Abstract
Primary malignant lymphomas originating in the adrenal gland, particularly of T-cell origin, are extremely rare. Here we present the primary unilateral adrenal anaplastic large cell lymphoma case. A 64-year-old Japanese male initially presented with fatigue and appetite loss. Computed tomography imaging revealed a unilateral adrenal mass with multiorgan invasion, posing challenges in differentiation from adrenal carcinoma. A biopsy from the metastatic site in the right lateral vastus muscle was obtained, and immunohistochemistry revealed that tumor cells were positive for CD30 and CD56 and negative for CD3, CD15, CD20, CD43, perforin, granzyme B, epithelial membrane antigen, and anaplastic lymphoma kinase. Ultimately, the patient was diagnosed with primary unilateral adrenal anaplastic large cell lymphoma. Although he achieved complete response to chemotherapy, he died 4 months after complete response due to cholecystitis and lymphoma recurrence.
Keywords: unilateral adrenal malignant lymphoma, anaplastic large cell lymphoma
Introduction
Primary malignant lymphomas (PALs) of the adrenal gland, especially of T-cell origin, are exceedingly rare. Most PALs exhibit a B-cell phenotype [1], and they involve both adrenal glands in 75% of cases [2]. As PALs typically lack excretory endocrine function, symptoms arise from the mass's pressure effect [3]. Common manifestations include B symptoms (unexplained fever, weight loss, and night sweats; 68%), vague abdominal pain (42%), and fatigue (36%) [2]. Adrenal insufficiency complicates 61% of PAL cases and is significantly associated with older age, bilaterality, and hyperpigmentation [2].
Here we report the case of primary unilateral adrenal anaplastic large cell lymphoma (ALCL), which posed challenges in differentiation from adrenocortical carcinoma.
Case Presentation
A 64-year-old Japanese male presented with fatigue and appetite loss. He had no history of hematologic or chronic inflammatory disease. Physical examination findings were unremarkable.
Diagnostic Assessment
Initial blood tests revealed elevated white blood cell count (11.2 × 103/μL [11.2 × 109/L], normal range: 4.5-11.0 × 103/μL [4.5-11.0 × 109/μL]). Blood chemistry tests showed elevated levels of γ-glutamyl transpeptidase (115 U/L, normal range: 9-50 U/L), aspartate aminotransferase (82 U/L, normal range: 10-40 U/L), alanine aminotransferase (84 U/L, normal range: 10-40 U/L), lactate dehydrogenase (LDH; 487 U/L [7.9 μkat/L], normal range: 124-222 U/L [1.3-3.8 μkat/L]), C-reactive protein (13.2 mg/dL [125.4 nmol/L], normal range: < 0.14 mg/dL [< 9.5 nmol/L]), ferritin (4940 ng/mL [11 115 pmol/L], normal range: 14.4-303.7 ng/mL [54-755 pmol/L]), IL-6 (117.0 pg/mL [117.0 ng/L], normal range: < 7.0 pg/mL [< 7.0 ng/L]), and soluble interleukin-2 receptor (sIL-2R; 2462 U/mL, normal range: 121-613 U/mL) (Table 1).
Table 1.
Laboratory data obtained upon patient admission
| Test | Result | Normal range | Test | Result | Normal range |
|---|---|---|---|---|---|
| WBC | 11.2 × 103/μL (11.2 × 109/L) |
4.5-11.0 × 103/μL (4.5-11.0 × 109/L) |
Insulin | 4.4 μU/mL (30.5 pmol/L) |
< 18.7 μU/mL (< 138.9 pmol/L) |
| Neu (%) | 62.4 (62.4) |
50.0-70.0 (50.0-70.0) |
ACTH | 35.7 pg/mL (7.8 pmol/L) |
< 46.0 pg/mL (2.2-13.2 pmol/L) |
| Lym (%) | 33.7 (33.4) |
16.0-49.5 (30.0-45.0) |
Cortisol | 7.5 μg/dL (210 nmol/L) |
6.2-19.4 μg/dL (140-690 nmol/L) |
| Eos (%) | 0.2 (0.2) |
0.0-3.0 (0.0-3.0) |
PRA | 4.5 ng/mL/h (106.6 pmol/L) |
0.2-2.7 ng/mL/h (7.11-59.25 pmol/L) |
| RBC | 3.34 × 106/μL (3.34 × 106/μL) |
4.2-5.9 × 106/μL (4.2-5.9 × 106/μL) |
PAC | 15.7 pg/mL (0.04 nmol/L) |
4.0-82.1 pg/mL (≦ 0.3 nmol/L) |
| Hb | 10.0 g/dL (100 g/L) |
13.5-17.0 g/dL (135-170 g/L) |
MN | < 20.0 pg/mL (< 0.1 nmol/L) |
< 130 pg/mL (< 0.5 nmol/L) |
| Plts | 363 × 103/μL (363/L) |
150-350 × 103/μL (150-350/L) |
NMN | 129.8 pg/mL (0.7 nmol/L) |
< 506.0 pg/mL (< 0.9 nmol/L) |
| CRP | 13.2 mg/dL (125.4 nmol/L) |
< 0.14 mg/dL (< 9.5 nmol/L) |
DHEA-S | 263 μg/dL (7.0 μmol/L) |
24-244 μg/dL (2.4-12.4 μmol/L) |
| ALP | 289 U/L (289 U/L) |
30-120 U/L (30-120 U/L) |
TSH | 2.89 μU/mL (2.89 mU/L) |
0.5-4.0 μU/mL (0.5-4.0 mU/L) |
| γ-GTP | 115 U/L (115 U/L) |
9-50 U/L (9-50 U/L) |
FT3 | 1.84 pg/mL (2.8 pmol/L) |
2.2-4.3 pg/mL (3.5-6.5 pmol/L) |
| AST | 82 U/L (82 U/L) |
10-40 U/L (10-40 U/L) |
FT4 | 1.03 ng/dL (13.2 pmol/L) |
0.8-1.6 ng/dL (10.3-23.2 pmol/L) |
| ALT | 84 U/L (84 U/L) |
10-40 U/L (10-40 U/L) |
GH | 1.27 ng/mL (1.27 μg/L) |
<5.0 ng/mL (< 5.0 μg/L) |
| LDH | 487 U/L (7.9 μkat/L) |
124-222 U/L (1.3-3.8 μkat/L) |
IGF-I | 73 ng/mL (9.5 nmol/L) |
74-228 ng/mL (9.3-38.0 nmol/L) |
| TP | 7.0 g/dL (70 mg/dL) |
5.5-9.0 g/dL (55-90 mg/dL) |
PRL | 16.2 ng/mL (16.2 μg/L) |
< 20.0 ng/mL (< 20.0 μg/L) |
| Alb | 2.8 g/dL (28 g/L) |
3.5-5.5 g/dL (35-55 g/L) |
LH | 4.6 mIU/mL (4.6 IU/L) |
2.0-9.0 mIU/L (2.0-9.0 IU/L) |
| UA | 4.7 mg/dL (279.6 μmol/L) |
2.3-7.0 mg/dL (178.5-416.5 μmol/L) |
FSH | 15.0 mIU/mL (15.0 IU/L) |
1.0-7.0 mIU/mL (1.0-7.0 IU/L) |
| BUN | 17 mg/dL (6.1 mmol/L) |
8.0-22.0 mg/dL (2.9-7.1 mmol/L) |
Testosterone | 3.5 ng/dL (121 pmol/L) |
10.8-56.9 ng/dL (243-1040 pmol/L) |
| Cr | 0.72 mg/dL (63.7 μmol/L) |
0.50-0.80 mg/dL (62-115 μmol/L) |
Urinary MN | 0.11 mg/day (0.55 μmol/day) |
0.04-0.20 mg/day (< 2.0 μmol/day) |
| D-dimer | 6.4 μg/mL (38.4 nmol/L) |
< 1.0 μg/mL (< 3.0 nmol/L) |
Urinary NMN | 0.91 mg/day (5.0 μmol/day) |
0.09-0.28 mg/day (< 5.0 μmol/day) |
| HbA1c, % | 5.4 (27.2 mmol/mol) |
4.6-6.2 (20.2-37.7 mmol/mol) |
|||
| IgG | 1943 mg/dL (19.4 g/L) |
800-1500 mg/dL (8-15 g/L) |
|||
| Fe | 20 μg/dL (3.6 μmol/L) |
49-219 μg/dL (9.0-27.0 μmol/L) |
|||
| Ferritin | 4940 ng/mL (11 115 pmol/L) |
14.4-303.7 ng/mL (54-755 pmol/L) |
|||
| IL-6 | 117 pg/mL (117 ng/L) |
< 7.0 pg/mL (< 7.0 ng/L) |
|||
| sIL-2R | 2462 U/mL (2462 U/mL) |
121-613 U/mL (121-613 U/mL) |
|||
| FPG | 97 mg/dL (5.4 mmol/L) |
69-109 mg/dL (3.9-5.5 mmol/L) |
Values in parenthesis are International System of Units.
Abbreviations: Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CPR, C-reactive protein; Cr, creatinine; DHEA-S, dehydroepiandrosterone sulfate; Eos, eosinophil; FPG, fasting plasma glucose; FT3, free T3; FT4, free T4; Hb, hemoglobin; LDH, lactate dehydrogenase; Lym, lymphocytes; MN, metanephrine; Neu, neutrophil; NMN, normetanephrine; PAC, plasma aldosterone concentration; Plt, platelet; PRA, plasma renin activity; PRL, prolactin; RBC, red blood cell; sIL-2R, soluble interleukin-2 receptor; TP, total protein; UA, uric acid; WBC, white blood cell; γ-GTP, γ-glutamyl transpeptidase.
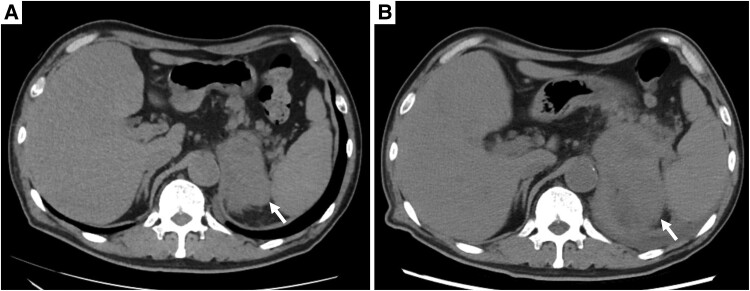
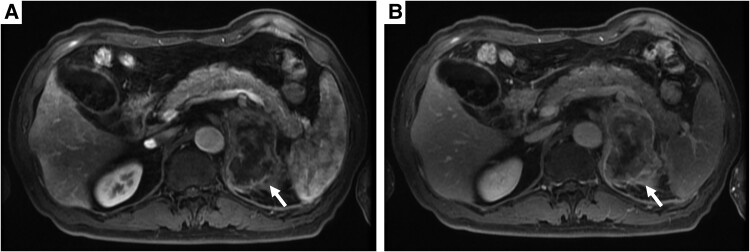
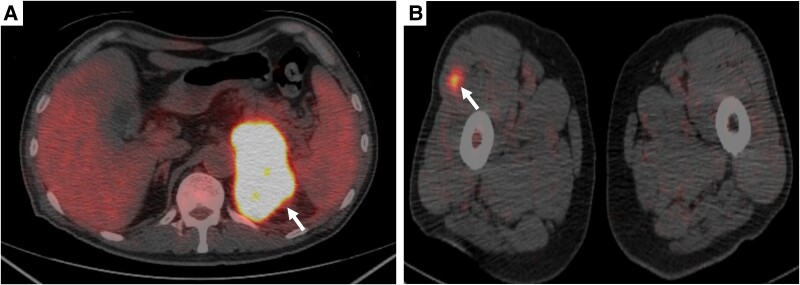
Abdominal computed tomography (CT) at initial examination (Fig. 1A) revealed a unilateral left adrenal mass (approximately 75 mm) without any other organ metastasis. Magnetic resonance imaging showed an internally heterogeneous mass with low T1 and high T2 signal intensity. The adrenal mass exhibited intense staining from the margins (Fig. 2). Owing to persistent low-grade fever and anorexia over a month, we scheduled fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET)/CT scan, and the patient was transferred to our hospital. Fifteen days after the initial examination, fluorine-18-FDG PET/CT (Fig. 3) indicated accumulation in the left adrenal gland [standardized uptake value (SUV) max: 22.5], the small intestine (SUV max: 7.5), the right lateral thigh (SUV max: 4.4), the lymph nodes in the genital area (SUV max: 2.5), and the right lateral vastus muscle (SUV max: 2.5).
Figure 1.
(A) Abdominal CT scan obtained during the initial examination. Left adrenal mass: 75 mm (arrow). Unenhanced attenuation of 35 HUs. (B) Abdominal CT scan obtained postadmission. The left adrenal mass was enlarged to 105 mm (arrow). Unenhanced attenuation of 40 HUs.
Abbreviations: CT, computed tomography; HU, Hounsfield unit.
Figure 2.
Contrast-enhanced magnetic resonance imaging. (A) Early phase; (B) late phase.
Figure 3.
Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. (A) Left adrenal mass (SUV max: 22.5); (B) right lateral vastus muscle (SUV max: 2.5).
Abbreviation: SUV, standardized uptake value.
A follow-up abdominal CT scan revealed progressive enlargement of the left adrenal mass to 105 mm with invasion into the gastric antrum, spleen, splenic vein, and left diaphragm within 1 month (Fig. 1B). On admission, he was initiated on apixaban for lower extremity deep vein thrombosis and required red blood cell transfusion for progressive anemia. Upper gastrointestinal endoscopy showed a gastric ulcer suspected to be due to cancer invasion. Declining food intake necessitated total parenteral nutrition by the eighth day of admission.
Adrenal function tests, including the ACTH–cortisol axis, renin–aldosterone axis, and catecholamine levels, revealed no impairment (plasma metanephrine < 20.0 pg/mL [< 0.1 nmol/L], normal range: < 130 pg/mL [< 0.5 nmol/L]; plasma normetanephrine 129.8 pg/mL [0.7 nmol/L], normal range: < 506.0 pg/mL [< 0.9 nmol/L]; urinary metanephrine 0.11 mg/day [0.55 μmol/day], normal range: 0.04-0.20 mg/day [< 2.0 μmol/day]; urinary normetanephrine 0.91 mg/day [5.0 μmol/day], normal range: 0.09-0.28 mg/day [< 5.0 μmol/day]; plasma ACTH 35.7 pg/mL [7.8 pmol/L], normal range: < 46.0 pg/mL [2.2-13.2 pmol/L]; plasma cortisol 7.5 μg/dL [210 nmol/L], normal range: 6.2-19.4 μg/dL [140-690 nmol/L]; dehydroepiandrosterone sulfate: 263 μg/dL [7.0 μmol/L], normal range: 24-244 μg/dL [2.4-12.4 μmol/L]). Midnight levels of ACTH and cortisol (26.7 pg/mL [5.8 pmol/L] and 7.2 μg/dL [201 nmol/L], respectively) were not sufficiently suppressed (Table 1).
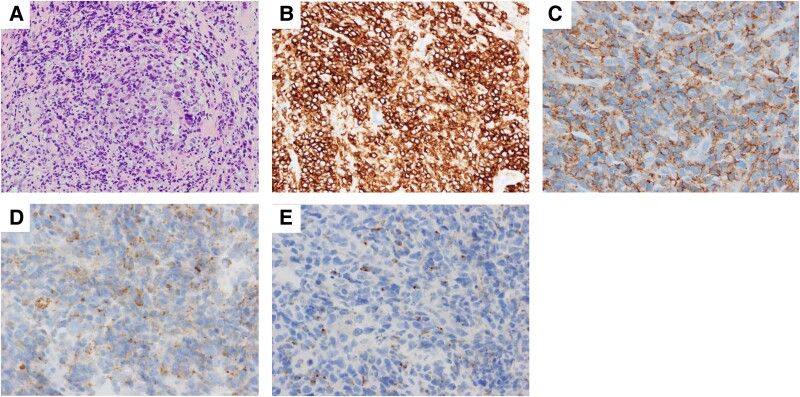
A biopsy from the metastatic site in the right lateral vastus muscle yielded a metastatic muscle mass (Fig. 4A–4C) with a solid and trabecular cell composition as well as numerous mitotic figures. Immunohistochemical analysis demonstrated CD30 and CD56 positivity and CD3, CD15, CD20, CD43, perforin, granzyme B, epithelial membrane antigen, and anaplastic lymphoma kinase (ALK) negativity in tumor cells. Similar histological findings were confirmed in CT-guided biopsy specimens obtained from the left adrenal mass (Fig. 4D and 4E). Based on these features, the patient was diagnosed with ALK-negative ALCL.
Figure 4.
Pathology from the right lateral vastus muscle metastasis site (A–C). (A) Hematoxylin and eosin stain; (B) CD30 stain; (C) CD56 stain. Pathology from left adrenal mass (D and E). (D) Perforin stain; (E) Granzyme stain.
Treatment
Treatment consisted of 8 cycles of brentuximab vedotin (a CD30-directed antibody–drug conjugate) combined with cyclophosphamide, doxorubicin, and prednisone (BV-CHP).
Outcome and Follow-up
The primary adrenal lymphoma decreased in size by 50% and 80% after the first and second cycles of BV-CHP therapy, respectively. The patient achieved complete response following completion of all 8 cycles.
However, 4 months after achieving complete response, the patient developed fever and anorexia. An abdominal CT scan revealed gallbladder enlargement and a bilateral adrenal mass. The patient was diagnosed with cholecystitis and lymphoma recurrence. Despite immediate initiation of IV broad-spectrum antibiotics, he died due to septic shock 10 months after the lymphoma diagnosis.
Discussion
PAL is rare among adrenal nodules, with approximately 250 cases in the English medical literature [3]. Among these cases, PAL with the T-cell phenotype is extremely rare, with only 10 documented cases [4-12], along with 2 cases of natural killer cell type [1, 13] and 1 case of adult T-cell lymphoma [14]. Table 2 summarizes the clinicopathological features of these cases. Previously, only 1 case of ALCL involving bilateral adrenal glands had been reported [15].
Table 2.
Summary of previously reported primary adrenal T/NK cell lymphoma cases
| Case | Age | Sex | Presenting symptoms | Site | Size | Adrenal function | Diagnosis | Pathology | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Schnitzer et al, 1986 |
74 | M | Fatigue, weight loss, fever |
Bilateral | 30 mm/ 40mm |
I | Biopsy | Diffuse large T-cell | No treatment | Early death |
| Oppong et al, 1991 |
63 | M | Weight loss, lethargy, abdominal pain |
Bilateral | NM | I | Biopsy | T-cell | Chemotherapy | Death 4 months later |
| Pimentel et al, 1997 |
42 | M | Vomiting, weight loss, fever |
Bilateral | NM | I | Biopsy | Diffuse large cleaved T-cell | Chemotherapy | Death 4 months later |
| May et al, 1998 |
59 | M | Asymptomatic | Right | NM | N | NM | Centroblastic T-cell | Surgery/radiation | Remission 8 years later |
| Nakatsuka et al, 2002 |
67 | M | Asymptomatic | Left | 110mm | I | Surgery | Peripheral T-cell, not specified | Surgery | Remission 10 months later |
| Nakatsuka et al, 2002 |
74 | M | Fatigue, fever | Bilateral | NM | NM | Autopsy | Peripheral T-cell, not specified | No treatment | Death 33 months later |
| Xu et al, 2003 |
NM | NM | Lumber pain, weight loss, fever |
Bilateral | NM | N | NM | T-cell | Surgery | Death within 3 months |
| Mizoguchi et al, 2005 |
17 | M | Fever, general fatigue | Bilateral | 48 mm/ 50mm |
NM | Autopsy | Nasal-type NK-cell | No treatment | Death 1 week later |
| Tomoyose et al, 2007 |
37 | F | Back pain, malaise, fever |
Bilateral | 92 mm/ 92mm |
N | Biopsy | ATLL | Chemotherapy | Death 11 months later |
| Sfaxi et al, 2008 |
70 | M | Anorexia, weight loss, fever |
Bilateral | 120 mm/ 74mm |
I | Surgery | T-cell lymphoma | Surgery | Death 4 days later |
| Tsukahara et al, 2012 |
79 | F | Cough, bloody sputum | Bilateral | 57 mm/ 74mm |
NM | Autopsy | Nasal-type NK-cell | No treatment | Death 11 days later |
| Bommannan et al, 2017 |
26 | M | Weight loss, blurred vision |
Bilateral | 93 mm/ 92mm |
N | Biopsy | T-cell | No treatment | Early death |
| Bedaiwi et al, 2020 |
71 | M | Anorexia, weight loss, eye pain |
Left | NM | NM | Biopsy | T-cell lymphoma | Chemotherapy | Death within 1 year |
| Frankel et al, 2000 |
62 | M | Fatigue | Bilateral | 150 mm/ 40mm |
NM | Biopsy | Anaplastic large cell | Surgery/ Chemotherapy |
Remission 23 months later |
| Our case | 64 | M | Weight loss, fever, anorexia, fatigue | Left | 75mm | N | Biopsy | Anaplastic large cell | Chemotherapy | Death 10 months later |
Abbreviations: ATLL, adult T-cell leukemia/lymphoma; I, insufficiency; N, normal; NK, natural killer; NM, not mentioned.
Here we presented the case of unilateral primary adrenal ALCL (Table 2). The patient's presentation with unilateral involvement, mild B symptoms, multiorgan infiltration, rapid tumor growth, and heterogeneous internal findings on imaging posed diagnostic challenges, as these features resemble those typically observed in adrenocortical carcinoma. However, elevated levels of LDH and sIL-2R suggested a PAL diagnosis. Historically, around half of T-cell phenotype PALs have been diagnosed histologically postmortem (3 cases) or through adrenalectomy (2 cases) (Table 2).
As the general diagnostic strategy, CT and magnetic resonance imaging HERE are equally effective for differential diagnosis of an adrenal mass because they can provide clear evidence for a benign tumor in most cases when performed according to the state of the art. In addition, FDG-PET, especially when combined with CT, may be highly valuable in patients with suspected adrenal cell carcinoma. High uptake of FDG-PET demonstrates increased glucose metabolism and indicates malignancy [16]. The only indication for a preoperative adrenal biopsy is to obtain histological evidence of secondary adrenal metastasis from a known primary adrenal lesion if doubt persists after all the investigations (thoracic-abdominal-pelvic CT, magnetic resonance imaging, and FDG-PET scan) [17]. The performance of a preoperative biopsy is generally contraindicated because of its low sensitivity and specificity for diagnosing adrenal cell carcinoma and the theoretical risk of capsular rupture and seeding on the needle track [17-19]. Therefore, in our present case, a biopsy from the metastatic site in the vastus lateralis muscle was performed for histological diagnosis.
The final diagnosis was ALCL/ALK-negative and CD30-positive lymphoma, confirmed later in the left adrenal mass. Generally, ALCL/ALK-negative lymphomas have poorer prognoses compared to ALCL/ALK-positive lymphomas [20]. However, outcomes are better than those for peripheral T-cell lymphomas, as BV-CHP treatment provides a clinically meaningful improvement in overall survival over CHOP, achieving a 5-year overall survival rate of 75.8% in patients with ALCL [21]. Conversely, PAL has a poor prognosis, with a 20% 1-year survival rate [22]. Most patients with T-cell phenotype PAL die within 1 year despite treatments, including chemotherapy, surgery, and radiotherapy (Table 2). Fortunately, our patient responded well to initial chemotherapy, resulting in rapid lymphoma regression. Continued accumulation of similar cases will enhance our understanding of the pathological features of primary adrenal ALCL/ALK-negative and CD30-positive lymphomas.
Although some malignant lymphomas may initially present unilaterally and exhibit rapid growth, cases like ours demonstrate that even with multiorgan involvement, prolonged survival is achievable with chemotherapy (Table 2). Biopsy from metastatic sites should be considered early for the diagnosis and treatment of primary adrenal T/natural killer cell lymphomas.
In conclusion, we report the first case of unilateral primary adrenal ALCL. Our literature review underscores the importance of understanding the clinicopathological features, treatment modalities, and prognosis of primary adrenal lymphomas, particularly given the challenge of distinguishing them from rapidly progressing unilateral PAL and adrenocortical carcinoma. Biopsy from metastatic sites played a critical role in enabling prompt diagnosis and initiating effective chemotherapy, thereby contributing to prolonged survival outcomes.
Learning Points
Primary malignant lymphomas originating in the adrenal gland, particularly of T-cell origin, are extremely rare.
In patients with adrenal mass and constitutional symptoms, elevated LDH and sIL-2R may help to distinguish adrenal lymphoma from adrenocortical carcinoma.
Biopsy from metastatic sites plays a critical role in enabling prompt diagnosis and initiating effective chemotherapy.
Acknowledgments
We thank Dr. Hiroko Ikeda for her assistance with the pathological diagnosis and Dr. Miki Okumura, Dr. Takeo Tanaka, and Dr. Kosuke Shima for meaningful discussions.
Contributor Information
Daisuke Goto, Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa 920-8640, Japan.
Yumie Takeshita, Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa 920-8640, Japan.
Kosuke Nagai, Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa 920-8640, Japan.
Hisanori Goto, Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa 920-8640, Japan.
Yujiro Nakano, Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa 920-8640, Japan.
Toshinari Takamura, Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa 920-8640, Japan.
Contributors
D.G. and Y.T. were the lead clinicians in patient management and wrote the manuscript. K.N. was a clinician in patient management. D.G., Y.T., K.N., H.G., Y.N., and T.T. discussed the case. Y.T. was responsible for patient care and edited the manuscript. All authors approved the final submitted version.
Funding
No public or commercial funding.
Disclosures
This case was previously presented at the Japanese Society of Internal Medicine meeting 2022 and the 34th JES Clinical Update on Endocrinology & Metabolism. The authors have nothing to disclose.
Informed Patient Consent for Publication
Signed informed consent was obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
- 1. Mizoguchi Y, Nakamura K, Miyagawa SI, Nishimura SI, Arihiro K, Kobayashi M. A case of adolescent primary adrenal natural killer cell lymphoma. Int J Hematol. 2005;81(4):330‐334. [DOI] [PubMed] [Google Scholar]
- 2. Rashidi A, Fisher SI. Primary adrenal lymphoma: a systematic review. Ann Hematol. 2013;92(12):1583‐1593 [DOI] [PubMed] [Google Scholar]
- 3. Xing Q, Hu C, Zhao Q, et al. Primary adrenal diffuse large B cell lymphoma with tumor thrombus in central adrenal vein: a case report and literature review. BMC Endocr Disord. 2023;23(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnitzer B, Smid D, Lloyd RV. Primary T-cell lymphoma of the adrenal glands with adrenal insufficiency. Hum Pathol. 1986;17(6):634‐636. [DOI] [PubMed] [Google Scholar]
- 5. Pimentel M, Johnston JB, Allan DR, Greenberg H, Bernstein CN. Primary adrenal lymphoma associated with adrenal insufficiency: a distinct clinical entity. Leuk Lymphoma. 1997;24(3-4):363‐367. [DOI] [PubMed] [Google Scholar]
- 6. May F, Bachor R, Hack M, Gottfried HW, Hautmann RE. Primary adrenal nonHodgkin's lymphoma: long-term survival. J Urol. 1998;160(2):487. [PubMed] [Google Scholar]
- 7. Oppong FC, Brodribb AJ, Hunt AC, Ring NE. Bilateral adrenal lymphoma presenting as Addison's disease and hypercalcaemia. Eur J Surg Oncol. 1991;17(4):395‐396. [PubMed] [Google Scholar]
- 8. Nakatsuka SI, Hongyo T, Syaifudin M, Nomura T, Shingu N, Aozasa K. Mutations of p53, c-kit, K-ras, and beta-catenin gene in non-Hodgkin's lymphoma of adrenal gland. Jpn J Cancer Res. 2002;93(3):267‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu A, Xiao X, Ye L, Hong B, Wang X. Primary adrenal lymphoma. Leuk Lymphoma. 2003;44(4):739‐740. [DOI] [PubMed] [Google Scholar]
- 10. Sfaxi M, Bouzouita A, Bouasker I, et al. Primary bilateral adrenal T-cell lymphoma. A case report rarer than B-cell lymphoma. Ann Endocrinol (Paris). 2008;69(3):249‐253. [DOI] [PubMed] [Google Scholar]
- 11. Bommannan K, Sachdeva MUS, Sekar A, Kumar R, Dey P. Primary adrenal T-cell lymphoma in a young adult presented with pseudo-hypopyon: a case report and literature review. Blood Res. 2017;52(3):227‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bedaiwi K, Alfawaz AM, Mohammed SF, et al. Intraocular T-cell lymphoma metastasizing from a primary adrenal T-cell lymphoma: case report. Ann Med Surg (Lond). 2020;60:646‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsukahara T, Takasawa A, Murata M, et al. NK/T-cell lymphoma of bilateral adrenal glands in a patient with pyothorax. Diagn Pathol. 2012;7(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomoyose T, Nagasaki A, Uchihara JN, et al. Primary adrenal adult T-cell leukemia/lymphoma: a case report and review of the literature. Am J Hematol. 2007;82(8):748‐752. [DOI] [PubMed] [Google Scholar]
- 15. Frankel WL, Shapiro P, Weidner N. Primary anaplastic large cell lymphoma of the adrenal gland. Ann Diagn Pathol. 2000;4(3):158‐164. [DOI] [PubMed] [Google Scholar]
- 16. Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23(2):273‐289. [DOI] [PubMed] [Google Scholar]
- 17. Gaujoux S, Weinandt M, Bonnet S, Reslinger V, Bertherat J, Dousset B. Surgical treatment of adrenal carcinoma. J Visc Surg. 2017;154(5):335‐343. [DOI] [PubMed] [Google Scholar]
- 18. Fassnacht M, Dekkers OM, Else T, et al. 2018 European Society of Endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2018;179(4):G1‐G46. [DOI] [PubMed] [Google Scholar]
- 19. Williams AR, Hammer GD, Else T. Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome. Eur J Endocrinol. 2014;170(6):829‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zerbini MC, Soares FA, Velloso ED, Paes RP. World Health Organization classification of tumors of hematopoietic and lymphoid tissues, 2008–major changes from the 3rd edition, 2001. Rev Assoc Med Bras. 2011;57(1):66‐73. [Google Scholar]
- 21. Horwitz S, O’Connor OA, Pro B, et al. The ECHELON-2 trial: 5-year results of a randomized, phase III study of Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol. 2022;33(3):288‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazzola M, Morini L, Bertoglio CL, Boniardi M, Pauna I. Primary adrenal lymphoma: when the attempt to cure becomes the way to make diagnosis. Case report and systematic review of the literature. J Oncol Res Ther. 2018;04:2574‐2710. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.