Abstract
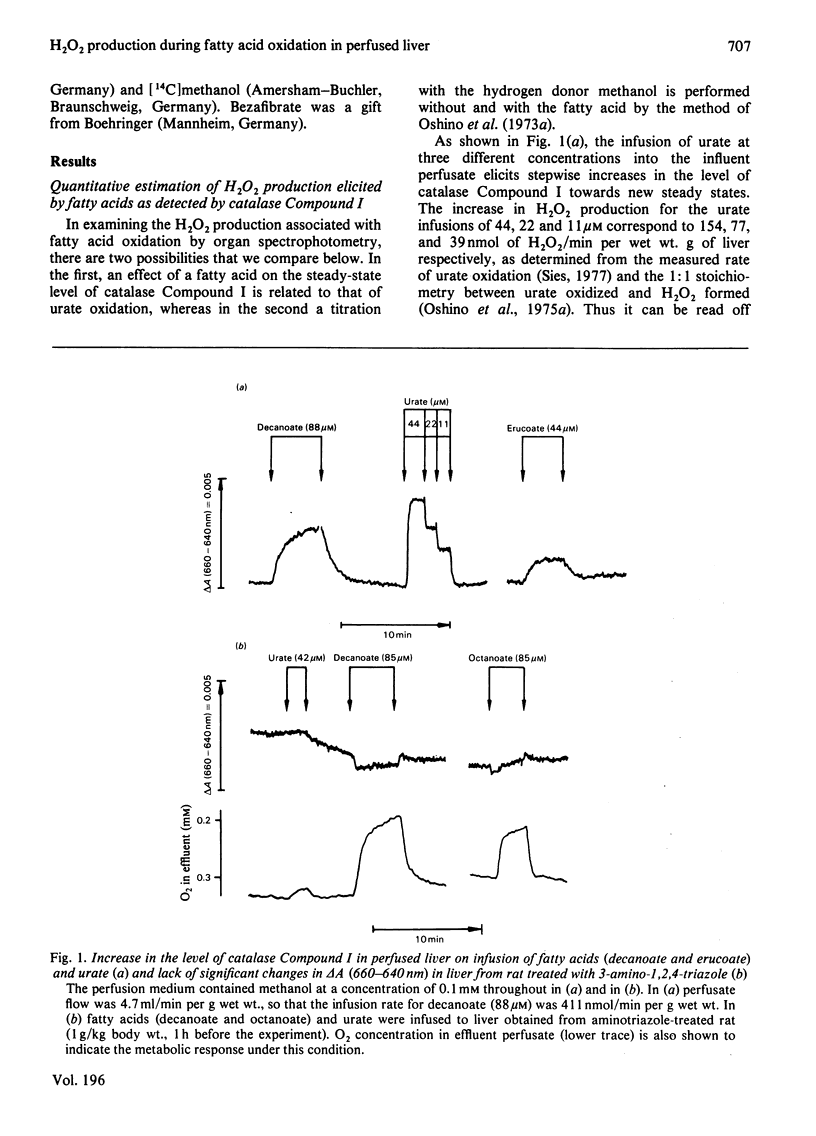
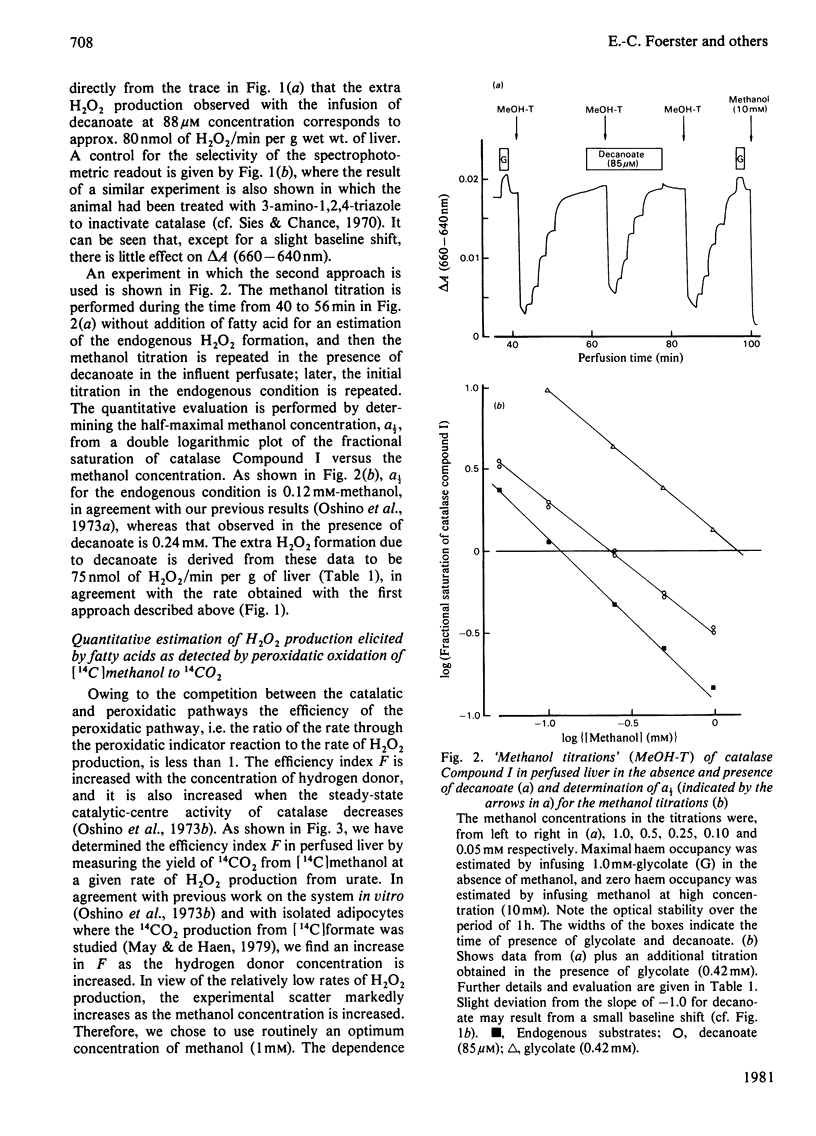
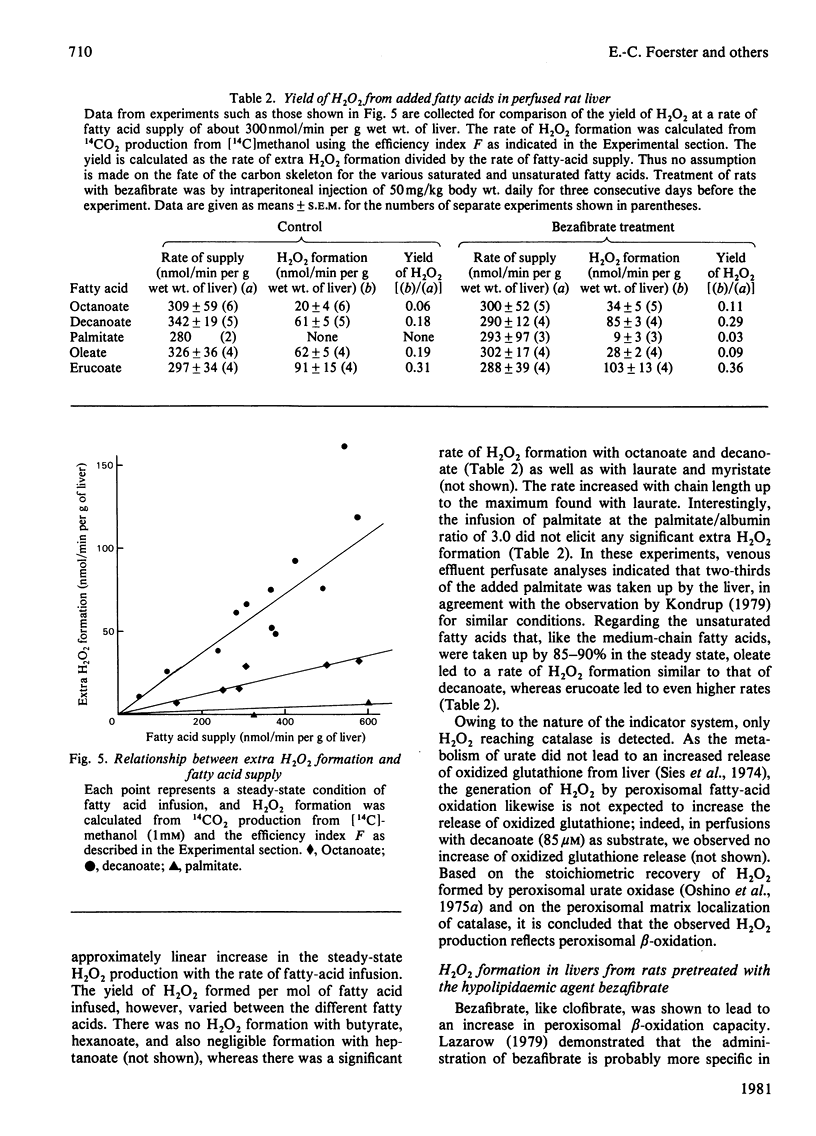
1. H2O2 formation associated with the metabolism of added fatty acids was quantitatively determined in isolated haemoglobin-free perfused rat liver (non-recirculating system) by two different methods. 2. Organ spectrophotometry of catalase Compound I [Sies & Chance (1970) FEBS Lett. 11, 172-176] was used to detect H2O2 formation (a) by steady-state titration with added hydrogen donor, methanol or (b) by comparison of fatty-acid responses with those of the calibration compound, urate. 3. In the use of the peroxidatic reaction of catalase, [14C]methanol was added as hydrogen donor at an optimal concentration of 1 mM in the presence of 0.2 mM-L-methionine, and 14CO2 production rates were determined. 4. Results obtained by the different methods were similar. 5. The yield of H2O2 formation, expressed as the rate of H2O2 formation in relation to the rate of fatty-acid supply, was less than 1.0 in all cases, indicating that, regardless of chain length, less than one acetyl unit was formed per mol of added fatty acid by the peroxisomal system. In particular, the standard substrate used with isolated peroxisomal preparations (C16:0 fatty acid) gave low yield (close to zero). Long-chain monounsaturated fatty acids exhibit a relatively high yield of H2O2 formation. 6. The hypolipidaemic agent bezafibrate led to slightly increased yields for most of the acids tested, but the yield with oleate was decreased to one-half the original yield. 7. It is concluded that in the intact isolated perfused rat liver the assayable capacity for peroxisomal beta-oxidation is used to only a minor degree. However, the observed rates of H2O2 production with fatty acids can account for a considerable share of the endogenous H2O2 production found in the intact animal.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelkvist E. L., Dallner G. Possible involvement of fatty acid binding protein in peroxisomal beta-oxidation of fatty acids. Biochim Biophys Acta. 1980 Jan 18;617(1):156–160. doi: 10.1016/0005-2760(80)90233-7. [DOI] [PubMed] [Google Scholar]
- Bronfman M., Inestrosa N. C., Leighton F. Fatty acid oxidation by human liver peroxisomes. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1030–1036. doi: 10.1016/0006-291x(79)91512-2. [DOI] [PubMed] [Google Scholar]
- Christiansen R. Z., Osmundsen H., Borrebaek B., Bremer J. The effects of clofibrate feeding on the metabolism of palmitate and erucate in isolated hepatocytes. Lipids. 1978 Jul;13(7):487–491. doi: 10.1007/BF02533618. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- DUNCOMBE W. G. THE COLORIMETRIC MICRO-DETERMINATION OF NON-ESTERIFIED FATTY ACIDS IN PLASMA. Clin Chim Acta. 1964 Feb;9:122–125. doi: 10.1016/0009-8981(64)90004-x. [DOI] [PubMed] [Google Scholar]
- Hajra A. K., Burke C. L., Jones C. L. Subcellular localization of acyl coenzyme A: dihydroxyacetone phosphate acyltransferase in rat liver peroxisomes (microbodies). J Biol Chem. 1979 Nov 10;254(21):10896–10900. [PubMed] [Google Scholar]
- Hryb D. J., Hogg J. F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Weiss L., Sies H. Activation of pyruvate dehydrogenase during metabolism of ammonium ions in hemoglobin-free perfused rat liver. Eur J Biochem. 1975 Apr 1;52(3):421–431. doi: 10.1111/j.1432-1033.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Bronfman M., Leighton F. Detection of peroxisomal fatty acyl-coenzyme A oxidase activity. Biochem J. 1979 Sep 15;182(3):779–788. doi: 10.1042/bj1820779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa N. C., Bronfman M., Leighton F. Properties of fatty acyl-CoA oxidase from rat liver, a peroxisomal flavoprotein. Life Sci. 1979 Sep 24;25(13):1127–1135. doi: 10.1016/0024-3205(79)90134-6. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Bronfman M., Leighton F. Purification of the peroxisomal fatty acyl-CoA oxidase from rat liver. Biochem Biophys Res Commun. 1980 Jul 16;95(1):7–12. doi: 10.1016/0006-291x(80)90696-8. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Hajra A. K. Properties of guinea pig liver peroxisomal dihydroxyacetone phosphate acyltransferase. J Biol Chem. 1980 Sep 10;255(17):8289–8295. [PubMed] [Google Scholar]
- Kawamoto S., Nozaki C., Tanaka A., Fukui S. Fatty acid beta-oxidation system in microbodies of n-alkane-grown Candida tropicalis. Eur J Biochem. 1978 Feb;83(2):609–613. doi: 10.1111/j.1432-1033.1978.tb12130.x. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Kondrup J. Metabolism of palmitate in perfused rat liver. Isolation of subcellular fractions containing triacylglycerol. Biochem J. 1979 Oct 15;184(1):63–71. doi: 10.1042/bj1840063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahling J. B., Gee R., Murphy P. A., Kirk J. R., Tolbert N. E. Comparison of fatty acid oxidation in mitochondria and peroxisomes from rat liver. Biochem Biophys Res Commun. 1978 May 15;82(1):136–141. doi: 10.1016/0006-291x(78)90587-9. [DOI] [PubMed] [Google Scholar]
- Kramar R., Hüttinger M., Gmeiner B., Goldenberg H. Beta-oxidation in peroxisomes of brown adipose tissue. Biochim Biophys Acta. 1978 Dec 22;531(3):353–356. doi: 10.1016/0005-2760(78)90217-5. [DOI] [PubMed] [Google Scholar]
- Krisans S. K., Mortensen R. M., Lazarow P. B. Acyl-CoA synthetase in rat liver peroxisomes. Computer-assisted analysis of cell fractionation experiments. J Biol Chem. 1980 Oct 25;255(20):9599–9607. [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. The immediate and long term effects of clofibrate on the metabolism of the perfused rat liver. Biochem Pharmacol. 1979 Sep 15;28(18):2813–2827. doi: 10.1016/0006-2952(79)90567-7. [DOI] [PubMed] [Google Scholar]
- Laurell S., Tibbling G. Colorimetric micro-determination of free fatty acids in plasma. Clin Chim Acta. 1967 Apr;16(1):57–62. doi: 10.1016/0009-8981(67)90269-0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Mannaerts G. P., Debeer L. J., Thomas J., De Schepper P. J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979 Jun 10;254(11):4585–4595. [PubMed] [Google Scholar]
- Masters C., Holmes R. Peroxisomes: new aspects of cell physiology and biochemistry. Physiol Rev. 1977 Oct;57(4):816–882. doi: 10.1152/physrev.1977.57.4.816. [DOI] [PubMed] [Google Scholar]
- May J. M., de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979 Apr 10;254(7):2214–2220. [PubMed] [Google Scholar]
- Murphy P. A., Krahling J. B., Gee R., Kirk J. R., Tolbert N. E. Enzyme activities of isolated hepatic peroxisomes from genetically lean and obese male mice. Arch Biochem Biophys. 1979 Mar;193(1):179–185. doi: 10.1016/0003-9861(79)90021-3. [DOI] [PubMed] [Google Scholar]
- Oshino N., Chance B., Sies H., Bücher T. The role of H 2 O 2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys. 1973 Jan;154(1):117–131. doi: 10.1016/0003-9861(73)90040-4. [DOI] [PubMed] [Google Scholar]
- Oshino N., Jamieson D., Chance B. The properties of hydrogen peroxide production under hyperoxic and hypoxic conditions of perfused rat liver. Biochem J. 1975 Jan;146(1):53–65. doi: 10.1042/bj1460053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Jamieson D., Sugano T., Chance B. Optical measurement of the catalase-hydrogen peroxide intermediate (Compound I) in the liver of anaesthetized rats and its implication to hydrogen peroxide production in situ. Biochem J. 1975 Jan;146(1):67–77. doi: 10.1042/bj1460067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Oshino R., Chance B. The characteristics of the "peroxidatic" reaction of catalase in ethanol oxidation. Biochem J. 1973 Mar;131(3):555–563. doi: 10.1042/bj1310555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmundsen H., Neat C. E., Borrebaek B. Fatty acid products of peroxisomal beta-oxidation. Int J Biochem. 1980;12(4):625–630. doi: 10.1016/0020-711x(80)90015-4. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Neat C. E., Norum K. R. Peroxisomal oxidation of long chain fatty acids. FEBS Lett. 1979 Mar 15;99(2):292–296. doi: 10.1016/0014-5793(79)80975-8. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Neat C. E. Regulation of peroxisomal fatty acid oxidation. FEBS Lett. 1979 Nov 1;107(1):81–85. doi: 10.1016/0014-5793(79)80468-8. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem Biophys Res Commun. 1978 Jul 28;83(2):479–485. doi: 10.1016/0006-291x(78)91015-x. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T., Ui N. Purification and properties of acyl-CoA oxidase from rat liver. J Biochem. 1980 Jun;87(6):1735–1746. doi: 10.1093/oxfordjournals.jbchem.a132918. [DOI] [PubMed] [Google Scholar]
- Schwab H., Sies H. A new organ spectrophotometer for sensitive dual-wavelength absorbance measurement and spectral scanning of intact perfused organs. Hoppe Seylers Z Physiol Chem. 1978 Mar;359(3):385–392. doi: 10.1515/bchm.1978.359.1.385. [DOI] [PubMed] [Google Scholar]
- Shindo Y., Hashimoto T. Acyl-Coenzyme A synthetase and fatty acid oxidation in rat liver peroxisomes. J Biochem. 1978 Nov;84(5):1177–1181. doi: 10.1093/oxfordjournals.jbchem.a132234. [DOI] [PubMed] [Google Scholar]
- Sies H., Bücher T., Oshino N., Chance B. Heme occupancy of catalase in hemoglobin-free perfused rat liver and of isolated rat liver catalase. Arch Biochem Biophys. 1973 Jan;154(1):106–116. doi: 10.1016/0003-9861(73)90039-8. [DOI] [PubMed] [Google Scholar]
- Sies H., Chance B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970 Dec;11(3):172–176. doi: 10.1016/0014-5793(70)80521-x. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxygen gradients during hypoxic steady states in liver. Urate oxidase and cytochrome oxidase as intracellular O2 indicators. Hoppe Seylers Z Physiol Chem. 1977 Aug;358(8):1021–1032. doi: 10.1515/bchm2.1977.358.2.1021. [DOI] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Thomas J., Debeer L. J., De Schepper P. J., Mannaerts G. P. Factors influencing palmitoyl-CoA oxidation by rat liver peroxisomal fractions. Substrate concentration, organelle integrity and ATP. Biochem J. 1980 Sep 15;190(3):485–494. doi: 10.1042/bj1900485. [DOI] [PMC free article] [PubMed] [Google Scholar]



