTIs and liver cyst infections in hospitalized ADPKD patients transplanted between 2000 and 2017 with a matched control group. In addition, we analyzed patient survival, graft survival, and graft function as well as risk factors for the corresponding endpoints.
More ADPKD patients experienced more episodes of UTI necessitating hospitalization during the entire observation period compared to controls. Increasing recipient age and the use of steroids increased the risk of UTI/urosepsis. During the initial hospitalization at transplantation no such difference was evident probably reflecting the routine use of antibiotic prophylaxis [16]. Notably, DJ stents were routinely removed at 3–6 weeks after Tx and nephrolithiasis did not play an important role, as it was detected in only two patients of each group. We deliberately decided to assess episodes of UTI and urosepsis requiring hospitalization because in our experience despite intense follow-up a considerable part of uncomplicated UTI episodes is treated by local nephrologists or general practitioners and may therefore escape our notice. In accordance with our results, Stiasny et al. [17] and Jänigen et al. [18] also observed an increased incidence of UTI in ADPKD patients, while Hadimeri et al. [19] and Jacquet et al. [20] did not. Detailed analyses on the necessity of inpatient treatment, the severity and frequency of infections as well as the underlying risk factors are not reported.
Sulikowski et al. described that nephrectomy before Tx may decrease the number of posttransplant UTIs [21]. Therefore, we included unilateral nephrectomy as independent variable in the risk factor analysis and censored patients from the time at which both native kidneys had been removed, i.e. the suggested “risk factor” was no longer present. We found that unilateral and bilateral nephrectomy of the native kidneys reduce the risk of UTI/urosepsis. To our knowledge, this is the first report confirming the generalized assumption that nephrectomy of the native kidneys may in fact reduce the risk of infection.
There is a considerable amount of literature concerning the timing and technique of native nephrectomy in ADPKD patients [22–26]. In our view, the timing and technique of native nephrectomy in these patients remains an individual decision in which the specific indication for nephrectomy and the availability of a potential donor play a central role. At our center, we do not perform simultaneous native nephrectomy and kidney transplantation.
Notably, 8/193 patients experienced liver cyst infection, and two of these patients even died of severe liver cyst infection, one patient with functioning graft and another patient soon after graft failure. Altogether, seven patients in the ADPKD cohort died with functioning graft because of infectious events compared to four patients of the control group. These data underline that ADPKD is a systemic disease and that infections of both kidney and liver cysts constitute serious complications after Tx.
Schellekens et al. recently described lower peripheral WBC counts in ADPKD patients as compared to non-ADPKD patients before and after transplantation [27]. Our results agree with these data. Whether or not lower WBC counts represent an additional risk factor for infection, which is independent of the underlying kidney disease and pathogenetically relevant is an interesting question that should be addressed in future prospective studies.
Patient survival in ADPKD patients was similar compared to non-ADPKD patients. In this regard, our results are consistent with most of the existing literature [17–20, 28–30]. Graft survival in ADPKD patients was superior compared to controls. Some previous studies also found improved graft survival in ADPKD patients [20, 27, 28] while others described comparable graft survival [17–19, 29, 30]. We therefore analyzed graft survival in depth and found that especially death-censored graft survival was superior in ADPKD patients. The missing risk of recurrence of the underlying kidney disease and a reduced risk of cardiorenal syndrome may partially explain this phenomenon. Major risk factors for death with functioning graft were waiting time and comorbidity; major risk factors for death-censored graft failure were donor age and rejection. UTI/urosepsis seemed to be a significant risk factor for death-censored graft failure in the univariable analysis, but not in the multivariable analysis. This can be explained by the fact that rejection episodes were usually treated with steroids, and steroid treatment increased the risk of UTI/urosepsis.
Most of the existing studies date back more than one [20, 28] or even two [17, 19, 29, 30] decades. Since that time the (peri)transplant procedure including immunosuppression has markedly changed. We deliberately included patients transplanted from 2000 onwards to assure that most patients received current standard immunosuppression including anti-IL-2R-induction together with steroids, calcineurin inhibitors, and mycophenolate. In a more recent study, Bhutani et al. compared PKD patients with non-PKD patients transplanted between 1994 and 2014 [31]. They also found that the risk of death-censored graft failure was lower in PKD patients.
Some of the previously mentioned studies used a matched-pair design [17, 19, 29] using three matching variables while others compared ADPKD patients with non-ADPKD patients without matching [20, 28, 30, 31]. Several studies excluded diabetics [17, 19, 28–30]. We decided not to exclude diabetics to generate a complete real-world picture.
Our study has several limitations, chief among them the fact that it represents a retrospective single-center study. Although we adjusted for a number of variables, residual confounding due to parameters, which may not have been completely accounted for in our statistical analysis cannot be excluded. We tried to outweigh these limitations by detailed and thorough data recording, by applying a matched-pair design, and by using complex statistical analyses.
In conclusion, our results indicate that ADPKD patients are at increased risk for UTI and liver cyst infection including sepsis requiring inpatient treatment after kidney transplantation compared to non-ADPKD patients. Nevertheless, patient survival seems to be similar and graft survival, especially death-censored graft survival even superior. Recipient age and steroid treatment were associated with an increased risk for UTI/urosepsis, while nephrectomy of native kidneys seems to reduce it. Individually tailored, steroid-free immunosuppression and nephrectomy of one or both native kidneys may help to reduce the risk of recurrent and severe infections in these patients.
Contributor Information
Johannes Waiser, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Jens Klotsche, German Rheumatism Research Center Berlin – a Leibniz Institute, Berlin, Germany.
Petra Glander, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Danilo Schmidt, Business Unit IT, Charité - Universitätsmedizin Berlin, Berlin, Germany.
Marcel Naik, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Lutz Liefeldt, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Klemens Budde, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Jan Halbritter, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Fabian Halleck, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Bianca Zukunft, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Robert Peters, Department of Urology, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Frank Friedersdorff, Department of Urology, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Nils Lachmann, Institute of Transfusion Medicine, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Kai-Uwe Eckardt, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Leonie d'Anjou, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Friederike Bachmann, Department of Nephrology and Medical Intensive Care, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
FUNDING
The authors received no funding for this study.
AUTHORS' CONTRIBUTIONS
J.W., J.K., L.d'A., and F.B. were responsible for conception and design of the work. J.W., P.G., D.S., M.N., N.L., L.d'A., and F.B. were responsible for data acquisition. J.K. and P.G. were responsible for data analysis. J.W., J.K., P.G., L.L., K.B., J.H., N.L., L.d'A., and F.B. were responsible for interpretation of data. J.W., J.K., P.G., L.L., K.B., J.H., F.H., B.Z., R.P., F.F., N.L., K.-U.E., L.d'A., and F.B. were involved in drafting and critically revising the manuscript. All authors reviewed and approved the final manuscript. J.W. and J.K. are co-first authors. L.d'A. and F.B. are co-senior authors.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bergmann C, Guay-Woodford LM, Harris PC et al. Polycystic kidney disease. Nat Rev Dis Primers 2018;4:50. 10.1038/s41572-018-0047-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet 2019;393:919–35. 10.1016/S0140-6736(18)32782-X [DOI] [PubMed] [Google Scholar]
- 3. Chapman AB, Devuyst O, Eckardt K-U et al. for conference participants . Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2015;88:17–27. 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spithoven EM, Kramer A, Meijer E et al. on behalf of the ERA EDTA Registry. ERA-EDTA is the acronym for the European Renal Association—European Dialysis and Transplantation Association. ERA-EDTA Registry collaborators Abad JM, de los Ángeles García BM, Caskey F. et al. Renal replacement therapy for ADPKD in Europe: prevalence and survival. An analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 2014;29 Suppl 4:iv15–v25. 10.1093/ndt/gfu017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanaan N, Devuyst O, Pirson Y. Renal transplantation in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2014;10:455–65. 10.1038/nrneph.2014.104 [DOI] [PubMed] [Google Scholar]
- 6. Deutsche Stiftung Organtransplantation . Jahresbericht Organspende und Transplantation in Deutschland 2021. April, 2022;87. [Google Scholar]
- 7. Schmidt D, Osmanodja B, Pfefferkorn M et al. TBase—an integrated electronic health record and research database for kidney transplant recipients. J Vis Exp 2021; e61971. 10.3791/61971 [DOI] [PubMed] [Google Scholar]
- 8. Pei Y, Obaji J, Dupuis A et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009;20:205–12. 10.1681/ASN.2008050507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dreger NM, Degener S, Ahmad-Nejad P et al. Urosepsis—etiology, diagnosis, and treatment. Dtsch Ärzteblatt Int 2015;112:837–47. 10.3238/arztebl.2015.0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jouret F, Lhommel R, Beguin C et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2011;6:1644–50. 10.2215/CJN.06900810 [DOI] [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 13. Hemmelgarn BR, Manns BJ, Quan H et al. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 2003;42:125–32. 10.1016/s0272-6386(03)00415-3 [DOI] [PubMed] [Google Scholar]
- 14. Grosso G, Corona D, Mistretta A et al. Predictive value of the Charlson Comorbidity Index in kidney transplantation. Transplant Proc 2012;44:1859–63. 10.1016/j.transproceed.2012.06.042 [DOI] [PubMed] [Google Scholar]
- 15. Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis 2005;46:136–42. 10.1053/j.ajkd.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 16. Bachmann F, Adam T, Friedersdorff F et al. Perioperative antibiotic prophylaxis in renal transplantation: a single-center comparison between two regimens and a brief survey among the Eurotransplant renal transplantation centers. World J Urol 2019;37:957–67. 10.1007/s00345-018-2440-2 [DOI] [PubMed] [Google Scholar]
- 17. Stiasny B, Ziebell D, Graf S et al. Clinical aspects of renal transplantation in polycystic kidney disease. Clin Nephrol 2002;58:16–24. 10.5414/cnp58016 [DOI] [PubMed] [Google Scholar]
- 18. Jänigen BM, Hempel J, Holzner P et al. Simultaneous ipsilateral nephrectomy during kidney transplantation in autosomal dominant polycystic kidney disease: a matched pair analysis of 193 consecutive cases. Langenbecks Arch Surg 2020;405:833–42. 10.1007/s00423-020-01939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hadimeri H, Nordén G, Friman S et al. Autosomal dominant polycystic kidney disease in a kidney transplant population. Nephrol Dial Transplant 1997;12:1431–6. 10.1093/ndt/12.7.1431 [DOI] [PubMed] [Google Scholar]
- 20. Jacquet A, Pallet N, Kessler M et al. Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 2011;24:582–7. 10.1111/j.1432-2277.2011.01237.x [DOI] [PubMed] [Google Scholar]
- 21. Sulikowski T, Tejchman K, Zietek Z et al. Experience with autosomal dominant polycystic kidney disease in patients before and after renal transplantation: a 7-year observation. Transplant Proc 2009;41:177–80. 10.1016/j.transproceed.2008.10.034 [DOI] [PubMed] [Google Scholar]
- 22. Jean RA, Alexandre M, Yoo P. Kidney transplantation with and without native nephrectomy for polycystic kidney disease: results of the National Inpatient Sample and the rationale for a 2-staged procedure. J Am Coll Surg 2018;226:1079–84. 10.1016/j.jamcollsurg.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 23. Patel P, Horsfield C, Compton F et al. Native nephrectomy in transplant patients with autosomal dominant polycystic kidney disease. Ann R Coll Surg Engl 2011;93:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasmussen A, Levine MA, Mandurah MM et al. Staged vs. simultaneous bilateral nephrectomy and kidney transplantation in patients with autosomal dominant polycystic kidney disease: outcomes and costs. Can Urol Assoc J 2022;16:424–9. 10.5489/cuaj.7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casteleijn NF, Geertsema P, Koorevaar IW et al. The need for routine native nephrectomy in the workup for kidney transplantation in autosomal dominant polycystic kidney disease patients. Urol Int 2023;107:148–56. 10.1159/000525575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas MN, Datta RR, Wahba R et al. Introduction of laparoscopic nephrectomy for autosomal dominant polycystic kidney disease as the standard procedure. Langenbecks Arch Surg 2023;408:8. 10.1007/s00423-022-02737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schellekens P, van Loon E, Coemans M et al. Leukopenia in autosomal dominant polycystic kidney disease: a single-center cohort of kidney transplant candidates with post-transplantation follow-up. Clin Kidney J 2023;16:2578–86. 10.1093/ckj/sfad165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston O, O'Kelly P, Donohue J et al. Favorable graft survival in renal transplant recipients with polycystic kidney disease. Ren Fail 2005;27:309–14. 10.1081/JDI-56606 [DOI] [PubMed] [Google Scholar]
- 29. Fitzpatrick PM, Torres VE, Charboneau JW et al. Long-term outcome of renal transplantation in autosomal dominant polycystic kidney disease. Am J Kidney Dis 1990;15:535–43. 10.1016/s0272-6386(12)80523-3 [DOI] [PubMed] [Google Scholar]
- 30. Florijn KW, Chang PC, van der Woude FJ et al. Long-term cardiovascular morbidity and mortality in autosomal dominant polycystic kidney disease patients after renal transplantation. Transplantation 1994;57:73–81. 10.1097/00007890-199401000-00014 [DOI] [PubMed] [Google Scholar]
- 31. Bhutani G, Astor BC, Mandelbrot DA et al. Long-term outcomes and prognostic factors in kidney transplant recipients with polycystic kidney disease. Kidney360 2020;2:312–24. 10.34067/KID.0001182019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.